-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown
Causes PKD-Phenotypes
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by
formation of renal cysts that destroy the kidney. Mutations in PKD1 and PKD2,
encoding polycystins-1 and -2, cause ADPKD. Polycystins are thought to function
in primary cilia, but it is not well understood how these and other proteins are
targeted to cilia. Here, we provide the first genetic and biochemical link
between polycystins and the exocyst, a highly-conserved eight-protein membrane
trafficking complex. We show that knockdown of exocyst component Sec10 yields
cellular phenotypes associated with ADPKD, including loss of flow-generated
calcium increases, hyperproliferation, and abnormal activation of MAPK. Sec10
knockdown in zebrafish phenocopies many aspects of polycystin-2
knockdown—including curly tail up, left-right patterning defects,
glomerular expansion, and MAPK activation—suggesting that the exocyst is
required for pkd2 function in vivo. We observe
a synergistic genetic interaction between zebrafish sec10 and
pkd2 for many of these cilia-related phenotypes.
Importantly, we demonstrate a biochemical interaction between Sec10 and the
ciliary proteins polycystin-2, IFT88, and IFT20 and co-localization of the
exocyst and polycystin-2 at the primary cilium. Our work supports a model in
which the exocyst is required for the ciliary localization of polycystin-2, thus
allowing for polycystin-2 function in cellular processes.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1001361
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001361Summary
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by
formation of renal cysts that destroy the kidney. Mutations in PKD1 and PKD2,
encoding polycystins-1 and -2, cause ADPKD. Polycystins are thought to function
in primary cilia, but it is not well understood how these and other proteins are
targeted to cilia. Here, we provide the first genetic and biochemical link
between polycystins and the exocyst, a highly-conserved eight-protein membrane
trafficking complex. We show that knockdown of exocyst component Sec10 yields
cellular phenotypes associated with ADPKD, including loss of flow-generated
calcium increases, hyperproliferation, and abnormal activation of MAPK. Sec10
knockdown in zebrafish phenocopies many aspects of polycystin-2
knockdown—including curly tail up, left-right patterning defects,
glomerular expansion, and MAPK activation—suggesting that the exocyst is
required for pkd2 function in vivo. We observe
a synergistic genetic interaction between zebrafish sec10 and
pkd2 for many of these cilia-related phenotypes.
Importantly, we demonstrate a biochemical interaction between Sec10 and the
ciliary proteins polycystin-2, IFT88, and IFT20 and co-localization of the
exocyst and polycystin-2 at the primary cilium. Our work supports a model in
which the exocyst is required for the ciliary localization of polycystin-2, thus
allowing for polycystin-2 function in cellular processes.Introduction
ADPKD is the most common potentially lethal monogenetic disorder, affecting 12 million people worldwide [1]. ADPKD is characterized by the development of numerous renal cysts, which greatly increase kidney size, perturb kidney function, and eventually lead to kidney failure. While we know that mutations in PKD1 and PKD2 cause ADPKD [2], [3], we are only beginning to understand how the proteins—polycystin-1 and polycystin-2—regulate the cellular phenotypes associated with cystogenesis.
Interactions between polycystin-2, a calcium-permeable cation channel [4], [5], and polycystin-1 may act to regulate calcium signaling in normal kidney cells [6]. Consistent with calcium regulation being relevant to cystogenesis, ADPKD cells show a lower basal intracellular calcium concentration [7]. Furthermore, altered calcium regulation has been linked, through cyclic AMP (cAMP) signaling, to phenotypes observed during cystogenesis, such as increased cell proliferation and abnormal fluid secretion. Addition of cAMP agonists cause ADPKD cells, but not normal kidney cells, to stimulate proliferation via the MAPK pathway [6], [8], [9].
Growing evidence suggests that the cilium is an important site of polycystin function. Kidney tubular epithelial cells have a single non-motile primary cilium that acts as a mechanosensor, triggering a rise in intracellular calcium in response to fluid flow [10], [11]. Polycystins-1 and -2 localize to the primary cilium of kidney cells [12], [13], and the calcium response to fluid flow requires polycystin function [14]. Consistent with the idea that mechanosensation is relevant to cystogenesis, ADPKD cells are unresponsive to fluid flow [15].
Research in animal models suggests that cilia play important roles, not only during adult kidney function, but also throughout early embryonic development (reviewed in [16]). Zebrafish has been increasingly used as a model organism to expand our understanding of the in vivo function of ciliary proteins through studies utilizing mutants that affect cilia, and morpholino antisense knockdown of ciliary proteins. Loss of intraflagellar transport proteins (reviewed in [17]), which are required for cilia assembly, results in body axis curvatures (“curly tails”), left-right defects, pronephric cysts, edema, and small eye phenotypes [18]-[21]. Other mutants that show disrupted cilia length or motility similarly show curly tails, left-right defects, and pronephric cysts [20], [22]-[26].
These phenotypes, which comprise the range of cilia-related phenotypes in zebrafish, suggest that proper cilia formation and/or function is required for multiple developmental processes. The mechanistic relationship connecting cilia to each phenotype is understood to differing degrees depending on the specific phenotype. The connection is well understood for left-right patterning and pronephric development. Left-right patterning governs the stereotypical positioning of organs, which is preceded and directed by left-sided expression of the Nodal signaling pathway (reviewed in [27]). The asymmetric expression of the nodal genes spaw, lefty1, and lefty2 in zebrafish is itself thought to be established by cilia-dependent fluid flow in Kupffer's vesicle [18], [28]. Indeed, mutants that show disrupted cilia length or flow in Kupffer's vesicle subsequently show randomized nodal gene expression and left-right defects [18], [28]. Cilia in the pronephric tubules are similarly thought to be important for pronephric development such that perturbations in motility result in tubule dilations and cystogenesis [25], [26].
Research into pkd2 function in zebrafish has further strengthened the idea that polycystin-2 functions in the cilium. Knockdown of pkd2 by morpholino [29]-[31] or in mutants [20], [31] produces phenotypes that are consistent with a role in cilia function: curly tails, left-right defects, pronephric cysts, and edema. Indeed, polycystin-2 is expressed in Kupffer's vesicle, and mutations in pkd2 lead to defects in left-right patterning in zebrafish and mice [29]-[32]. However, pkd2 is unique in zebrafish for a number of reasons. First, it is the only reported mutant to consistently display a curly tail up phenotype [20], [29]-[31], as opposed to the typical curly tail down phenotype of other cilia mutants. Secondly, pkd2 knockdown does not produce observable defects in cilia structure [29]-[31] or motility [25], [30]. Therefore, pkd2 is likely to be important for cilia function in a way that is distinct from a role in cilia formation, maintenance, or motility. For example, it has been proposed that pkd2 may play a specific mechanosensory role related to calcium regulation during left-right patterning in mice [33].
While we are beginning to identify the roles ciliary proteins play in diverse biological processes, there is little known about how these proteins are transported to the cilium [34]. The exocyst, originally identified in S. cerevisiae [35], is a highly conserved 750kD eight-protein complex known for the targeting and docking of vesicles carrying membrane proteins [36]. It is comprised of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 [37]. Notably, in addition to being found near the tight junction, we localized exocyst proteins to the primary cilium in kidney cells [38], [39]. Sec10 and Sec15 are the most vesicle-proximal of the exocyst components. Sec10 has been shown to directly bind to Sec15, which, in turn, directly binds Sec4, a Rab GTPase on the surface of transport vesicles. Sec10 then acts as a “linker”, by binding the other exocyst components through Sec5 [40]. Our previous studies suggested that the exocyst would no longer be able to bind Sec15 and target/dock transport vesicles without Sec10, and would, instead, disintegrate and be degraded. Importantly, we showed that knockdown of exocyst Sec10 in Madin-Darby canine kidney (MDCK) cells abrogated ciliogenesis, while Sec10 overexpression enhanced ciliogenesis. Furthermore, Sec10 knockdown caused abnormal cystogenesis when the cells were grown in a collagen matrix, and decreased the levels of other exocyst components and the intraflagellar transport protein 88 (IFT88). This was in contrast to knockdown of exocyst components Sec8 and Exo70, which had no effect on ciliogenesis, cystogenesis, or levels of other exocyst components [39]. These data uncovered a role for the exocyst, and especially the Sec10 component, in building the primary cilium. Given its known role in trafficking proteins to the plasma membrane [41]-[44], we have proposed that Sec10 and the exocyst may be required in the cilium to target and dock vesicles carrying proteins important for ciliogenesis.
Here we show that Sec10 knockdown, in vitro in MDCK cells and in vivo in zebrafish, results in phenotypes associated with loss of polycystins and ADPKD. We specifically demonstrate a genetic and biochemical interaction between Sec10 and polycystin-2, as well as show co-localization at the primary cilium, providing further evidence that the exocyst is important for polycystin-2 function. Furthermore, we show biochemical interactions between Sec10 and the ciliogenesis proteins IFT88 and IFT20. Our results demonstrate that the exocyst is required for pkd2 function in the cell. Together with our previous results, these data suggest that the exocyst is important for maintaining both cilia structure and function. Exocyst dysfunction may therefore contribute to ciliopathies including ADPKD, and Sec10 may represent a novel target for the development of effective treatments.
Results
Exocyst Sec10 knockdown leads to a cellular phenotype similar to ADPKD cells
Given that loss of polycystin-2 leads to ADPKD, we first determined whether exocyst Sec10 knockdown or overexpression in MDCK cells produced ADPKD-like phenotypes. Primary cultures of ADPKD cells fail to show the expected rise in calcium levels in response to a shear flow [7], [15]. After growing these MDCK cell lines to confluent monolayers on Transwell filters, conditions that we have previously shown results in ciliation [39], we measured steady state levels of intracellular calcium using the Fura-2 indicator. We then tested whether cells exposed to a constant 5 ml/minute flow rate over the apical surface responded appropriately with an increase in calcium levels. Sec10 knockdown cells showed a significantly lower basal calcium level than both control MDCK and Sec10-overexpressing cells, and calcium levels in the Sec10 knockdown cells also failed to increase in response to fluid flow (0.2% increase in Sec10 knockdown versus 5.8% in control and 26.2% in Sec10-overexpressing cells) (Figure 1A). Thus, Sec10 knockdown cells do indeed produce phenotypes similar to that observed in ADPKD cells [7], [15]. The reason for the limited increase in calcium in response to fluid flow in control T23 MDCK cells, that constitutively express the tetracycline transactivator that drives the Sec10 shRNA, is likely due to the fact that ciliogenesis, for unknown reasons, is sporadic—with the literature describing from ∼30% of T23 MDCK cells being ciliated (as we see [39]), to as few as 14.6% being ciliated [45]. The loss of mechanosensation in Sec10 knockdown cells is consistent with the loss of cilia in these cells [39], [46]. Similarly, since Sec10-overexpressing cells display longer cilia [39], the increased calcium response may reflect a heightened mechanosensory capability of those cilia.
Fig. 1. Sec10 knockdown in MDCK cells leads to ADPKD-like phenotypes. 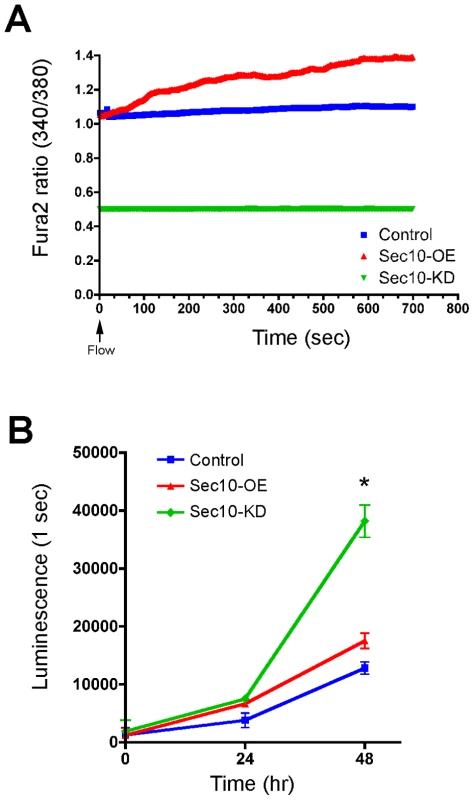
Cellular hyperproliferation is another major feature of ADPKD cells [47], so we investigated whether the abnormal calcium level observed in Sec10 knockdown cells was associated with hyperproliferation. Using a luminescence-based viability assay, Sec10 knockdown cells showed an increased rate of proliferation after 48 hours (Figure 1B). Sec10-overexpressing cells, by contrast, showed a relatively normal rate of proliferation.
Exocyst Sec10 knockdown leads to activation of MAPK
The mitogen activated protein kinase (MAPK) pathway is activated during cell proliferation and kidney development [48], [49]. It has been shown in some mouse models of ADPKD that the MAPK pathway is activated, and that blockage of extracellular-signal regulated kinase (ERK) slows the development of polycystic kidney disease [50]. It has been theorized that the decreased intracellular calcium in primary ADPKD cells, resulting from the dysfunctional primary cilia, leads to increased cyclic AMP (cAMP) and, therefore, protein kinase A (PKA) activity, with downstream MAPK pathway hyperactivation [51]-[53]. We therefore tested whether the low intracellular calcium and hyperproliferative phenotypes of Sec10 knockdown cells were accompanied by activation of the MAPK pathway. The MAPK pathway involves the phosphorylation cascade of Raf, MEK, and ERK. By Western blot analysis, phosphorylated ERK (pERK)—a measure of MAPK activation—was increased in Sec10 knockdown cells relative to normal cells by 4.6-fold (Figure 2A and 2B). This increase in pERK was blocked completely with a 1-hour treatment of a MEK inhibitor (U0126) and a src-family inhibitor (PP2). Treatment with a cAMP-activated PKA inhibitor (H-89) partially blocked activated ERK, restoring levels of pERK to approximately that of control MDCK cells. Treatment with other kinase inhibitors, including a PKC inhibitor (BIM) and an mTOR inhibitor (Rapamycin), were ineffective in blocking overactivation of pERK in Sec10 knockdown cells (Figure 2C and 2D), suggesting that the pERK increase was specific for the MAPK pathway. These data support the idea that the increased pERK observed in Sec10 knockdown cells is due to the combined upstream activities of PKA, Src, and MEK.
Fig. 2. Sec10 knockdown in MDCK cells leads to activation of MAPK. 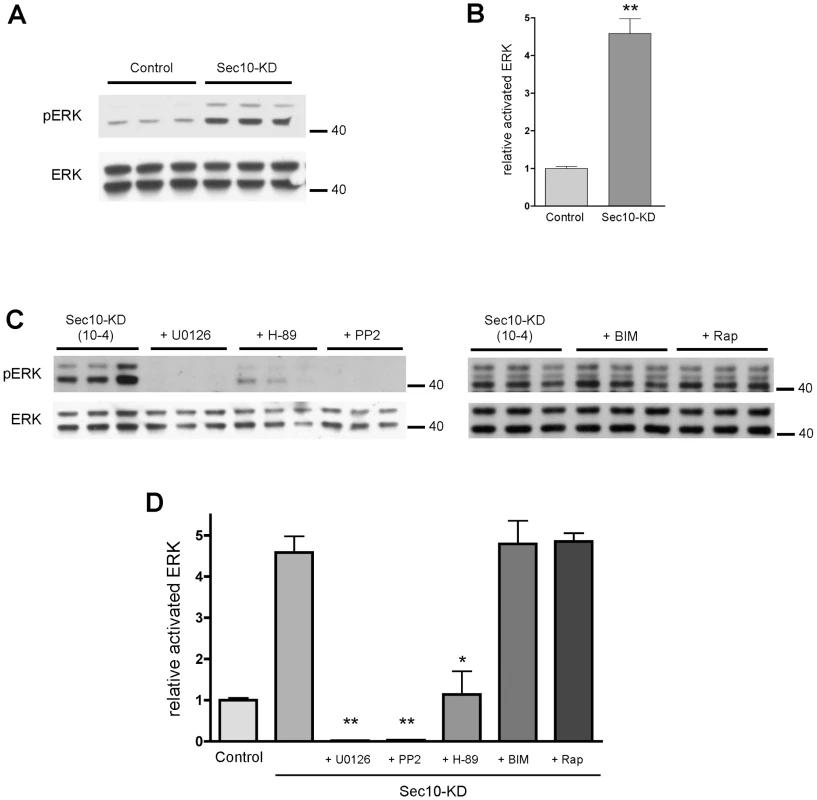
Together, our in vitro results show that Sec10 knockdown cells display many cellular phenotypes shared with ADPKD cells: from abnormal calcium regulation associated with an insensitivity to fluid flow, to increased proliferation associated with MAPK activity.
Exocyst Sec10 is required for normal pronephric development in zebrafish
To determine how sec10 affects cilia and cilia-related processes in vivo, we utilized morpholinos (MOs) to knockdown zebrafish Sec10 (zfSec10) levels. Our first start-site morpholino was ineffective at knocking down zfSec10 levels (see Materials and Methods). Since exocyst Sec8 knockout mice display very early embryonic lethality, well before kidney development occurs [54], we did not focus on developing working start-site morpholinos because these would affect both maternal and zygotic transcripts and could cause early phenotypes that would preclude any analysis of specific phenotypes. Thus, we utilized splice-site morpholinos, which would bypass any early general requirement for the exocyst and allow us to focus on later tissue-specific effects of Sec10 knockdown.
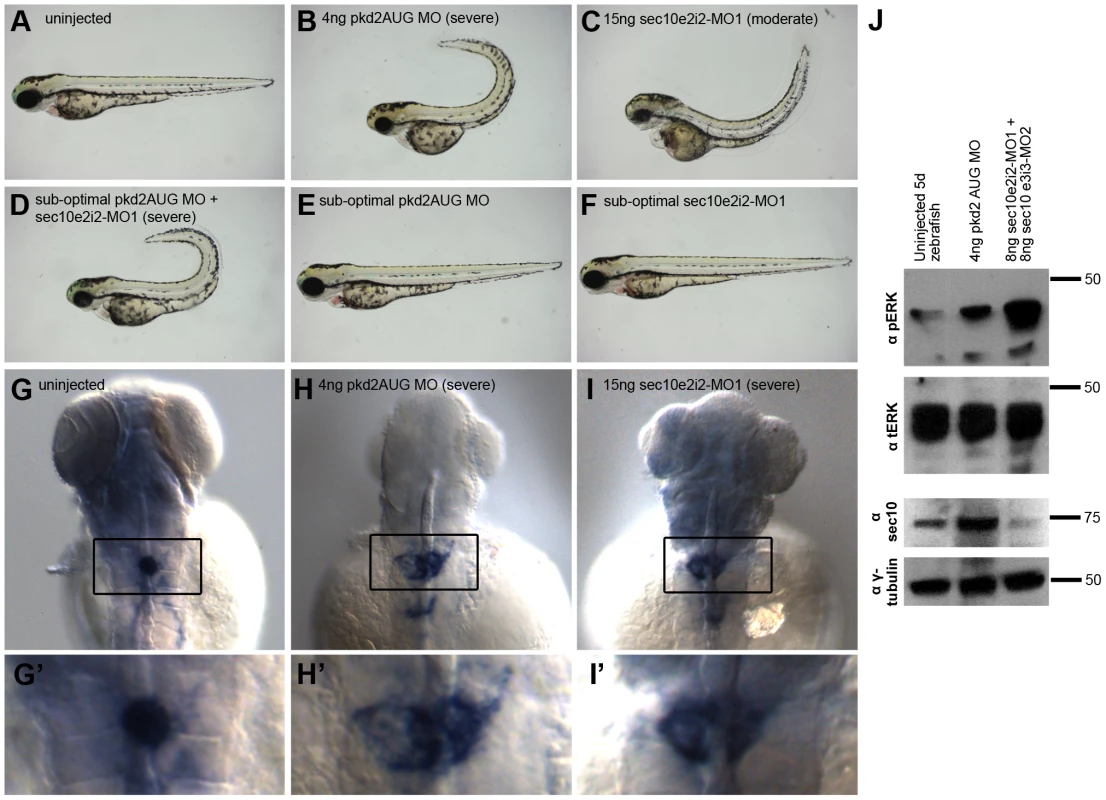
Two splice-site morpholinos (MOs) against zebrafish sec10 were designed and injected either alone, 15 ng sec10e2i2-MO1, or as a combined dose of 8 ng sec10e2i2-MO1 +8 ng sec10e3i3-MO2. Hereafter, we will use the following shorthand for such morpholino-injected embryos: “15ng sec10MO embryos” and “8+8ng sec10MO embryos”, respectively. Aberrant splicing was verified by sequencing transcripts from 24 hours post fertilization (hpf) cDNA libraries made from sec10MO embryos. Multiple splicing variants were not observed. Sequencing of the transcript from 15ng sec10MO embryos revealed a 25 bp deletion in exon 2. The same 25 bp deletion and an additional 27 bp deletion in exons 3 and 4 were observed in the 8+8ng sec10MO injected embryos. Both cases result in a truncated 33 amino acid protein product. Furthermore, the level of Sec10 knockdown was assayed directly by Western blot with antibody against human Sec10 (hSec10) [39] (Figure 3A). While some zfSec10 remained at 1 day post fertilization (dpf) in sec10MO embryos, levels were significantly reduced soon thereafter. Therefore, these sec10 splice-site morpholinos can be used to effectively knockdown Sec10.
Fig. 3. sec10MO embryos show abnormal pronephric development. 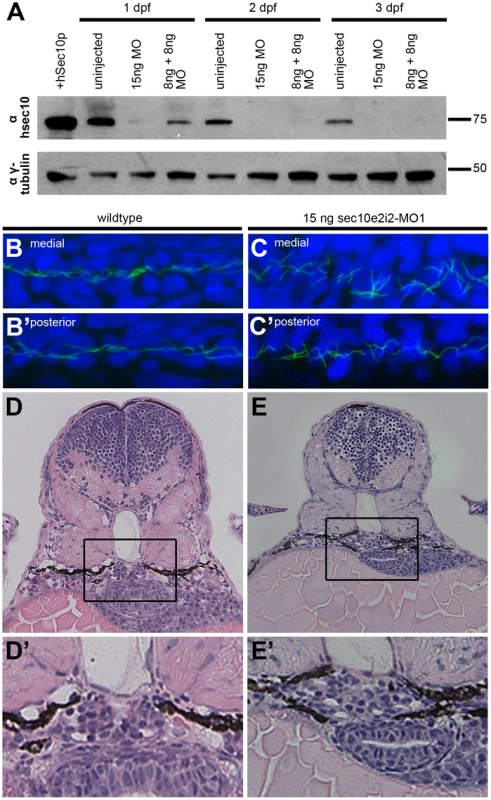
Tab. 1. sec10MO embryos show variable gross phenotypes at 3 dpf. 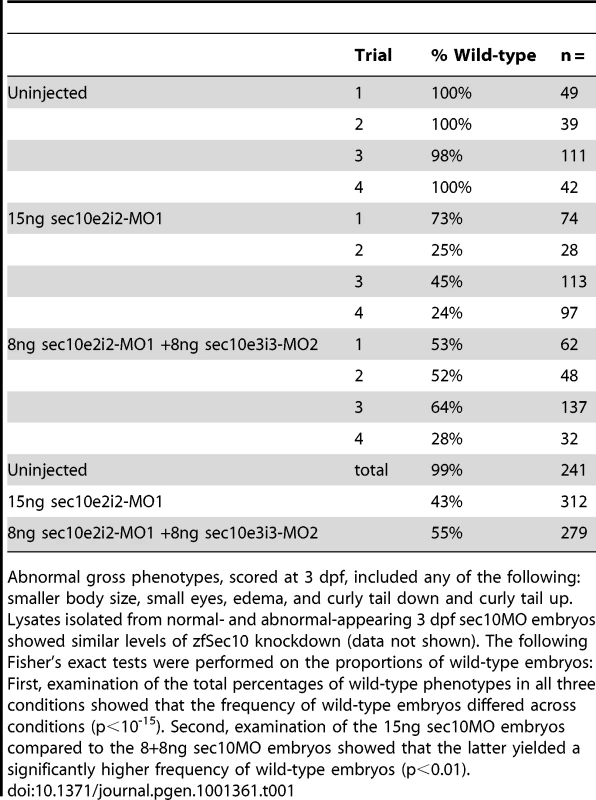
Given the ciliogenesis defects observed with Sec10 knockdown in vitro [39], we predicted that pronephric cilia would be shorter in sec10MO embryos. Surprisingly, pronephric cilia length at 1 dpf appeared normal by immunofluorescence (Figure 3B-3C′). We then assayed whether cilia motility was affected. Whereas mammalian kidney cilia are non-motile, pronephric cilia in the zebrafish are motile [18], [25]. We assayed cilia motility at 2 dpf and, surprisingly, found that it was intact in sec10MO embryos (Videos S1 and S2).
The discrepancy in ciliogenesis phenotypes between Sec10 knockdown in vitro and in vivo may be explained by incomplete knockdown of Sec10 protein in zebrafish. Since we utilized a splice-site morpholino, it is likely that maternally-deposited RNA and/or protein was sufficient to allow for establishment of the cilia at 1 dpf. Indeed, our Western blot analysis detected Sec10 protein at this time (Figure 3A). Therefore, pronephric cilia may only require Sec10 for initial ciliogenesis, but not for later maintenance of the structure. This may also be true for cilia motility.
Unexpectedly, sec10MO embryos showed defects in pronephric development despite the absence of pronephric cilia structure and motility defects. At 1 dpf, the cilia were disordered specifically within the medial pronephros (uninjected: n = 0/3 disorganized; 15ng sec10MO: n = 1/3 disorganized; 8+8ng sec10MO: n = 3/5 disorganized, compare Figure 3B and 3C), where we have observed dilations and pronephric cysts in other zebrafish cilia mutants [20], [25]. Since the disorganization suggested pronephric tubule dilation, we performed histological analysis to look directly for pronephric defects. At 3 dpf, sec10MO embryos did not show obvious dilations in the pronephros; however, the morphology of the glomerulus was abnormal. Instead of a normal compact U-shaped glomerulus, sec10MO embryos showed disorganization, which may be due to increased cell number (uninjected: n = 0/1 disorganized; 15ng sec10MO: n = 3/5 disorganized; 8+8ng sec10MO: n = 1/2 disorganized, compare Figure 3D′ and 3E′).
Therefore, while in vivo Sec10 knockdown did not affect pronephric cilia structure or motility, we still observed defects in pronephric development.
Knockdown of sec10 partially phenocopies loss of pkd2
Though sec10 knockdown did not affect ciliogenesis in vivo, sec10 knockdown may still perturb other aspects of cilia function, even if cilia structure and motility are intact. Consistent with this possibility, sec10MO embryos displayed a range of gross phenotypes that have been observed in other zebrafish cilia mutants, including smaller body size, small eyes, edema, and curly tail up [18]-[21], [25], [26], [31]. These phenotypes were variably penetrant (Table 1), despite similar levels of protein knockdown (Figure 3A, data not shown). These phenotypes suggest that while a residual level of maternal Sec10 protein was adequate to maintain cilia structure in sec10MO embryos, higher levels are required for full wild-type cilia function.
One way in which sec10 could be important for cilia function is through regulating pkd2 function. Notably, pkd2 knockdown in zebrafish does not produce defects in cilia structure [29]-[31] or motility [25], [30]—similar to what we observed with Sec10 knockdown by splice-site morpholinos. Since pkd2 is specifically known to be important for cilia function, and our in vitro analysis revealed ADPKD-like behaviour in Sec10 knockdown cells, we wanted to determine whether Sec10 knockdown would share phenotypes associated with pkd2 knockdown in vivo as well. Importantly, we noticed that the curly tail up phenotype of sec10MO embryos was reminiscent of the unique curly tail up observed from loss of pkd2 in zebrafish (uninjected: 0% curly tail up, n = 42; compared to 15ng sec10MO: 51%, n = 97; and 8+8ng sec10MO: 6%, n = 32; Figure 4A and 4C) [20], [29]-[31].
To determine whether sec10MO embryos shared other phenotypes with pkd2MO embryos, we investigated whether sec10MO embryos displayed left-right defects. Indeed, like pkd2MO embryos, sec10MO embryos show defects in left-right patterning with respect to the positioning of the visceral organs (Table 2). Additionally, we observed defects in asymmetric nodal gene expression in sec10MO embryos (Table 2), as would be expected if cilia function was disrupted. Ciliary function in Kupffer's vesicle is known to be upstream of asymmetric nodal expression [28]. Thus, loss of sec10 may affect left-right patterning indirectly through its effects on cilia and/or polycystin-2 function.
Tab. 2. Left-right defects in sec10MO embryos. 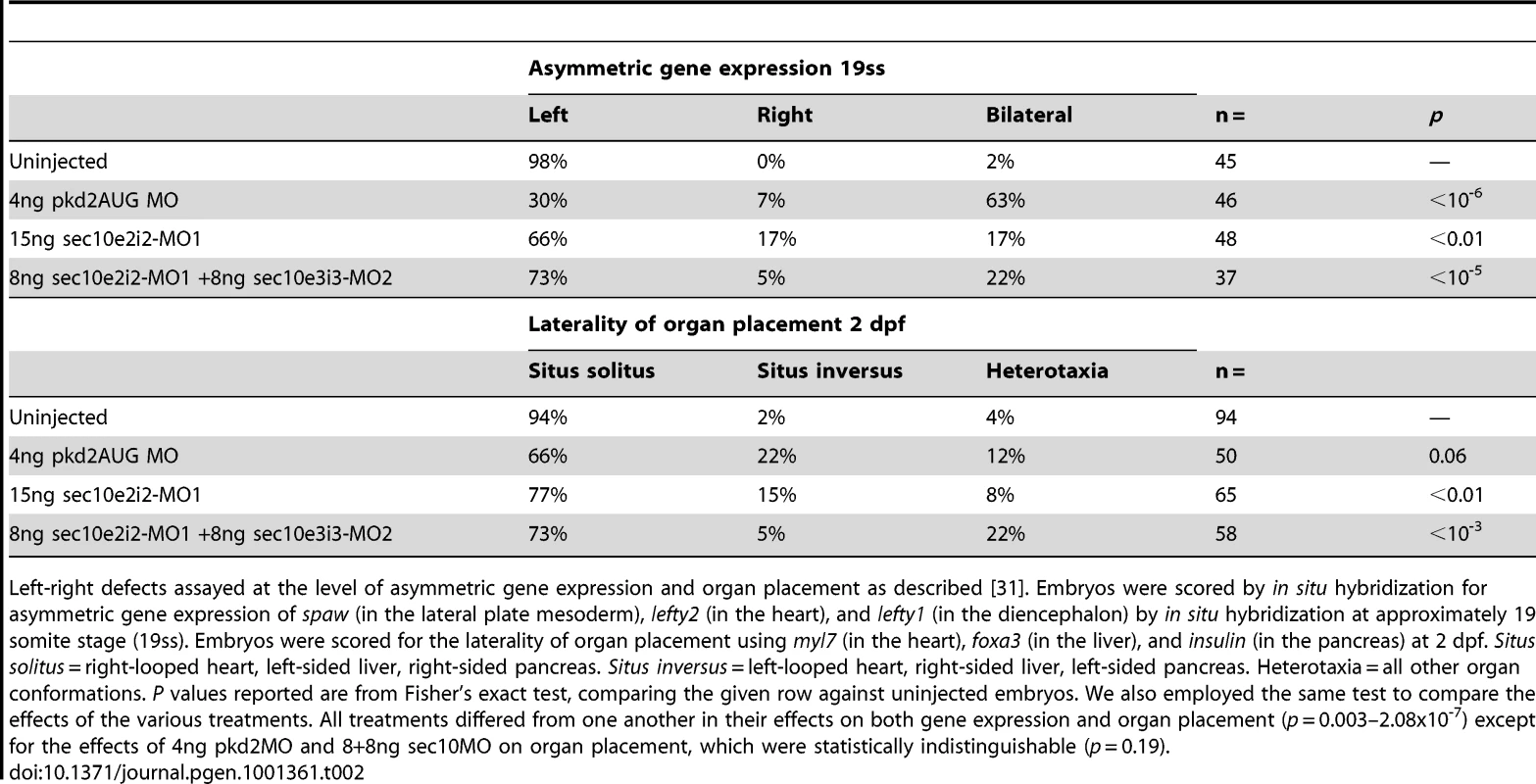
Also similar to pkd2MO embryos [29], sec10MO embryos showed glomerular expansion in the pronephros by in situ hybridization with the glomerular marker, Wilm's tumor 1a (wt1a) at 3 dpf (Figure 4G-4I′). Wild-type embryos showed a condensed glomerular stain (100% condensed, n = 30). By contrast, only 50% of 15ng sec10MO embryos showed a condensed stain (50% condensed, 31% moderate enlargement, 19% severe enlargement, n = 16), similar to 4ng pkd2MO embryos (35% condensed, 59% moderate enlargement, 6% severe enlargement, n = 32). Given the glomerular disorganization we observed by histology (Figure 3E′), the expansion in the wt1a stain in sec10MO embryos may be due to increased cell proliferation.
In spite of the expanded glomerulus, sec10MO embryos did not display obvious glomerular dilations by histology, nor did they show pronephric cysts. While we note that the lack of dilation in sec10MO embryos contrasts with the glomerular dilation observed in pkd2MO embryos [20], [25], [30], zygotic pkd2 mutants do not show glomerular dilation either [20], [31]. It is likely that maternal contribution of polycystin-2 and Sec10 explains why we do not observe glomerular dilation or pronephric cysts in pkd2 mutants and sec10MO embryos, respectively.
To determine whether these in vivo pronephric defects were accompanied by the cellular phenotypes we observed with Sec10 knockdown in vitro, we assayed pERK levels by Western blot to determine the level of MAPK activation. Consistent with our in vitro results, pkd2MO and sec10MO embryos showed abnormally increased pERK levels at 5 dpf, relative to uninjected embryos, at 5 dpf, (Figure 4J). MAPK activation was more pronounced in sec10MO than in pkd2MO embryos.
Therefore, sec10MO embryos share curly tail up, left-right, pronephric, and cellular phenotypes with pkd2MO embryos. Consistent with our in vitro analysis, we observed that loss of sec10 partially phenocopies loss of pkd2, supporting the idea that exocyst function is required for polycystin-2 function. Furthermore, our observation of these cilia-related phenotypes is consistent with a role for sec10 in cilia function, even though overt defects in cilia length and motility were not observed upon knockdown.
sec10 and pkd2 genetically interact for cilia-related phenotypes
Our in vitro and in vivo analyses together support a link between exocyst sec10 and the ADPKD gene pkd2. While the shared curly tail up phenotype is more specific to pkd2, the left-right defects and wt1a expansion phenotypes shared between sec10MO and pkd2MO embryos have also been observed upon knockdown of other ciliary proteins [18], [24]. We therefore wanted to directly test for a specific genetic interaction between these two genes. We titrated both sec10 and pkd2 morpholinos to find suboptimal doses that did not result in strong gross phenotypes on their own. Interestingly, when we co-injected both morpholinos at these reduced doses we observed a striking synergistic effect on the curly tail up phenotype (Figure 4D-4F, Figure S1A, S1A′). Co-injection of 0.25ng pkd2MO and 7.5ng sec10MO yielded curly tail up phenotypes when each morpholino alone produced completely wild-type tails. Likewise, co-injection with a slightly higher dose of pkd2MO (2ng pkd2MO) shifted almost all embryos from a range of curly up phenotypes into a severe curly up phenotype. We also observed effects upon the left-right defect (Figure S1B and S1B′) and the wt1a glomerular expansion (Figure S1C and S1C′) phenotypes. We had to use different suboptimal doses of pkd2 morpholino for the phenotypes because left-right defects, curly tail, and pronephric phenotypes are extremely dose-sensitive to pkd2 levels [31]. The genetic interaction we observed between sec10 and pkd2 morpholinos suggests that sec10 may play a role in pkd2 function in multiple cilia-related processes.
The observed genetic interaction can be interpreted in two ways: pkd2 and sec10 may act in parallel pathways, or in the same pathway. In the former case, redundancy between two parallel pathways—one requiring pkd2 and one requiring sec10—explains the lack of phenotypes in the single suboptimal dose conditions; a slight reduction of both pathways upon co-injection of both morpholinos leads to a failure to complement. In the latter case, where pkd2 and sec10 act in the same pathway, reduction of function at two steps prevents wild-type function in a dosage-sensitive manner. This latter interpretation is supported by a similar synergistic effect that was observed between the two ciliary proteins Seahorse and Inversin [23]; these proteins were then shown to biochemically interact, supporting the idea that they are likely to act in the same pathway [23], [24]. Previously, we proposed that Sec10 and the exocyst are important for transporting proteins important for ciliary structure [39]. We, therefore, suggest a similar model for transporting proteins important for ciliary function, like polycystin-2. If Sec10 is similarly required to transport polycystin-2, then morpholino co-injection would further impair ciliary polycystin-2 levels beyond that seen following direct knockdown of polycystin-2 by a sub-optimal dose of morpholino, because a reduced amount of Sec10 would be present to effectively transport the remaining polycystin-2.
The exocyst and polycystin-2 interact biochemically and co-localize in the primary cilium
In light of the genetic interaction we observed between sec10 and pkd2, we wanted to determine whether we could detect a biochemical interaction between Sec10 and polycystin-2. Using lipofectamine, we transfected a cDNA encoding human polycystin-2-myc into human embryonic kidney 293 (HEK293) cells. Western blotting of the transfected HEK293 cell lysates with antibodies against both polycystin-2 (not shown) and the myc epitope tag (Figure 5A), identified a band at approximately twice the molecular weight of polycystin-2, suggesting it was in polymeric form. Purified Sepharose-immobilized Sec10-GST was then used as an affinity resin to pull down specific binding proteins from polycystin-2-myc transfected HEK293 cell lysates. Western blotting, using antibodies directed against both polycystin-2 and the myc epitope tag, identified polycystin-2 as a Sec10-GST binding protein that was not recovered on the GST resin alone (Figure 5B). Using another technique, we showed that exocyst Sec8 co-immunoprecipitated with polycystin-2, but not the isotype control, from intracellular vesicles isolated from mouse kidney lysate (Figure 5C). Having shown that the exocyst and polycystin-2 can interact in cell lysates, we next investigated if native polycystin-2 would co-localize with the exocyst at the primary cilium. We previously showed by immunofluorescence and electron gold microscopy that the exocyst localized to the primary cilium in MDCK cells [39]. Using a polyclonal polycystin-2 antibody that recognizes canine polycystin-2 [55], we first demonstrated that polycystin-2 co-localizes with acetylated alpha tubulin at the primary cilium in MDCK cells (Figure S2). We then showed that exocyst Sec8 co-localizes along the length of the primary cilium with polycystin-2 (Figure 5D).
Fig. 5. Sec10 biochemically interacts with polycystin-2. 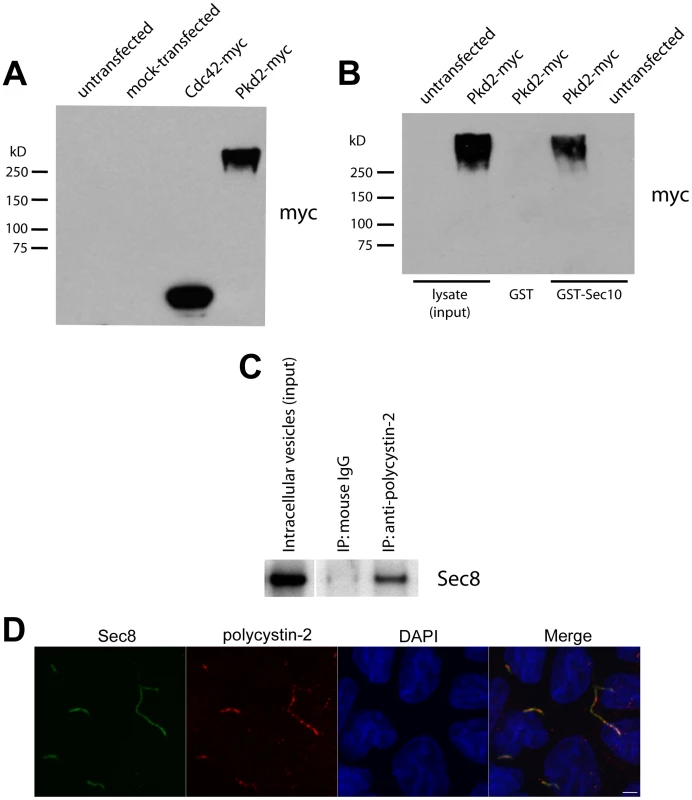
Consistent with our phenotypic and genetic analyses in zebrafish, we observe that Sec10 and polycystin-2 biochemically interact and co-localize in the cilia of cultured renal tubule epithelial cells.
Sec10 biochemically interacts with the ciliogenesis proteins IFT88 and IFT20
Since we observed a biochemical interaction between the exocyst and polycystin 2, we wanted to determine whether the exocyst biochemically interacts with other ciliary proteins. We focused on ciliary proteins such as IFT88 and IFT20, because they have both been found to interact with polycystin-2 [56], [57].
Previously we demonstrated that levels of IFT88 were reduced upon Sec10 knockdown in vitro [39]. IFT88 is required for cilia structure [58] and is mutated in the orpk mouse model of PKD [59]. Interestingly, IFT88 has been shown to be in a complex with polycystin-2 and possibly trafficked together with it in the cell [57]. Consistent with this interpretation, IFT88 is not itself required for polycystin-2 localization to primary cilia in cultured cells [56]. We used GST-pulldown assays to test for biochemical interactions, as relatively larger amounts of binding proteins can be obtained from the affinity column. A Sec10-GST fusion protein was purified on glutathione Sepharose and used as an affinity matrix for the purification of specific binding proteins from HEK293 cell lysates. IFT88 interacted with Sec10 and was found in the pulldown fraction (Figure 6A). To demonstrate specificity, we probed for GAPDH, a protein not known to interact with the exocyst. In Figure 6A (bottom), GAPDH is seen in the cell lysate, but not in the Sec10-GST pulldown fractions. The glutathione-Sepharose immobilized Sec10 also pulled down exocyst Sec8 from the cell lysate, serving as a positive control for exocyst binding (Figure 6A).
Fig. 6. Sec10 interacts with IFT88 and IFT20. 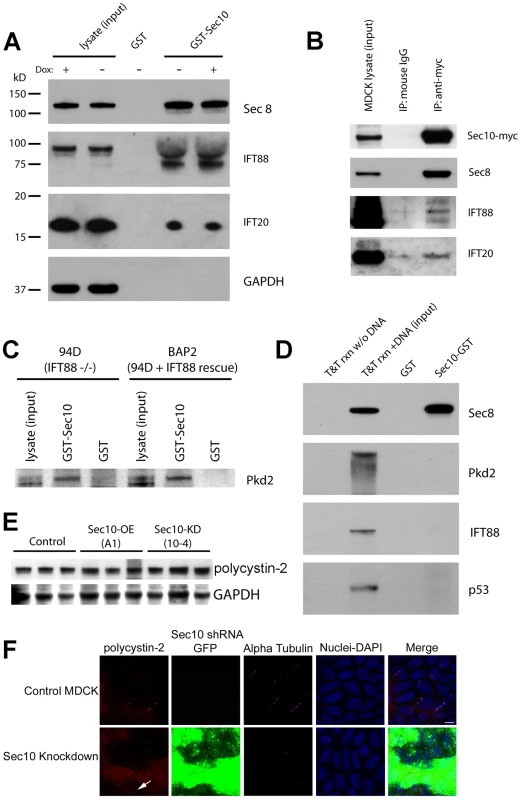
IFT20 is a ciliary protein known to be trafficked to cilia on vesicles, and knockout of IFT20 leads to polycystic kidney disease in mice [56], [60]. Interestingly, IFT20 has been shown to be important for polycystin-2 localization to the primary cilium [56]. IFT20 was also found in the pulldown fraction, and is, therefore, a Sec10 binding partner (Figure 6A). As a negative control, in all the pulldown experiments bead-immobilized GST alone was used and no proteins were detected in the pulldown fractions.
Using another technique, we showed that Sec8, IFT88, and IFT20 all co-immunoprecipitated with Sec10-myc from MDCK cell lysates, but not the isotype control (Figure 6B). Therefore, Sec10 biochemically interacts not only with proteins important in cilia function (like polycystin-2), but also with proteins implicated in cilia formation and trafficking, such as IFT88 and IFT20.
IFT88 is not required for the biochemical interaction between Sec10 and polycystin-2
Given that IFT88 has been shown to interact with polycystin-2 [57], we next investigated if IFT88 might act as a direct bridging protein between Sec10 and polycystin-2. An immortalized cell line, 94D, derived from the cortical collecting duct cells of an Oak Ridge Polycystic Kidney mutant mouse (orpk), and deficient in IFT88, was generated by Yoder and colleagues [61]. An immortalized IFT88 “rescue” cell line, BAP2, was also generated with endogenous levels of IFT88. To determine if IFT88 is necessary for the interaction between polycystin-2 and Sec10, Sec10-GST pulldowns using lysate from both the 94D and BAP2 cell lines were performed. There was no difference in the amount of polycystin-2 pulled down from the IFT88-deficient or -replete cell lines (Figure 6C). Therefore, IFT88 is not required for the Sec10 interaction with polycystin-2.
We then performed in vitro translation of Sec8, polycystin-2, IFT88, and p53, followed by pulldown with Sec10-GST. We demonstrated an interaction between Sec10 and Sec8 (our positive control), but not p53 (our negative control), polycystin-2, or IFT88 (Figure 6D). Therefore, while the exocyst biochemically interacts with polycystin-2 and IFT88, these interactions are not direct, indicating there are remaining proteins yet to be identified in these complexes.
Polycystin-2 is mislocalized upon knockdown of sec10
As determined by Western blot, equal amounts of polycystin-2 are seen in control, Sec10-overexpressing, and Sec10 knockdown MDCK cells (Figure 6E). If Sec10 is required for polycystin-2 localization, we would expect to see a change in polycystin-2 localization in Sec10 knockdown MDCK cells. Indeed, Sec10 knockdown cells show a loss of native polycystin-2 localization at the primary cilium by immunofluorescence staining (Figure 6F). However, it should be noted that this result is not surprising given that Sec10 knockdown cells, as we previously reported, have no, or few, cilia [39]. Therefore, the loss of polycystin-2 localization could well be an indirect effect of the ciliogenesis defect.
Discussion
Here, we describe the first genetic and biochemical link between the exocyst and a human disease gene, PKD2. Phenotypic analyses of sec10MO embryos support a role for sec10 in multiple cilia-related processes, which is surprising given the absence of obvious defects in cilia structure or motility. Thus, while our previous work indicated a role for Sec10 in ciliogenesis [39], the results presented here suggest that Sec10 is important for cilia function as well. Furthermore, we demonstrate a specific genetic interaction with pkd2, a gene that influences cilia function without affecting cilia structure. Knockdown of Sec10 in vitro and in vivo partially phenocopies knockdown of the ADPKD protein polycystin-2. Consistent with these results, we observe that Sec10 and polycystin-2 co-localize in the primary cilium in vitro. We further report biochemical interactions between multiple exocyst proteins and ciliary proteins—polycystin-2, IFT88, and IFT20. Together with our previous work [39], our studies demonstrate that the exocyst protein Sec10 is likely to be important for both cilia formation and function.
We demonstrate that Sec10 knockdown also leads to phenotypes associated with ADPKD. In vitro, we show that knockdown of Sec10 leads to decreased basal intracellular calcium levels and lack of a calcium response to fluid flow. Conversely, Sec10-overexpressing cells showed a significantly increased calcium response to fluid flow. These results were not unexpected given the defects in ciliogenesis in Sec10 knockdown cells, and the increased ciliogenesis seen in Sec10-overexpressing cells [39]. We also showed increased cell proliferation in Sec10 knockdown cells. Increased cell proliferation is a well-known characteristic of ADPKD cells and plays a major role in the formation of the cysts that destroy the kidney, leading some to refer to ADPKD as “neoplasia in disguise” [47].
Our analysis of the role sec10 plays in zebrafish development then revealed that Sec10 may also be required for cilia function, separate from a role in cilia formation. When we used morpholinos to knockdown Sec10 levels in vivo in zebrafish, we did not observe a gross defect in pronephros cilia morphology or motility. While this was initially surprising because in vitro knockdown results in severe ciliogenesis defects [39], we believe that maternal Sec10—which is unaffected by our splice-site morpholinos—is sufficient to allow for cilia assembly. However, we believe this residual maternal protein was unable to restore complete cilia function during zebrafish development because sec10MO embryos still showed cilia-related phenotypes that have been observed in other cilia mutants in zebrafish—such as left-right patterning defects and glomerular expansion. Therefore, we believe the partial knockdown of Sec10 levels in vivo with splice-site morpholinos allowed us to uncover a role for exocyst Sec10 in cilia function.
If true, we would expect that future studies knocking down both maternal and zygotic Sec10 with a start-site morpholino might recapitulate a ciliogenesis defect like that observed in vitro. We tested one start-site morpholino but it did not effectively knockdown Sec10 (see Materials and Methods). It should also be noted that even translation blocking morpholinos do not always produce maternal and zygotic losses seen in actual maternal zygotic mutants. For example, translation blocking MOs against IFT88 in fish show almost complete loss of protein by Western blot [62], and yet still do not display phenotypes observed in the maternal-zygotic ift88/oval zebrafish mutant [63]. Thus, a maternal-zygotic sec10 mutant would be required to definitively say whether or not Sec10 is required for ciliogenesis in zebrafish, as it is in MDCK cells.
Finally, we provide phenotypic and genetic evidence that sec10 may be important specifically for pkd2 function in these cilia-related processes. This is interesting because pkd2 is one of the causative genes for ADPKD and could explain the ADPKD-like phenotypes we observed upon Sec10 knockdown in vitro. Multiple lines of evidence support our interpretation that sec10 is important for pkd2 function in vivo: 1) pkd2 knockdown has been implicated in multiple cilia-related processes and is known to affect cilia function, but not structure or motility [14], [29]-[31], [33]; 2) Similar to pkd2 knockdown, sec10MO embryos share several cilia-related phenotypes—including the curly tail up and MAPK activation phenotypes, even though they similarly do not show defects in cilia length or motility; 3) We observed specific genetic interactions between sec10 and pkd2 for multiple cilia-related phenotypes—including curly tail up, left-right defects, and aberrant wt1a glomerular expansion.
It will be important to tease apart the extent to which sec10 phenotypes are explained solely by inhibition of pkd2 function. We have provided evidence supporting the idea that many of the sec10 phenotypes are likely due to its regulation of pkd2. But it plausible that sec10 may be important for the function of other ciliary proteins as well. This is supported by the fact that sec10MO embryos possess cilia-related phenotypes, such as small eyes, that are not observed upon loss of pkd2.
While we favour a model where sec10 is required for ciliary function, we recognize that Sec10 may play a different role since some proteins implicated in cilia function also have cilia-independent functions. Many ciliary proteins are not exclusively localized to the cilium, and it has recently been argued that polycystin function in the endoplasmic reticulum is more relevant for the observed curly tail phenotype [64]. Indeed, multiple IFT proteins have recently been shown to have important functions in non-ciliated cells [65].
We observed activation of ERK (MAPK) following knockdown of Sec10 both in vitro and in vivo. Similar to polycystin-2 knockout in mouse [66], we report that polycystin-2 knockdown in zebrafish results in elevated pERK levels. Since we propose that polycystin-2 function requires the exocyst, it is not surprising that we also observed increased pERK levels upon Sec10 knockdown. It remains to be determined how MAPK activation relates to cystogenesis, since sec10MO embryos did not show pronephric cysts. We did, however, observe glomerular disorganization that could have been the result of unchecked proliferation. So it is possible that while MAPK activity may regulate proliferation associated with cystogenesis, MAPK hyperactivation alone is not sufficient to cause cystogenesis. If true, this may explain conflicting results others have observed using MAPK inhibitors to abrogate cystogenesis. On the one hand, inhibition of ERK activation with the oral MAP/ERK kinase inhibitor, PD184352, largely prevented cystogenesis in the pcy mouse model of polycystic kidney disease [50]. On the other hand, inhibition of ERK activation with the MEK inhibitor, U0126, failed to prevent cystogenesis in a Pkd1 mouse model of ADPKD [66]. The effectiveness of these inhibitors may depend on the specific genetic background and the role MAPK activation plays in cystogenesis in that background. While we observed MAPK hyper-activation in both sec10MO and pkd2MO embryos, only pkd2MO embryos show cysts [20], [25], [30].
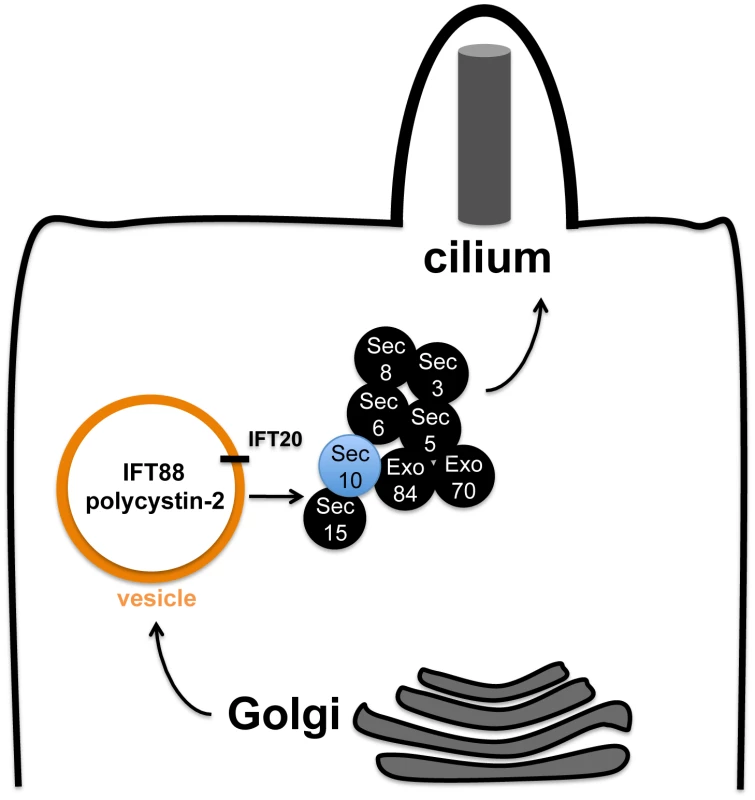
In light of its known role in trafficking basolateral proteins to the plasma membrane [41]-[44], we propose that the exocyst is required for the delivery of ciliary proteins to the cilium, including polycystin-2. This model would explain why Sec10 knockdown results in defects in both cilia formation and function.
If our model were true, identification of the IFT B proteins IFT88 and IFT20 in complex with exocyst proteins could mean one of several possibilities: 1) IFT B and polycystin-2 proteins are both being trafficked by the exocyst; 2) The IFT B complex is directly responsible for trafficking polycystin-2 and the exocyst is only directly responsible for trafficking IFT B. We favour the first model (Figure 7) for the following reasons: 1) The biochemical interaction between Sec10 and polycystin-2 that we report did not require IFT88; 2) If the exocyst was required in an indirect fashion for pkd2 function, through the action of IFT proteins, then it should not have been possible to observe cilia-related phenotypes in vivo in the absence of pronephric ciliary structural defects.
Relatedly, IFT20 may play a special role in bridging these complexes since it has been implicated in Golgi-to-cilium vesicular traffic [56]. Unlike other IFT proteins, which are only localized to the cilium, IFT20 is also observed in the Golgi. Pazour and colleagues have proposed that IFT20 may mark vesicles carrying proteins destined for the cilium [56]. IFT20 has been specifically implicated in localizing polycystin-2 to the cilium [56], and polycystin-2 may be trafficked as part of a larger complex containing IFT88 and IFT57 [57]. Our detection of an interaction between Sec10 and IFT20 suggests that IFT20-marked vesicles may utilize the exocyst to dock at the cilium, or that the exocyst may chaperone IFT20-positive vesicles from the Golgi to the primary cilium (Figure 7). Interestingly, IFT20 knockdown by splice-site morpholinos in zebrafish does not significantly disrupt pronephric cilia structure, even though it results in ciliary loss in the otic vesicle [50]. Therefore, loss of IFT20 may result in milder ciliogenesis defects then are observed following knockdown of other IFT proteins in zebrafish.
However, future studies are needed to clarify the interactions among the exocyst, IFT proteins, and the polycystins. Detailed immunofluorescence studies are needed to demonstrate that the exocyst is directly involved in ciliary trafficking. If our model were true, we would expect to see a reduction in the ciliary localization of polycystin-2 upon Sec10 knockdown. Unfortunately, we are unable to test this in vitro, which is a limitation of our study, because of the ciliogenesis defects upon Sec10 knockdown in MDCK (Figure 6F) and ARPE-19 cells (data not shown). In vivo, we were unable to consistently detect sub-cellular localization of polycystin-2 in the cilium by immunofluorescence utilizing multiple polycystin-2 antibodies (data not shown), thus we were unable to look at polycystin-2 localization in the cilia that remain in sec10MO embryos. Therefore, it remains to be seen how the biochemical interactions we observe between the exocyst and ciliary proteins relate to their trafficking to the cilium.
In summary, we have shown that Sec10, a conserved and crucial component of the exocyst complex, is likely to promote pkd2 function in many cilia-related phenotypes. These data support an additional role for the exocyst, not only in ciliogenesis [39], but also for cilia function. We report a novel genetic interaction between sec10 and pkd2, which supports the ADPKD-like and pkd2-like phenotypes we observed upon Sec10 knockdown in vitro and in vivo. Our data support a model in which the exocyst is required for the trafficking of proteins essential for ciliary function and structure, possibly in conjunction with IFT20. Future work will reveal the extent to which the exocyst participates in ciliary trafficking, and whether it can be utilized as a novel target for therapeutic intervention in ADPKD, a disease for which there are currently no approved treatments beyond supportive care.
Materials and Methods
Ethics statement
All zebrafish experiments were approved by the Institutional Animal Care and Use Committee at Princeton University.
Cell culture and reagents
All MDCK cell lines used were derived from low passage type II MDCK cells that were obtained from Dr. K. Mostov (UCSF, San Francisco, CA), and which were originally cloned by Dr. D. Louvard (European Molecular Biology Laboratory, Heidelberg, Germany). The monoclonal MDCK type II cell line with silenced expression of Sec10 through stable transfection of shRNA designed against the canine Sec10 gene, as well as the Sec10 overexpression MDCK type II cell line, have been previously described [39]. MDCK cells were cultured on 0.4 µm Transwell filters in modified Eagle's minimal essential medium (MEM) containing Earl's balanced salt solution and L-glutamine, with 5% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin.
The IFT88-deficient and –rescued cell lines, 94D and BAP2, respectively, a generous gift of Dr. Brad Yoder, were derived from the cortical collecting duct cells of the orpk mouse, and have been previously described [61]. The orpk mutant mouse has an insertional mutation in the Ift88/Tg737/polaris gene, which results in a hypomorphic allele, with extremely low Ift88 protein levels. This well-studied mutant mouse was crossed with the ImmortoMouse (Charles River Laboratories) and an immortalized cortical collecting duct cell line was established. These cells were transfected with a wild-type Ift88 gene to restore Ift88 expression (BAP2 line), or with an empty vector to retain hypomorphic mutant Ift88 cells (94D line). To keep these cell lines undifferentiated and immortalized, cells were grown at 33°C with 10 U/ml interferon-γ in collecting duct media (DMEM/F-12, 10% FBS, 1.3 µg/l sodium selenite, 1.3 µg/l T3, 5 mg/l insulin, 5 mg/l transferrin, 2.5 mM L-glutamine, 5 µM dexamethasone, 100 U/ml penicillin, and 100 µg/ml streptomycin). To differentiate these cells and inactivate SV40 large-T antigen, cells were grown at 37°C in the absence of interferon-γ for 3 days before performing Sec10-GST pulldowns.
Small molecule inhibitors, which were incubated with cells at the manufacturer recommended concentrations for one hour, include: the MEK inhibitor U0126 at 10 µM (Promega, Madison, WI), PKA inhibitor H89 at 10 µM (Calbiochem, San Diego, CA), Src family inhibitor PP2 at 10 µM (Calbiochem), PKC inhibitor bisindolylmaleimide I (BIM) at 1 µM (Calbiochem), and mTOR inhibitor Rapamycin (Rap) at 20 nM (LC Laboratories, Woburn, MA).
Cellular assays
Live cell fluorescent imaging and intracellular calcium concentration measurements were done as previously described [46]. Briefly, cells were grown to confluent monolayers on 0.4 µm clear polyester permeable supports in a perfusion chamber, which allows precisely controlled shear fluid flow across the apical surface of the epithelium. After 5-7 days in confluent monolayers, cells were loaded with fura 2 (10 µM fura 2/AM; Teflabs, Austin, TX), and transferred to the incubation chamber on the microscope, where a constant temperature of 37°C was maintained, with a 5% CO2 concentration. Cells were then perfused with Ringer's solution with 1 mM probenecid. We performed dual-excitation wavelength fluorescence microscopy (Photon Technologies, Birmingham, NJ) with a Nikon microscope, x20 S Fluor long-working distance objective, and a cooled SenSys charged-coupled camera (Photometrics, Tucson, AZ). Fura 2 was excited at wavelengths of 340 and 380 nm and emitted fluorescence was measured at 510 nm. Data were obtained in each experiment from a grid of 20 regions of interest each containing 8–10 cells. Cells were maintained in the absence of apical flow for 10 minutes followed by an abrupt increase to a flow rate of 5 ml/min. The basolateral flow rate remained constant at 1.5 ml/min.
For cell proliferation measurements, MDCK cells were seeded in parallel 96-well plates in triplicates at 4000 cells/well, and were allowed to attach for 24 h at 37°C. The medium was replenished on all plates, and the cells on one plate were counted using the CellTiter-Glo viability assay (Promega) and considered cell population at t = 0 h. Cells on other plates were grown for 24 h and 48 h at 37°C, and then counted using the same method. Luminescence was measured using a Becton Dickson microplate reader, and proliferation rates were calculated by dividing cell number at a given timepoint by the initial number of seeded cells (t = 0).
Western analysis
For MDCK cell lysates, immunoblotting was performed as previously described [39]. To make zebrafish protein lysates, embryos were washed in E3 buffer and re-suspended in Ringer's solution. To remove the yolk protein, the sample was vortexed five times in 30 seconds bursts and the supernatant was removed following a gentle centrifugation at 300g for 1 minute at 4°C. The pellet was re-suspended in lysis buffer [67] supplemented with a protease inhibitor cocktail (P1860, Sigma) and 200 µM PMSF. The protein lysate supernatant was removed following centrifugation at 14,000g for 10 minutes at 4°C. Protein samples were mixed with Laemmli buffer and boiled for 5 minutes. Immunoblotting was performed using standard protocols.
The antibodies used in this study are: rabbit polyclonal anti-Sec10 which was described previously [39], rabbit polyclonal anti-phospho-ERK1/2 (#9101, Cell Signaling, Danvers, MA), rabbit polyclonal anti-total ERK1(/2) (sc-94, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse monoclonal anti-GAPDH (G8795, Sigma, St. Louis, MO), rabbit polyclonal anti-γ tubulin (T5192, Sigma), mouse monoclonal anti-acetylated α-tubulin (T6793, Sigma), rabbit polyclonal anti-IFT88 ([68]), mouse monoclonal anti-Sec8 (Assay Designs, Ann Arbor, MI), mouse monoclonal anti-myc (#2276, Cell Signaling), rabbit polyclonal anti-IFT20 ([56], a generous gift from Dr. Greg Pazour), mouse monoclonal anti-polycystin-2 (D-3, Santa Cruz Biotechnology, Inc.), rabbit polyclonal anti-TRPM4 (Santa Cruz Biotechnology), rabbit polyclonal anti-polycystin-2 (a gift from the Johns Hopkins Research and Clinical Core Center), normal isotype mouse IgG control (Santa Cruz), and normal isotype rabbit IgG control (Santa Cruz).
Zebrafish injections and morpholinos
Embryos were injected at the one-to-8-cell stage, and morpholinos were diluted with phenol red tracer at 5 μg/μL phenol and injected at 500 picoLiter or 1 nanoLiter/embryo. Splice-site morpholinos designed against zebrafish sec10 were purchased from Gene Tools, LLC. (Philomath, OR): sec10e2i2-MO1 (5′ - AATATTCTGTAACTCACTTCTTAGG -3′), sec10e3i3-MO2 (5′-CAAATGTAAAGACGACTGACTTGGT-3′), and sec10AUG-MO1 (5′ - CGAATAATTGAGCTGTCGTAGCCAT-3′). Sec10e2i2-MO1 was designed to target the exon 2-intron 2 boundary (hence “e2i2”). Sec10e3i3-MO2 was targeted against the exon 3-intron 3 boundary (hence “e3i3”). Splice-site morpholinos were injected either as a single dose of 15 ng sec10e2i2-MO1 (designated in the text as “15ng sec10MO”) or a combined dose of 8 ng sec10e2i2-MO1 +8 ng sec10e3i3-MO2 (designated in the text as “8+8ng sec10MO”) per embryo. Developmental delay was more noticeable with 8+8ng sec10MO. Sec10e3i3-MO2 did not show gross phenotypes when injected alone. sec10AUG-MO1 was designed to target the ATG (hence “AUG”, complementary sequence underlined above). sec10AUG-MO1 was tested but did not knockdown zfSec10 by Western blot analysis even at doses of 15 ng per embryo (data not shown); since some MOs fail to work for inexplicable reasons, this was not pursued further. pkd2AUG MO (5′-AGGACGAACGCGACTGGAGCTCATC-3′), start site [20], was injected at 4 ng per embryo.
Immunofluorescence, histology, and in situ hybridizations
Immunostaining of MDCK cells grown on Transwell filters was performed as previously described [39], except the cells were fixed with 4% paraformaldehyde for 15 minutes at 37°C. Immunofluorescence for pronephric cilia and histology in zebrafish was performed similar to previously published protocols [25]. The antibodies used in this study for immunofluorescence studies: mouse monoclonal anti-acetylated α-tubulin (T6793, Sigma), rabbit polyclonal anti-polycystin-2 antibody (a gift from the Johns Hopkins Research and Clinical Core Center, [55]), goat-anti-mouse IgG2b/g2b chain specific-FITC (Southern Biotech#1090-02, Birmingham, AL). In situ hybridization in zebrafish was performed using standard protocols [69]. Probes used: wt1a, myl7, foxa3, ins, spaw, lefty1, and lefty2.
Imaging
All images were captured in TIF format and processed in Adobe Photoshop CS4. For immunofluorescence, zebrafish embryos were imaged on a Zeiss LSM 510 Confocal Microscope with a 40x water objective and captured at 2x zoom, and the LSM Image Browser application; TIFs were captured at 150 ppi. For histology and in situ hybridization, samples were imaged using a Leica DM RA2 Microscope, a Leica DFC490 digital camera, and the Leica Application Suite v 3.1.0 software; TIFs were captured at 96 ppi. For live images, embryos were imaged on a Leica MZ FLIII Stereo-Fluorescence Microscope using a Jenoptik LaserOptikSysteme ProgResC14 digital camera and Picture Frame v2.3 software; TIFs were captured at 150 ppi. For video microscopy of pronephric cilia, embryos were imaged as previously described [25], using an Olympus BX51 Upright Microscope with a 60x water immersion objective, an Andor Technology Luca EMCCD digital camera, and Matlab software.
GST pull-downs and co-immunoprecipitations
Full-length human Sec10 cDNA was cloned in frame into the plasmid pGEX-4T-1 (Amersham Biosciences, Piscataway, NJ), and transformed into the DE3 strain of Escherichia coli (Stratagene, La Jolla, CA). GST fusion protein expression was induced by adding isopropyl-1-thio-β-D-galactopyranoside to growing cultures and shaking for an additional 3 h at 37°C. Recombinant proteins were purified with glutathione-Sepharose (Amersham Biosciences) following bacterial cell lysis. For pull-down experiments, lysates from wild-type HEK293 cells, HEK293 cells transfected with PKD2-myc (a generous gift from Dr. S. Solmo), or differentiated 94D and BAP2 cell lines, were incubated overnight with Sec10-GST, or GST only, bound to glutathione-Sepharose. Pull-downs were washed extensively, and then resuspended in Laemmli buffer and boiled, and equal amounts were electrophoresed by SDS-PAGE. Bound IFT88, IFT20, GAPDH, Sec8, and polycystin-2-myc were detected by Western blot analysis.
Co-immunoprecipitations for polycystin-2 were performed from intracellular vesicle fractions of mouse kidney lysates, isolated as described [70] . Isolated vesicles were incubated overnight with polycystin-2 antibody, or equal amounts of control mouse IgG, and protein complexes were precipitated with ProteinG Dynabeads (Invitrogen). After washing five times in vesicle isolation buffer, the precipitated protein complexes were analyzed with SDS-PAGE and Western blotting. Co-immunoprecipitations for Sec10 were performed from confluent MDCK cells overexpressing Sec10 containing a myc epitope tag, since our Sec10 antibody cannot be used for immunoprecipitation. Cells were grown 5-7 days past confluency, washed with PBS, and incubated with 1 mM of the membrane-permeable chemical crosslinker dithiobis(succinimidylpropionate) (DSP) (Thermo Scientific) for 30 minutes at room temperature. The cells were quenched with TBS for 15 min, and then lysed in Co-IP lysis buffer (20 mM HEPES pH 7.4, 120 mM NaCl, 1 mM EDTA, 1% IGEPAL CA-630). Soluble proteins from the lysates were incubated overnight with 2 µg of various antibodies in parallel with equal amounts of isotype control IgG. Protein-G agarose (Invitrogen) was used to precipitate the protein complexes, and after five washes with the Co-IP buffer, the agarose resin was resuspended in Laemmli buffer. Equal amounts of samples were electrophoresis by SDS-PAGE, and co-immunoprecipitated proteins were detected by Western blot analysis utilizing the Trueblot-HRP secondary antibodies (eBioscience).
Statistical analysis
One-way ANOVAs, with post-hoc Tukey test for statistical significance, were performed to compare band intensities from Western blots and cell proliferation rates using the Prism statistical software (Graphpad, San Diego, CA). Fisher's exact tests for statistical significance were performed to compare phenotypic analyses in zebrafish using R statistical software.
Supporting Information
Zdroje
1. Smyth
BJ
Snyder
R
Balkovetz
DF
Lipschutz
JH
2003
Recent advances in the cell biology of polycystic kidney
disease.
Jeon
KW
Int Rev Cytol
San Diego
Elsevier Inc
52
89
2. Consortium
TIPD
1995
Polycystic kidney disease: The complete structure of the PKD1
gene and its protein.
Cell
81
289
298
3. Mochizuki
T
Wu
G
Hayashi
T
Xenophontos
SL
Veldhuisen
B
1996
PKD2, a gene for polycystic kidney disease that encodes an
integral membrane protein.
Science
272
1339
1342
4. Gonzalez-Perrett
S
Kim
K
Ibarra
C
Damiano
AE
Zotta
E
2001
Polycystin-2, the protein mutated in autosomal dominant
polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective
cation channel.
Proc Natl Acad Sci U S A
98
1182
1187
5. Vassilev
PM
Guo
L
Chen
XZ
Segal
Y
Peng
JB
2001
Polycystin-2 is a novel cation channel implicated in defective
intracellular Ca(2+) homeostasis in polycystic kidney
disease.
Biochem Biophys Res Commun
282
341
350
6. Hanaoka
K
Qian
F
Boletta
A
Bhunia
AK
Piontek
K
2000
Co-assembly of polycystin-1 and -2 produces unique
cation-permeable currents.
Nature
408
990
994
7. Yamaguchi
T
Hempson
SJ
Reif
GA
Hedge
AM
Wallace
DP
2006
Calcium restores a normal proliferation phenotype in human
polycystic kidney disease epithelial cells.
J Am Soc Nephrol
17
178
187
8. Yamaguchi
T
Nagao
S
Wallace
DP
Belibi
FA
Cowley
BD
2003
Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from
autosomal-dominant polycystic kidneys.
Kidney Int
63
1983
1994
9. Yamaguchi
T
Pelling
JC
Ramaswamy
NT
Eppler
JW
Wallace
DP
2000
cAMP stimulates the in vitro proliferation of renal cyst
epithelial cells by activating the extracellular signal-regulated kinase
pathway.
Kidney Int
57
1460
1471
10. Praetorius
HA
Frokiaer
J
Nielsen
S
Spring
KR
2003
Bending the primary cilium opens Ca2+-sensitive
intermediate-conductance K+ channels in MDCK cells.
J Membr Biol
191
193
200
11. Praetorius
HA
Spring
KR
2001
Bending the MDCK cell primary cilium increases intracellular
calcium.
J Membr Biol
184
71
79
12. Pazour
GJ
San Agustin
JT
Follit
JA
Rosenbaum
JL
Witman
GB
2002
Polycystin-2 localizes to kidney cilia and the ciliary level is
elevated in orpk mice with polycystic kidney disease.
Curr Biol
12
R378
380
13. Yoder
BK
Hou
X
Guay-Woodford
LM
2002
The polycystic kidney disease proteins, polycystin-1,
polycystin-2, polaris, and cystin, are co-localized in renal
cilia.
J Am Soc Nephrol
13
2508
2516
14. Nauli
SM
Alenghat
FJ
Luo
Y
Williams
E
Vassilev
P
2003
Polycystins 1 and 2 mediate mechanosensation in the primary
cilium of kidney cells.
Nat Genet
33
129
137
15. Nauli
SM
Rossetti
S
Kolb
RJ
Alenghat
FJ
Consugar
MB
2006
Loss of polycystin-1 in human cyst-lining epithelia leads to
ciliary dysfunction.
J Am Soc Nephrol
17
1015
1025
16. Goetz
SC
Anderson
KV
2010
The primary cilium: a signalling centre during vertebrate
development.
Nat Rev Genet
11
331
344
17. Pedersen
LB
Rosenbaum
JL
2008
Intraflagellar transport (IFT) role in ciliary assembly,
resorption and signalling.
Curr Top Dev Biol
85
23
61
18. Kramer-Zucker
AG
Olale
F
Haycraft
CJ
Yoder
BK
Schier
AF
2005
Cilia-driven fluid flow in the zebrafish pronephros, brain and
Kupffer's vesicle is required for normal organogenesis.
Development
132
1907
1921
19. Krock
BL
Perkins
BD
2008
The intraflagellar transport protein IFT57 is required for cilia
maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate
photoreceptors.
J Cell Sci
121
1907
1915
20. Sun
Z
Amsterdam
A
Pazour
GJ
Cole
DG
Miller
MS
2004
A genetic screen in zebrafish identifies cilia genes as a
principal cause of cystic kidney.
Development
131
4085
4093
21. Tsujikawa
M
Malicki
J
2004
Intraflagellar transport genes are essential for differentiation
and survival of vertebrate sensory neurons.
Neuron
42
703
716
22. Duldulao
NA
Lee
S
Sun
Z
2009
Cilia localization is essential for in vivo functions of the
Joubert syndrome protein Arl13b/Scorpion.
Development
136
4033
4042
23. Kishimoto
N
Cao
Y
Park
A
Sun
Z
2008
Cystic kidney gene seahorse regulates cilia-mediated processes
and Wnt pathways.
Dev Cell
14
954
961
24. Serluca
FC
Xu
B
Okabe
N
Baker
K
Lin
SY
2009
Mutations in zebrafish leucine-rich repeat-containing six-like
affect cilia motility and result in pronephric cysts, but have variable
effects on left-right patterning.
Development
136
1621
1631
25. Sullivan-Brown
J
Schottenfeld
J
Okabe
N
Hostetter
CL
Serluca
FC
2008
Zebrafish mutations affecting cilia motility share similar cystic
phenotypes and suggest a mechanism of cyst formation that differs from pkd2
morphants.
Dev Biol
314
261
275
26. Zhao
C
Malicki
J
2007
Genetic defects of pronephric cilia in zebrafish.
Mech Dev
124
605
616
27. Shen
MM
2007
Nodal signaling: developmental roles and
regulation.
Development
134
1023
1034
28. Essner
JJ
Amack
JD
Nyholm
MK
Harris
EB
Yost
HJ
2005
Kupffer's vesicle is a ciliated organ of asymmetry in the
zebrafish embryo that initiates left-right development of the brain, heart
and gut.
Development
132
1247
1260
29. Bisgrove
BW
Snarr
BS
Emrazian
A
Yost
HJ
2005
Polaris and Polycystin-2 in dorsal forerunner cells and
Kupffer's vesicle are required for specification of the zebrafish
left-right axis.
Dev Biol
287
274
288
30. Obara
T
Mangos
S
Liu
Y
Zhao
J
Wiessner
S
2006
Polycystin-2 immunolocalization and function in
zebrafish.
J Am Soc Nephrol
17
2706
2718
31. Schottenfeld
J
Sullivan-Brown
J
Burdine
RD
2007
Zebrafish curly up encodes a Pkd2 ortholog that restricts
left-side-specific expression of southpaw.
Development
134
1605
1615
32. Pennekamp
P
Karcher
C
Fischer
A
Schweickert
A
Skryabin
B
2002
The ion channel polycystin-2 is required for left-right axis
determination in mice.
Curr Biol
12
938
943
33. McGrath
J
Somlo
S
Makova
S
Tian
X
Brueckner
M
2003
Two populations of node monocilia initiate left-right asymmetry
in the mouse.
Cell
114
61
73
34. Emmer
BT
Maric
D
Engman
DM
2010
Molecular mechanisms of protein and lipid targeting to ciliary
membranes.
J Cell Sci
123
529
536
35. Novick
P
Field
C
Schekman
R
1980
Identification of 23 complementation groups required for
post-translational events in the yeast secretory pathway.
Cell
21
205
221
36. Lipschutz
JH
Mostov
KE
2002
The many masters of the exocyst.
Curr Biol
12
R212
R214
37. Guo
W
Grant
A
Novick
P
1999
Exo84p is an exocyst protein essential for
secretion.
J Biol Chem
274
23558
23564
38. Rogers
KK
Wilson
PD
Zhang
X
Guo
W
Burrow
CR
2004
The exocyst localizes to the primary cilium in MDCK
cells.
Biochem Biophys Res Comm
319
39. Zuo
X
Guo
W
Lipschutz
JH
2009
The exocyst protein Sec10 is necessary for primary ciliogenesis
and cystogenesis in vitro.
Mol Biol Cell
20
2522
2529
40. Guo
W
Roth
D
Walch-Solimena
C
Novick
P
1999
The exocyst is an effector for Sec4p, targeting secretory
vesicles to sites of exocytosis.
EMBO J
18
1071
1080
41. Grindstaff
KK
Yeaman
C
Anandasabapathy
N
Hsu
S
Rodriguez-Boulan
R
1998
Sec6/8 complex is recruited to cell-cell contacts and specifies
transport vesicle delivery to the basal-lateral membrane in epithelial
cells.
Cell
93
731
740
42. Lipschutz
JH
Guo
W
O'Brien
LE
Nguyen
YH
Novick
P
2000
Exocyst is involved in cystogenesis and tubulogenesis and acts by
modulating synthesis and delivery of basolateral plasma membrane and
secretory proteins.
Mol Biol Cell
11
4259
4275
43. Lipschutz
JH
Lingappa
VR
Mostov
KE
2003
The exocyst affects protein synthesis by acting on the
translocation machinery of the endoplasmic reticulum.
J Biol Chem
278
20954
20960
44. Moskalenko
S
Henry
DO
Rosse
C
Mirey
G
Camonis
JH
2002
The exocyst is a Ral effector complex.
Nat Cell Biol
4
66
72
45. Shalom
O
Shalva
N
Altschuler
Y
Motro
B
2008
The mammalian Nek1 kinase is involved in primary cilium
formation.
FEBS Lett
582
1465
1470
46. Siroky
BJ
Ferguson
WB
Fuson
AL
Xie
Y
Fintha
A
2006
Loss of primary cilia results in deregulated and unabated apical
calcium entry in ARPKD collecting duct cells.
Am J Physiol Renal Physiol
290
F1320
1328
47. Grantham
JJ
1990
Polycystic kidney disease: neoplasia in disguise.
Am J Kidney Dis
15
110
116
48. Lipschutz
JH
1998
The molecular development of the kidney: a review of the results
of gene disruption studies.
Am J Kid Dis
31
383
397
49. O'Brien
LE
Tang
K
Kats
ES
Schutz-Geschwender
A
Lipschutz
JH
2004
ERK and MMPs sequentially regulate distinct stages of epithelial
tubule development.
Dev Cell
7
21
32
50. Omori
S
Hida
M
Fujita
H
Takahashi
H
Tanimura
S
2006
Extracellular signal-regulated kinase inhibition slows disease
progression in mice with polycystic kidney disease.
J Am Soc Nephrol
17
1604
1614
51. Calvet
JP
2008
Strategies to inhibit cyst formation in ADPKD.
Clin J Am Soc Nephrol
3
1205
1211
52. Sweeney
WE
Jr
Avner
ED
2006
Molecular and cellular pathophysiology of autosomal recessive
polycystic kidney disease (ARPKD).
Cell Tissue Res
326
671
685
53. Torres
VE
Harris
PC
Pirson
Y
2007
Autosomal dominant polycystic kidney disease.
Lancet
369
1287
1301
54. Friedrich
GA
Hildebrand
JD
Soriano
P
1997
The secretory protein Sec8 is required for paraxial mesoderm
formation in the mouse.
Dev Biol
192
364
374
55. Boletta
A
Qian
F
Onuchic
LF
Bhunia
AK
Phakdeekitcharoen
B
2000
Polycystin-1, the gene product of PKD1, induces resistance to
apoptosis and spontaneous tubulogenesis in MDCK cells.
Mol Cell
6
1267
1273
56. Follit
JA
Tuft
RA
Fogarty
KE
Pazour
GJ
2006
The intraflagellar transport protein IFT20 is associated with the
Golgi complex and is required for cilia assembly.
Mol Biol Cell
17
3781
3792
57. Jurczyk
A
Gromley
A
Redick
S
San Agustin
J
Witman
G
2004
Pericentrin forms a complex with intraflagellar transport
proteins and polycystin-2 and is required for primary cilia
assembly.
J Cell Biol
166
637
643
58. Pazour
GJ
Dickert
BL
Vucica
Y
Seeley
ES
Rosenbaum
JL
2000
Chlamydomonas IFT88 and its mouse homologue, polycystic kidney
disease gene tg737, are required for assembly of cilia and
flagella.
J Cell Biol
151
709
718
59. Moyer
JH
Lee-Tischler
MJ
Kwon
HY
Schrick
JJ
Avner
ED
1994
Candidate gene associated with a mutation causing recessive
polycystic kidney disease in mice.
Science
264
1329
1333
60. Jonassen
JA
San Agustin
J
Follit
JA
Pazour
GJ
2008
Deletion of IFT20 in the mouse kidney causes misorientation of
the mitotic spindle and cystic kidney disease.
J Cell Biol
183
377
384
61. Yoder
BK
Tousson
A
Millican
L
Wu
JH
Bugg
CE
Jr
2002
Polaris, a protein disrupted in orpk mutant mice, is required for
assembly of renal cilium.
Am J Physiol Renal Physiol
282
F541
552
62. Lunt
SC
Haynes
T
Perkins
BD
2009
Zebrafish ift57, ift88, and ift172 intraflagellar transport
mutants disrupt cilia but do not affect hedgehog signaling.
Dev Dyn
238
1744
1759
63. Huang
P
Schier
AF
2009
Dampened Hedgehog signaling but normal Wnt signaling in zebrafish
without cilia.
Development
136
3089
3098
64. Fu
X
Wang
Y
Schetle
N
Gao
H
Putz
M
2008
The subcellular localization of TRPP2 modulates its
function.
J Am Soc Nephrol
19
1342
1351
65. Finetti
F
Paccani
SR
Riparbelli
MG
Giacomello
E
Perinetti
G
2009
Intraflagellar transport is required for polarized recycling of
the TCR/CD3 complex to the immune synapse.
Nat Cell Biol
11
1332
1339
66. Shibazaki
S
Yu
Z
Nishio
S
Tian
X
Thomson
RB
2008
Cyst formation and activation of the extracellular regulated
kinase pathway after kidney specific inactivation of Pkd1.
Hum Mol Genet
17
1505
1516
67. Mintzer
KA
Lee
MA
Runke
G
Trout
J
Whitman
M
2001
Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally
and zygotically in dorsoventral pattern formation.
Development
128
859
869
68. Taulman
PD
Haycraft
CJ
Balkovetz
DF
Yoder
BK
2001
Polaris, a protein involved in left-right axis patterning,
localizes to basal bodies and cilia.
Mol Biol Cell
12
589
599
69. Thisse
C
Thisse
B
2008
High-resolution in situ hybridization to whole-mount zebrafish
embryos.
Nat Protoc
3
59
69
70. Barile
M
Pisitkun
T
Chou
C
Verbalis
M
Knepper
M
2005
Large-scale protein identification in intracellular aquaporin-2
vesicles from renal inner medullary collecting duct.
Mol Cell Prot
4
1095
1106
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání