-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaParental Genome Dosage Imbalance Deregulates Imprinting in
In mammals and in plants, parental genome dosage imbalance deregulates embryo growth and might be involved in reproductive isolation between emerging new species. Increased dosage of maternal genomes represses growth while an increased dosage of paternal genomes has the opposite effect. These observations led to the discovery of imprinted genes, which are expressed by a single parental allele. It was further proposed in the frame of the parental conflict theory that parental genome imbalances are directly mirrored by antagonistic regulations of imprinted genes encoding maternal growth inhibitors and paternal growth enhancers. However these hypotheses were never tested directly. Here, we investigated the effect of parental genome imbalance on the expression of Arabidopsis imprinted genes FERTILIZATION INDEPENDENT SEED2 (FIS2) and FLOWERING WAGENINGEN (FWA) controlled by DNA methylation, and MEDEA (MEA) and PHERES1 (PHE1) controlled by histone methylation. Genome dosage imbalance deregulated the expression of FIS2 and PHE1 in an antagonistic manner. In addition increased dosage of inactive alleles caused a loss of imprinting of FIS2 and MEA. Although FIS2 controls histone methylation, which represses MEA and PHE1 expression, the changes of PHE1 and MEA expression could not be fully accounted for by the corresponding fluctuations of FIS2 expression. Our results show that parental genome dosage imbalance deregulates imprinting using mechanisms, which are independent from known regulators of imprinting. The complexity of the network of regulations between expressed and silenced alleles of imprinted genes activated in response to parental dosage imbalance does not support simple models derived from the parental conflict hypothesis.
Published in the journal: . PLoS Genet 6(3): e32767. doi:10.1371/journal.pgen.1000885
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000885Summary
In mammals and in plants, parental genome dosage imbalance deregulates embryo growth and might be involved in reproductive isolation between emerging new species. Increased dosage of maternal genomes represses growth while an increased dosage of paternal genomes has the opposite effect. These observations led to the discovery of imprinted genes, which are expressed by a single parental allele. It was further proposed in the frame of the parental conflict theory that parental genome imbalances are directly mirrored by antagonistic regulations of imprinted genes encoding maternal growth inhibitors and paternal growth enhancers. However these hypotheses were never tested directly. Here, we investigated the effect of parental genome imbalance on the expression of Arabidopsis imprinted genes FERTILIZATION INDEPENDENT SEED2 (FIS2) and FLOWERING WAGENINGEN (FWA) controlled by DNA methylation, and MEDEA (MEA) and PHERES1 (PHE1) controlled by histone methylation. Genome dosage imbalance deregulated the expression of FIS2 and PHE1 in an antagonistic manner. In addition increased dosage of inactive alleles caused a loss of imprinting of FIS2 and MEA. Although FIS2 controls histone methylation, which represses MEA and PHE1 expression, the changes of PHE1 and MEA expression could not be fully accounted for by the corresponding fluctuations of FIS2 expression. Our results show that parental genome dosage imbalance deregulates imprinting using mechanisms, which are independent from known regulators of imprinting. The complexity of the network of regulations between expressed and silenced alleles of imprinted genes activated in response to parental dosage imbalance does not support simple models derived from the parental conflict hypothesis.
Introduction
In mammals and plants, mothers differentiate distinctive structures specialized in the transport of maternal nutrients to the embryo, the mammalian placenta and the plant seed endosperm [1]. Thus, unilateral maternal contribution of nutrients results in an imbalanced parental contribution to the offspring. Such imbalance has been considered, in the frame of the kinship theory, as a potential cause for parental conflict of interest over allocation of resources to embryos [2],[3]. This hypothesis has gained support in mammals and in plants from the effects of parental genome dosage imbalance on embryo growth in plants and animals [4]–[8]. These observations were followed by the discovery of imprinted genes expressed preferentially from one parental allele [1],[9],[10]. The parental conflict hypothesis, derived from the kinship theory, proposes a competition over resource allocation to the embryo between imprinted genes encoding paternally expressed enhancers of embryo growth (PEGs) and maternally expressed inhibitors of embryo growth (MIGs) [11]. This hypothesis further suggests that increased maternal genome dosage results in increased levels of MIGs transcripts causing reduced embryo growth. A symmetrical increased paternal genome dosage is expected to result in increased levels of PEGs transcripts producing larger embryo. Although the parental conflict hypothesis was supported to a certain extent [2], [9], [10], [12]–[16], computational analyses on the origin of the selection of imprinting at the MEA locus did not lead to unequivocal support [17]–[19]. However, the response to dosage imbalanced is likely involved in deregulation of imprinted genes leading to sexual reproductive barriers [14] as suggested by studies involving Arabidopsis relatives [20],[21]. Although recent evidence suggested that a mutation causing the production of diploid male gametes deregulates imprinted gene expression when crossed to diploid wild type [22], the expression of imprinted genes in response to genome dosage imbalance in a wild type Arabidopsis background remained to be tested in order to provide experimental evidence for the parental conflict theory in plants.
Currently the regulation of five maternally expressed imprinted genes have been characterized in Arabidopsis, the Polycomb Group (PcG) gene MEDEA (MEA) [23], the gene MATERNALLY EXPRESSED PAB C-TERMINAL (MPC) [24], the PcG gene FERTILIZATION INDEPENDENT SEED 2 (FIS2) [25], the transcription factor FWA [26], and the actin regulator FORMIN5 [27]. The overall effect of loss-of-function of MEA and FIS2 causes enhanced endosperm growth [28],[29] leading to the conclusion that these two genes represent potential MIGs as predicted by the parental conflict hypothesis. By contrast, FORMIN5 loss of function leads to a reduction of endosperm growth and does not conform to the prediction of the parental conflict theory [27]. The transcription factor PHERES1 (PHE1) is a paternally expressed imprinted gene in Arabidopsis, which could play a role as a PEG [30],[31]. Additional imprinted genes have been characterized in Arabidopsis [32] but their function remains to be determined.
Plant reproduction is initiated by a double fertilization event [33]. Two haploid sperms are delivered to the female gametes, the egg cell and the central cell. Fertilization of the haploid egg cell leads to embryogenesis. The second sperm cell fuses with the central cell producing the endosperm. The endosperm can be considered as an embryonic annex, which nurtures embryo development [10]. Parental imbalance of genome dosage in maize affects endosperm growth, which in turn influences embryo and seed growth [4],[8]. In Arabidopsis, increased maternal genome dosage in seeds resulting from crosses between ovules from tetraploid plants and pollen from diploid plants (4nmat×2npat) leads to production of smaller endosperm, embryo and seed [5]. Reciprocal crosses (2nmat×4npat) cause the opposite effect. These results have suggested that collectively increased dosage of the expressed maternal allele of MIGs reduces endosperm growth while increased dosage of the expressed paternal allele of PEGs increases endosperm growth [11].
Although it was assumed that parental dosage imbalances would be directly mirrored by variations in the expression of the PEGs and MEGs [2],[3],[11],[14], it became apparent that MEA and PHE1 expression were regulated by FIS2 [31], [34]–[36]. This cross-regulation between imprinted genes could thus impact on the expression levels of MEA, FIS2, FWA and PHE1 in seeds resulting from interploid crosses. We performed quantitative RT-PCR to assess the expression of imprinted genes in endosperm produced by crosses between diploid and tetraploid plants and observed a global deregulation of expression levels of imprinted genes accompanied by an unexpected loss of parental imprinting for some genes. However the expression of known key regulators of imprinting were not affected. Our results suggest that parental dosage imbalance disrupts imprinting through interactions between imprinted genes and other unidentified regulators.
Results/Discussion
Increased paternal dosage causes silencing of FIS2 controlled by DNA methylation
Increased maternal dosage is expected to increase the level of expression of the active maternal allele of FIS2 and FWA. Conversely, the global level of expression of these genes should not be affected by an increased dosage of inactive paternal alleles. We used quantitative RT-PCR to investigate the effect of increased parental dosages in crosses between tetraploid and diploid plants. We measured the expression at 2 days after pollination (2DAP) when the imprinted genes studied are highly expressed and control the timing of endosperm development [28]. Between fertilization and 2 days after pollination the developmental pattern and size of endosperm size is not affected, [5] suggesting that we could observe direct consequences of parental genome imbalances. We performed the experiments in two genetic backgrounds C24 (Figure 1, Table S1) and Columbia (Col) (Figure S1, Table S3) and obtained similar results. We observed variations of higher amplitude in Col background and conservatively took into account only significant changes observed in both backgrounds and supported by statistical tests (Tables S2 and S4). We investigated the effects of genome dosage imbalance in non-imprinted genes expressed in the seed as GAPC or more specifically in endosperm as MINI3 [37] and did not observe significant fluctuation of their expression levels (Figure 1E and 1F and Figure S1E and S1F). Similarly we did not observe significant changes in the expression of the two essential regulators of imprinting encoding DEMETER (DME) [38] and the DNA METHYLTRANSFERASE1 (MET1) [25],[26],[39] (Figure 1C and 1D and Figure S1C and S1D). The expression of these two genes is also not imprinted (data not shown). Our measurements thus indicated that parental genome dosage imbalance did not affect transcription globally and did not affect regulators of DNA methylation, which control imprinting.
Fig. 1. Effects of interploid crosses on the expression of DNA methylation-dependent imprinted genes. 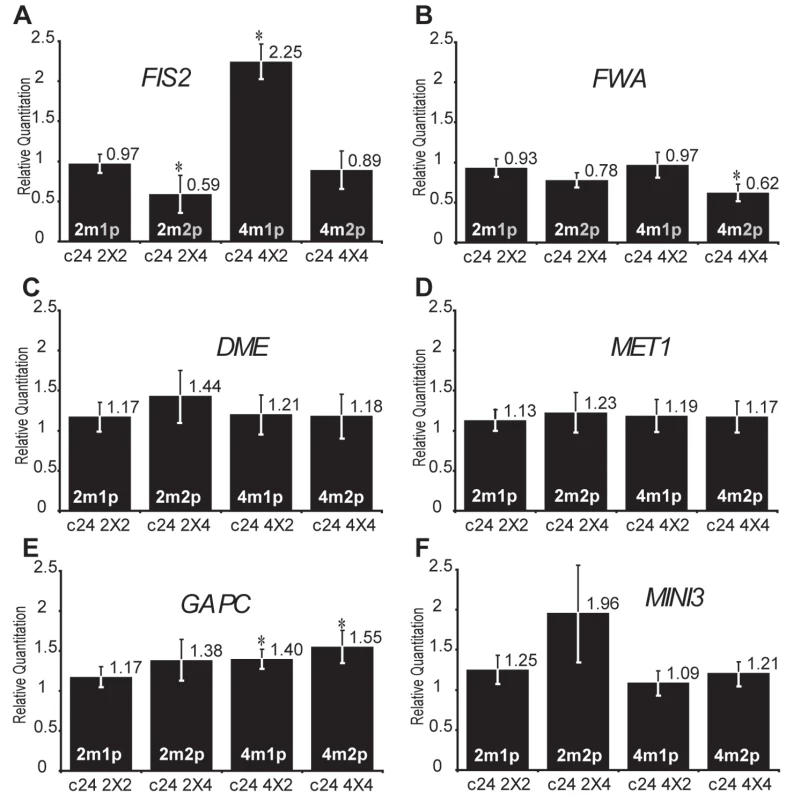
Quantitative PCR measurements of FIS2 (A), FWA (B), DME (C), MET1 (D), GAPC (E), and MINI3 (F) mRNAs were performed on total mRNAs extracted from siliques produced by crosses between diploid and tetraploid parents (2 DAP, C24 ecotype). Each point represents the average RQ value obtained for four independent biological samples (values can be found in Table S1). Error bars represent the standard deviation. * represents p<0.05 of Student's t-test using C24 2X2 as a reference, All p values can be found in Table S2. The genome copy number in the endosperm is represented inside each bar (active copy in white, inactive copy in grey). We tested the effect of genome dosage imbalance on maternally expressed imprinted genes FIS2 and FWA, which are silenced by DNA methylation [25],[26]. We observed hardly any changes in levels of FWA expression (Figure 1B and Figure S1B). By contrast to FWA, FIS2 expression levels were very sensitive to parental genome imbalance. Levels of expression in self-fertilized 2n and 4n crosses were comparable (Figure 1A). As expected, supplementary doses of active maternal FIS2 alleles produced by (4nmat×2npat) crosses increased FIS2 mRNA levels in endosperm (Figure 1A). Surprisingly, although (2nmat×4npat) crosses did not change the dosage of transcriptionaly active maternal FIS2 alleles, FIS2 expression was reduced in comparison to seeds produced by self-fertilized diploid plants. (Figure 1A). We obtained similar results in Columbia background (Figure S1A). A similar decrease of FIS2 expression was reported from 3 to 5 DAP in Landsberg erecta background using the meiotic jason (jas) mutant, which produces a proportion of diploid pollen [22]. Increased paternal dosage also reduced expression of the transcriptional reporter pFIS2-GUS, which contains the transcriptional regulatory cis-elements required for imprinting regulation [25],[40] (Figure S2). Thus, increased paternal genome dosage down-regulates FIS2 expression irrespective of its genomic context.
Trans-silencing in Arabidopsis and maize [41]–[44] has been associated with the production of small interfering RNAs [45]. However, non-coding RNAs have not been shown to affect FIS2 expression and the down-regulation of FIS2 expression in response to increased dosage of inactive paternal alleles likely result from a distinct mechanism. We propose that the unexpected silencing of the maternal alleles of FIS2 in endosperm produced by (2nmat×4npat) crosses could originate from increased paternal dosage of a paternally expressed imprinted inhibitor of FIS2 or from the activity of yet unidentified cis-elements.
Interploid crosses cause deregulation of imprinting
We assessed the imprinted status of FIS2 and FWA in interploid crosses. Both genes remained strictly maternally expressed in (4nmat×2npat) crosses (Figure 2A and 2B). This indicated that the increased dosage of active maternal alleles was directly responsible for the increased expression levels of FIS2. The opposite (2nmat×4npat) crosses did not affect the FWA imprinting status (Figure 2B) but caused an unexpected paternal expression of FIS2, resulting in the loss of FIS2 imprinting (Figure 2A). Loss of FIS2 imprinting was not restricted to RLD 2n X Col 4n crosses as it also occurred in crosses using Ler 2n and C24 2n (data not shown). We thus concluded that increased paternal dosage decreases the overall expression of FIS2 while both parental alleles become expressed. Such rather paradoxical effect is difficult to interpret. A negative interaction between MET1 activity, which maintains silencing marks and the trans-silencing mechanisms activated by the increased dosage of silenced paternal allele may cause removal of the silencing marks on the paternal allele of FIS2. Alternatively in response to reduced FIS2 expression, a transcriptional activator of FIS2 might be over-expressed and overcome silencing of the paternal allele.
Fig. 2. Effect of interploid crosses on imprinted status. 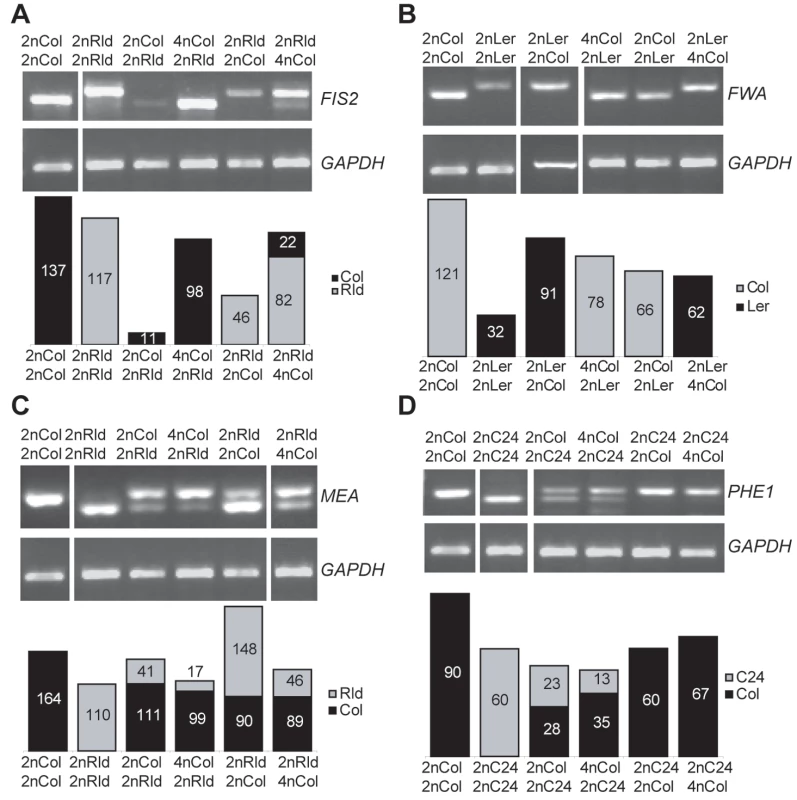
(A) The imprinting of FIS2 is detected by a size polymorphism between the strains RLD and Columbia (Col). The parent indicated at the top is the mother. (B) The imprinting of FWA is detected by a restriction polymorphism between the strains Ler and Col. (C) The imprinting of MEA is detected by a restriction polymorphism between the strains RLD and Col. (D) The imprinting of PHE1 is detected by a restriction polymorphism between the strains C24 and Col. GAPDH is used as control. The band quantification is represented below each gel as a percentage of the GAPDH band intensity. We tested whether parental genome dosage imbalance would also deregulate imprinting of the genes MEA and PHE1. MEA was predominantly expressed from the maternal allele in (4nmat×2npat) crosses as in control diploid crosses (Figure 2C). Surprisingly in (2nmat×4npat) crosses the expression from the maternal allele decreased causing a predominant paternal expression of MEA leading to an apparent inversion of MEA imprinted expression (Figure 2C). PHE1 imprinted status was not altered in response to paternal genome increase (Figure 2D). However PHE1 imprinting is hardly observed in crosses between Col females and C24 males [31], and we could not assess the effect of increased maternal dosage on PHE1 imprint (Figure 2D). In conclusion we observed that at least two out of four genes studied lost imprinting as a result of dosage imbalance. These results suggest that increasing the dosage of the silenced allele of an imprinted gene causes the removal of the imprinting marks on the silenced allele.
Dosage imbalances effect on MEA and PHE1 indicate crosstalk between imprinted gene regulations
We further tested the effect of dosage imbalance on the maternally expressed imprinted gene MEA, which is silenced by PcG mediated H3K27 trimethylation of its paternal allele [35],[36]. Although MEA is maternally expressed, MEA expression was repressed when the maternal genome dosage increased in (4nmat×2npat) crosses in C24 background (Figure 3A, Table S1 and Table S2) but not in Col background (Figure S3A, Table S3 and Table S4). A modest increased MEA expression in response to increased paternal dosage was observed in Col background (Figure S3A). A mild increase was also observed at 1 DAP in Ler background using jas mutant mimicking (2nmat×4npat) crosses [22].
Fig. 3. Effects of interploid crosses on the expression of genes imprinted by Polycomb group activity. 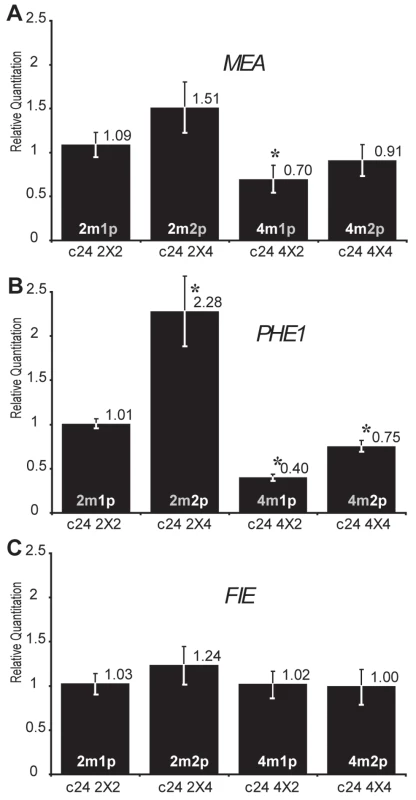
Quantitative PCR measurements of MEA (A), PHE1 (B), and FIE (C) mRNAs were performed on total mRNAs extracted from siliques produced by crosses between diploid and tetraploid parents (2 DAP, C24 ecotype). Each point represents the average RQ value obtained for four independent biological samples (values can be found in Table S1). Error bars represent the standard deviation. * represents p<0.05 of Student's t-test using C24 2X2 as a reference, p values can be found in Table S2. The genome copy number in the endosperm is represented inside each bar (active copy in white, inactive copy in grey). Parental dosage imbalances strongly perturbed PHE1 expression following the trends exhibited by MEA expression levels (Figure 3B and Figure S3B) although PHE1 is paternally expressed. A strong increase of PHE1 expression was also observed after 3 DAP in Ler background using jas mutant as pollen donor [22]. These results could be explained by the common regulation of MEA and PHE1 expression by the PcG complex, which contains FIS2 and MEA and is active in endosperm [35],[36],[46]. FIS2 encodes a Suppressor of zeste 12 (Su(z)12) Polycomb group subunit [47]. Since the two other members of the Su(z)12 family are not expressed in Arabidopsis endosperm [40] the reduction of FIS2 expression levels in (2nmat×4npat) crosses could become limiting for Polycomb group activity, leading to increased expression of MEA and PHE1. This effect would also be directly responsible for the inversion of MEA imprinting (Figure 3C) as previously shown for the effect of reduced Polycomb activity in loss of function mutants for FERTILIZATION INDEPENDENT ENDOSPERM (FIE) [35],[36]. We verified that parental dosage imbalance and tetraploidy do not affect FIE expression levels (Figure 3C and Figure S3C). We further tested whether alterations of FIS2 expression would mimic the effects observed on MEA and PHE1 in response to parental genome dosage imbalance. We used the loss of function allele fis2-6 to decrease the levels of FIS2 expression and a transgenic line expressing a complementing FIS2-YFP fusion protein to increase the levels of FIS2 expression [48] (Figure 4, Table S5). Manipulating FIS2 mRNA levels (Figure 4A) did not affect FWA expression (Figure 4B). We did not observe any effect of increased FIS2 levels on PHE1 expression levels (Figure 4C). However we observed that decreased FIS2 expression causes increased PHE1 expression but did not affect MEA expression. Despite the fact that MEA and PHE1 are over-expressed in response to decreased FIS PcG activity [36], [46].
Fig. 4. Effects of FIS2 mRNA levels on the expression of imprinted genes. 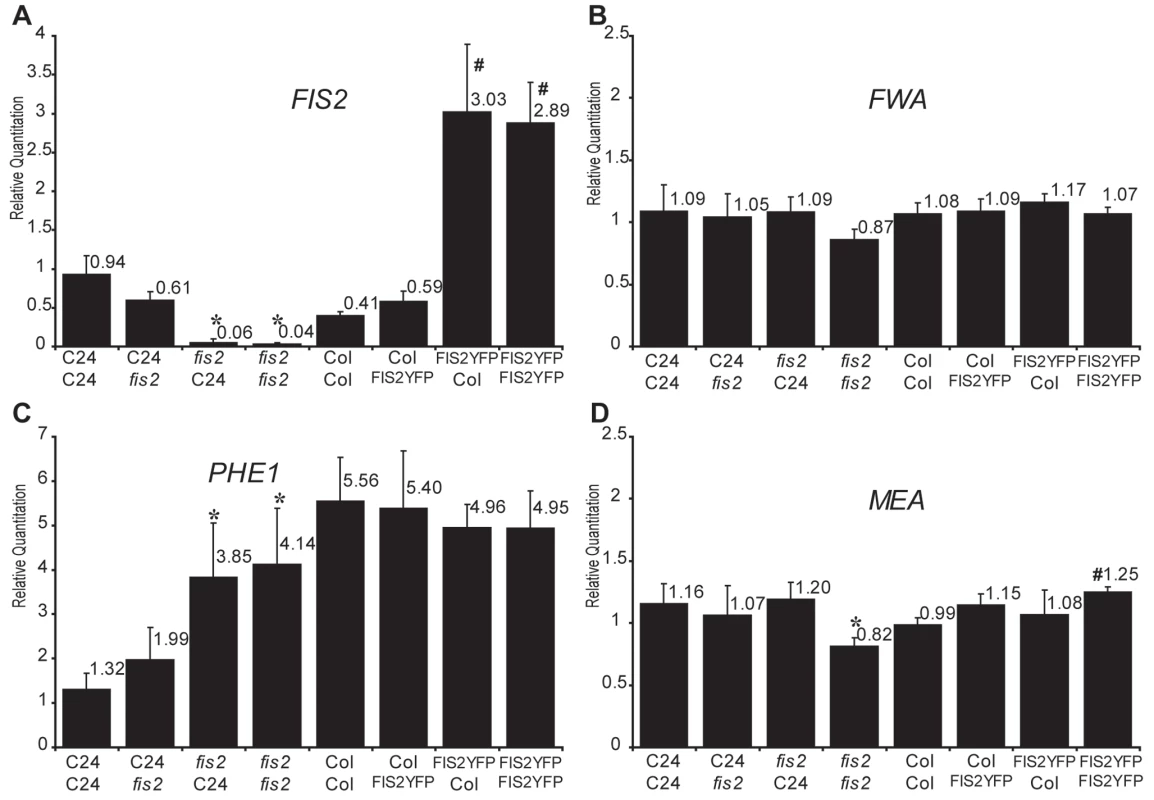
Quantitative PCR measurements of FIS2 (A), FWA (B), PHE1 (C), and MEA (D). mRNAs were performed on total mRNAs extracted from siliques produced by crosses between C24 and fis2-6 parents (2 DAP, C24 ecotype) to test for the effect of FIS2 down-regulation and between Col and FIS2YFP (2DAP, Col ecotype) to test the effect of increased FIS2 expression. Each point represents the average RQ value obtained for three independent biological samples. Error bars represent the standard deviation. * represents p<0.05 of Student's t-test using C24 2X2 as a reference, # represents p<0.05 of Student's t-test using Col 2X2 as a reference, p values can be found in Table S5. We conclude that increased FIS2 expression caused by maternal genome dosage increase is not directly responsible for the decreased expression of MEA and PHE1. By contrast, paternal genome dosage causes an unexpected decrease of FIS2 expression, which in turn could directly or indirectly increase PHE1 expression. As an alternative explanation, increased dosage of PHE1 copy number might rather directly increase PHE1 expression in response to paternal dosage increase.
Conclusions
Reciprocal changes of parental dosage do not cause the symmetrical variations of expression of imprinted genes predicted by previous studies. A similar complex phenomenon was observed in mammals [48]–[52]. However parthenogenetic embryos used in mice to investigate parental dosage imbalance do not allow a direct test for the interactions between the paternal and maternal allele. In addition parthenogenotes are produced via complex in vitro manipulations and other factors may perturb silencing at imprinted loci. In plants dosage imbalances are created in vivo in undisturbed reproductive tissues and their consequences are unlikely to reflect the consequence of experimental manipulations. In response to parental imbalance we observed unexpected non-symmetrical deregulation of the expression of imprinted genes coupled with a loss of imprinting in two out of four imprinted genes studied. The modulation of seed size by dosage imbalance does not result in variations of PEGs and MIGs expression parallel to variation of the dosage of the respective parental genome. In addition, the mode of perturbation may vary during later development stages in endosperms produced by crosses involving jas mutant that produces a fraction of diploid pollen [22].
After 6 DAP, paternal excess dosage causes endosperm developmental defects similar to loss of FIS complex activity [5]. The fact that MEA ectopic expression rescues late endosperm developmental defects in crosses with jas pollen [22] suggests that the late perturbations of imprinted genes expression in response to jas pollination may result rather from an indirect deregulation of endosperm developmental timing caused by loss of FIS activity [28]. At early stages of endosperm development we do not observe a strong link between FIS2 expression and the perturbation of MEA and PHE1 expression. Thus our data do not support that the FIS PcG complex directly deregulates imprinted genes expression in response to dosage imbalance a couple of days after fertilization. In addition parental genome dosage imbalance does not affect expression of FIE, the essential component of the FIS PcG complex. Parental genome dosage imbalance does not affect expression of the regulators of DNA methylation MET1 and DME. Hence, parental dosage imbalance does not directly affect the known major controls of imprinting. Nevertheless, we propose that parental dosage imbalance deregulates FIS2 and MEA expression, which causes late endosperm developmental defects including over-proliferation and ectopic expression of PHE1. A similar phenotype has been observed in crosses between A. thaliana and A. arenosa. Such deregulation compromise seed viability and likely contribute to species isolation mechanisms involving tetraploidization [21], [43], [53].
We do not currently understand the mechanisms that cause the immediate response to dosage imbalance and deregulation of FIS2, MEA and PHE1 expression. Such mechanisms could involve other controls of DNA methylation [54] or small non-coding RNAs inherited maternally [55] or paternally [56]. The apparent parental conflict linked to imprinting in plants and in mammals likely results from a complex series of non-symmetrical regulations during zygotic development. Nevertheless these mechanisms could involve imprinted regulators controlled in a dosage dependent manner predicted by the kinship theory [2], [57].
Materials and Methods
Plant lines
The wild-type control lines C24, Col, Ler, and RLD were obtained from the ABRC stock center. The tetraploid lines in C24 and in Col ecotypes were kindly provided by Rod Scott [5] and Luca Comai [58]. The reporter line pFIS2-GUS (C24 accession) was kindly provided by Abed Chaudhury [40]. FIS2YFP line was kindly provided by Ramin Yadegari [48] and fis2-6 was previously identified in our laboratory [59].
Allele-specific RT–PCR and quantitative real-time RT–PCR
Siliques two days after pollination (2DAP) were collected from Arabidopsis plants and frozen in liquid nitrogen. Total RNAs were extracted using the RNeasy mini kit (Qiagen). After DNAse treatment using DNase free kit (Ambion), RNAs were reverse-transcribed using Stratascript RT kit (Stratagene).
Allele-specific RT-PCR reactions were performed as previously described [23],[25],[26],[60]. Band quantification was performed using the ImageJ software (http://rsbweb.nih.gov/ij/).
Real-time PCR assays were performed using a PCR Master Mix (Applied Biosystems, Foster City, CA). One µl of RT product was used to perform each PCR reaction. Amplification reaction was carried out using specific primers at a concentration of 0.5 mM in a 10 µl reaction. Sequence of specific primer pairs can be found in Table S6. The specificity of the amplification product was determined by performing a dissociation curve analysis. PCR efficiency was determined using the LinReg program [61]. The PCR reaction and quantitative measurements were achieved with 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Thermal cycling parameters were 2 min at 50°C, 10 min at 95°C and 50 cycles of 15 sec at 95°C, 60 sec at 60°C. We performed four biological replicates, with three technical replicates for each sample. For each PCR reaction the ΔCt was calculated using ACT11 gene as endogenous control except for Figure 4 were FIE was used as endogenous control. Relative Quantitation values (RQ) were calculated using the 2−ΔΔCt method (RQ = 2−ΔΔCt) [62]. Values given in Figure 1, Figure 3, Figure 4, Figure S1 and Figure S3 represent the average of RQ values obtained for four or three biological replicates for each point and the error bars represent the standard deviation of the biological replicates. Tables of RQ values used to make the graphs can be found in Table S1 for C24 accession and Table S3 for Col accession.
Supporting Information
Zdroje
1. FeilR
BergerF
2007 Convergent evolution of genomic imprinting in plants and mammals. Trends Genet 23 192 199
2. HaigD
2004 Genomic imprinting and kinship: how good is the evidence? Annu Rev Genet 38 553 585
3. WilkinsJF
HaigD
2003 What good is genomic imprinting: the function of parent-specific gene expression. Nat Rev Genet 4 359 368
4. LinB-Y
1984 Ploidy barrier to endosperm development in maize. Genetics 107 103 115
5. ScottRJ
SpielmanM
BaileyJ
DickinsonHG
1998 Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 125 3329 3341
6. SuraniMA
BartonSC
NorrisML
1984 Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308 548 550
7. McGrathJ
SolterD
1984 Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 37 179 183
8. LeblancO
PointeC
HernandezM
2002 Cell cycle progression during endosperm development in Zea mays depends on parental dosage effects. Plant J 32 1057 1066
9. ScottRJ
SpielmanM
2004 Epigenetics: imprinting in plants and mammals, the same but different? Curr Biol 14 R201 203
10. BergerF
ChaudhuryA
2009 Parental memories shape seeds. Trends Plant Sci 14 550 556
11. SpielmanM
VinkenoogR
DickinsonHG
ScottRJ
2001 The epigenetic basis of gender in flowering plants and mammals. Trends Genet 17 705 711
12. de JongTJ
ScottRJ
2007 Parental conflict does not necessarily lead to the evolution of imprinting. Trends Plant Sci 12 439 443
13. GehringM
ChoiY
FischerRL
2004 Imprinting and seed development. Plant Cell16Suppl S203 213
14. DilkesBP
ComaiL
2004 A differential dosage hypothesis for parental effects in seed development. Plant Cell 16 3174 3180
15. KinoshitaT
IkedaY
IshikawaR
2008 Genomic imprinting: a balance between antagonistic roles of parental chromosomes. Semin Cell Dev Biol 19 574 579
16. ReikW
WalterJ
2001 Genomic imprinting: parental influence on the genome. Nature Reviews 2 21 32
17. KawabeA
FujimotoR
CharlesworthD
2007 High diversity due to balancing selection in the promoter region of the Medea gene in Arabidopsis lyrata. Curr Biol 17 1885 1889
18. MiyakeT
TakebayashiN
WolfDE
2009 Possible diversifying selection in the imprinted gene, MEDEA, in Arabidopsis. Mol Biol Evol
19. SpillaneC
SchmidKJ
Laoueille-DupratS
PienS
Escobar-RestrepoJM
2007 Positive darwinian selection at the imprinted MEDEA locus in plants. Nature 448 349 352
20. JosefssonC
DilkesB
ComaiL
2006 Parent-dependent loss of gene silencing during interspecies hybridization. Curr Biol 16 1322 1328
21. WaliaH
JosefssonC
DilkesB
KirkbrideR
HaradaJ
2009 Dosage-dependent deregulation of an AGAMOUS-LIKE gene cluster contributes to interspecific incompatibility. Curr Biol 19 1128 1132
22. ErilovaA
BrownfieldL
ExnerV
RosaM
TwellD
2009 Imprinting of the polycomb group gene MEDEA serves as a ploidy sensor in Arabidopsis. PLoS Genet 5 e1000663 doi:10.1371/journal.pgen.1000663
23. KinoshitaT
YadegariR
HaradaJJ
GoldbergRB
FischerRL
1999 Imprinting of the MEDEA polycomb gene in the Arabidopsis endosperm. Plant Cell 11 1945 1952
24. TiwariS
SchulzR
IkedaY
DythamL
BravoJ
2008 MATERNALLY EXPRESSED PAB C-TERMINAL, a novel imprinted gene in Arabidopsis, encodes the conserved C-terminal domain of polyadenylate binding proteins. Plant Cell 20 2387 2398
25. JullienPE
KinoshitaT
OhadN
BergerF
2006 Maintenance of DNA Methylation during the Arabidopsis Life Cycle Is Essential for Parental Imprinting. Plant Cell 18 1360 1372
26. KinoshitaT
MiuraA
ChoiY
KinoshitaY
CaoX
2004 One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303 521 523
27. Fitz GeraldJN
HuiPS
BergerF
2009 Polycomb group-dependent imprinting of the actin regulator AtFH5 regulates morphogenesis in Arabidopsis thaliana. Development 136 3399 3404
28. IngouffM
HaseloffJ
BergerF
2005 Polycomb group genes control developmental timing of endosperm. Plant J 42 663 674
29. KiyosueT
OhadN
YadegariR
HannonM
DinnenyJ
1999 Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. Proc Natl Acad Sci USA 96 4186 4191
30. KohlerC
HennigL
SpillaneC
PienS
GruissemW
2003 The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes and development 17 1540 1553
31. MakarevichG
VillarCB
ErilovaA
KohlerC
2008 Mechanism of PHERES1 imprinting in Arabidopsis. J Cell Sci 121 906 912
32. GehringM
BubbKL
HenikoffS
2009 Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science 324 1447 1451
33. BergerF
HamamuraY
IngouffM
HigashiyamaT
2008 Double fertilization - caught in the act. Trends Plant Sci 13 437 443
34. BarouxC
GagliardiniV
PageDR
GrossniklausU
2006 Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev 20 1081 1086
35. GehringM
HuhJH
HsiehTF
PentermanJ
ChoiY
2006 DEMETER DNA Glycosylase Establishes MEDEA Polycomb Gene Self-Imprinting by Allele-Specific Demethylation. Cell 124 495 506
36. JullienPE
KatzA
OlivaM
OhadN
BergerF
2006 Polycomb Group Complexes Self-Regulate Imprinting of the Polycomb Group Gene MEDEA in Arabidopsis. Curr Biol 16 486 492
37. LuoM
DennisES
BergerF
PeacockWJ
ChaudhuryA
2005 MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc Natl Acad Sci U S A 102 17531 17536
38. ChoiY
GehringM
JohnsonL
HannonM
HaradaJJ
2002 DEMETER, a DNA Glycosylase Domain Protein, Is Required for Endosperm Gene Imprinting and Seed Viability in Arabidopsis. Cell 110 33 42
39. FinneganEJ
PeacockWJ
DennisES
1996 Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc Natl Acad Sci U S A 93 8449 8454
40. LuoM
BilodeauP
DennisES
PeacockWJ
ChaudhuryA
2000 Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc Natl Acad Sci USA 97 10637 10642
41. ChanSW
ZhangX
BernatavichuteYV
JacobsenSE
2006 Two-step recruitment of RNA-directed DNA methylation to tandem repeats. PLoS Biol 4 e363 doi:10.1371/journal.pbio.0040363
42. ChandlerVL
2007 Paramutation: from maize to mice. Cell 128 641 645
43. ComaiL
2005 The advantages and disadvantages of being polyploid. Nat Rev Genet 6 836 846
44. Mittelsten ScheidO
AfsarK
PaszkowskiJ
2003 Formation of stable epialleles and their paramutation-like interaction in tetraploid Arabidopsis thaliana. Nat Genet 34 450 454
45. AllemanM
SidorenkoL
McGinnisK
SeshadriV
DorweilerJE
2006 An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442 295 298
46. MakarevichG
LeroyO
AkinciU
SchubertD
ClarenzO
2006 Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep 7 947 952
47. LuoM
BilodeauP
KoltunowA
DennisES
PeacockWJ
1999 Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci U S A 96 296 301
48. WangD
TysonMD
JacksonSS
YadegariR
2006 Partially redundant functions of two SET-domain polycomb-group proteins in controlling initiation of seed development in Arabidopsis. Proc Natl Acad Sci U S A 103 13244 13249
49. AllenND
BartonSC
HiltonK
NorrisML
SuraniMA
1994 A functional analysis of imprinting in parthenogenetic embryonic stem cells. Development 120 1473 1482
50. SasakiH
Ferguson-SmithAC
ShumAS
BartonSC
SuraniMA
1995 Temporal and spatial regulation of H19 imprinting in normal and uniparental mouse embryos. Development 121 4195 4202
51. SotomaruY
KatsuzawaY
HatadaI
ObataY
SasakiH
2002 Unregulated expression of the imprinted genes H19 and Igf2r in mouse uniparental fetuses. J Biol Chem 277 12474 12478
52. SotomaruY
KawaseY
UedaT
ObataY
SuzukiH
2001 Disruption of imprinted expression of U2afbp-rs/U2af1-rs1 gene in mouse parthenogenetic fetuses. J Biol Chem 276 26694 26698
53. HenryIM
DilkesBP
YoungK
WatsonB
WuH
2005 Aneuploidy and genetic variation in the Arabidopsis thaliana triploid response. Genetics 170 1979 1988
54. ChanSW
HendersonIR
JacobsenSE
2005 Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat Rev Genet 6 351 360
55. MosherRA
MelnykCW
KellyKA
DunnRM
StudholmeDJ
2009 Uniparental expression of PolIV-dependent siRNAs in developing endosperm of Arabidopsis. Nature 460 283 286
56. SlotkinRK
VaughnM
BorgesF
TanurdzicM
BeckerJD
2009 Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136 461 472
57. DittRF
KerrKF
de FigueiredoP
DelrowJ
ComaiL
2006 The Arabidopsis thaliana transcriptome in response to Agrobacterium tumefaciens. Mol Plant Microbe Interact 19 665 681
58. MadlungA
TyagiAP
WatsonB
JiangH
KagochiT
2005 Genomic changes in synthetic Arabidopsis polyploids. Plant J 41 221 230
59. GuittonAE
PageDR
ChambrierP
LionnetC
FaureJE
2004 Identification of new members of Fertilisation Independent Seed Polycomb Group pathway involved in the control of seed development in Arabidopsis thaliana. Development 131 2971 2981
60. KohlerC
PageDR
GagliardiniV
GrossniklausU
2005 The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet 37 28 30
61. RuijterJM
RamakersC
HoogaarsWM
KarlenY
BakkerO
2009 Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37 e45
62. LivakKJ
SchmittgenTD
2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402 408
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 3
-
Všechny články tohoto čísla
- Parental Genome Dosage Imbalance Deregulates Imprinting in
- Identification and Functional Analysis of the Vision-Specific BBS3 (ARL6) Long Isoform
- HAP2(GCS1)-Dependent Gamete Fusion Requires a Positively Charged Carboxy-Terminal Domain
- Initial Genomics of the Human Nucleolus
- Role of RecA and the SOS Response in Thymineless Death in
- PPS, a Large Multidomain Protein, Functions with Sex-Lethal to Regulate Alternative Splicing in
- Mislocalization of XPF-ERCC1 Nuclease Contributes to Reduced DNA Repair in XP-F Patients
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Human Population Differentiation Is Strongly Correlated with Local Recombination Rate
- Local-Scale Patterns of Genetic Variability, Outcrossing, and Spatial Structure in Natural Stands of
- Arginylation-Dependent Neural Crest Cell Migration Is Essential for Mouse Development
- HP1 Recruitment in the Absence of Argonaute Proteins in
- MiR-218 Inhibits Invasion and Metastasis of Gastric Cancer by Targeting the Robo1 Receptor
- Bias and Evolution of the Mutationally Accessible Phenotypic Space in a Developmental System
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Rapid Assessment of Genetic Ancestry in Populations of Unknown Origin by Genome-Wide Genotyping of Pooled Samples
- Regulation of Lifespan, Metabolism, and Stress Responses by the SH2B Protein, Lnk
- KRAB–Zinc Finger Proteins and KAP1 Can Mediate Long-Range Transcriptional Repression through Heterochromatin Spreading
- Identification of the Regulatory Logic Controlling Pathoadaptation by the SsrA-SsrB Two-Component System
- Drosophila Xpd Regulates Cdk7 Localization, Mitotic Kinase Activity, Spindle Dynamics, and Chromosome Segregation
- Multiple Signals Converge on a Differentiation MAPK Pathway
- In the Tradition of Science: An Interview with Victor Ambros
- Association of the Polymorphism His615Arg with Melanin Content in East Asian Populations: Further Evidence of Convergent Evolution of Skin Pigmentation
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Derepression of the Plant Chromovirus Induces Germline Transposition in Regenerated Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



