-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHAP2(GCS1)-Dependent Gamete Fusion Requires a Positively Charged Carboxy-Terminal Domain
HAP2(GCS1) is a deeply conserved sperm protein that is essential for gamete fusion. Here we use complementation assays to define major functional regions of the Arabidopsis thaliana ortholog using HAP2(GCS1) variants with modifications to regions amino(N) and carboxy(C) to its single transmembrane domain. These quantitative in vivo complementation studies show that the N-terminal region tolerates exchange with a closely related sequence, but not with a more distantly related plant sequence. In contrast, a distantly related C-terminus is functional in Arabidopsis, indicating that the primary sequence of the C-terminus is not critical. However, mutations that neutralized the charge of the C-terminus impair HAP2(GCS1)-dependent gamete fusion. Our results provide data identifying the essential functional features of this highly conserved sperm fusion protein. They suggest that the N-terminus functions by interacting with female gamete-expressed proteins and that the positively charged C-terminus may function through electrostatic interactions with the sperm plasma membrane.
Published in the journal: . PLoS Genet 6(3): e32767. doi:10.1371/journal.pgen.1000882
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000882Summary
HAP2(GCS1) is a deeply conserved sperm protein that is essential for gamete fusion. Here we use complementation assays to define major functional regions of the Arabidopsis thaliana ortholog using HAP2(GCS1) variants with modifications to regions amino(N) and carboxy(C) to its single transmembrane domain. These quantitative in vivo complementation studies show that the N-terminal region tolerates exchange with a closely related sequence, but not with a more distantly related plant sequence. In contrast, a distantly related C-terminus is functional in Arabidopsis, indicating that the primary sequence of the C-terminus is not critical. However, mutations that neutralized the charge of the C-terminus impair HAP2(GCS1)-dependent gamete fusion. Our results provide data identifying the essential functional features of this highly conserved sperm fusion protein. They suggest that the N-terminus functions by interacting with female gamete-expressed proteins and that the positively charged C-terminus may function through electrostatic interactions with the sperm plasma membrane.
Introduction
The fusion of gamete plasma membranes is a critical event in fertilization, but despite the ubiquity of the process among sexually reproducing eukaryotes, no conserved mechanism for gamete fusion has been described. At least two factors contribute to our lack of mechanistic insight. First, many proteins that mediate binding and fusion of complementary gametes evolve rapidly, thereby reinforcing barriers to interspecific hybridization [1]. Second, gamete fusion is a transient event occurring between two cells, limiting the ability to observe fusion and to study it using biochemical methods.
Genetic analysis in Arabidopsis (Arabidopsis thaliana, At) identified HAP2(GCS1), a sperm-expressed gene that is essential for fertilization [2]–[4]. In flowering plants, two genetically identical haploid sperm are delivered by a pollen tube to female gametes that develop within an ovule. One sperm fuses with the egg to produce a zygote while the other fuses with the central cell to produce endosperm, a tissue that supports the developing embryo. Both fertilization events are required to initiate development of a seed (reviewed in [5]–[6]). HAP2(GCS1), for HAPLESS2 [2],[4] and synonym GENERATIVE CELL SPECIFIC1 [3], is required for both sperm fusion events occurring during double fertilization.
The role of HAP2(GCS1) in fertilization may be widespread in eukaryotes as orthologs are present in several protist, animal, and plant genomes [7]–[8]. Loss of HAP2(GCS1) function in male gametes also blocks fertilization in Plasmodium berghei (sperm affected [7],[9]) and Chlamydomonas reinhardtii (Cr, minus gametes affected [7]), suggesting it plays a similar role at fertilization throughout eukaryotes. Key observations made in Chlamydomonas suggest a specific role for CrHAP2(GCS1) in gamete fusion [7]: (i) The Cr hap2(gcs1) loss-of-function mutation prevents fertilization, despite the ability of gametes to bind one another and bring opposing membranes into close proximity and (ii) CrHAP2(GCS1) is enriched at the tip of the minus mating projection just prior to fusion.
All predicted HAP2(GCS1) orthologs share a common primary architecture. Each is divided into two regions by a single pass transmembrane domain and contains a HAP2-GCS1 domain of about 50 amino acids in the large region amino(N)-terminal to the transmembrane domain (Figure 1A and Figure 2A). The carboxy(C)-terminus is enriched in charged residues that do not follow a defined sequence: histidine is dominant in flowering plants while other basic residues (lysine, arginine) are enriched in other species [3]–[4], [7]–[8]. Primary sequence analysis of HAP2(GCS1) has not detected other known motifs or functional domains.
Fig. 1. Protocol for analyzing complementation of hap2-1 fertilization defects using HAP2(GCS1) CDS variants. 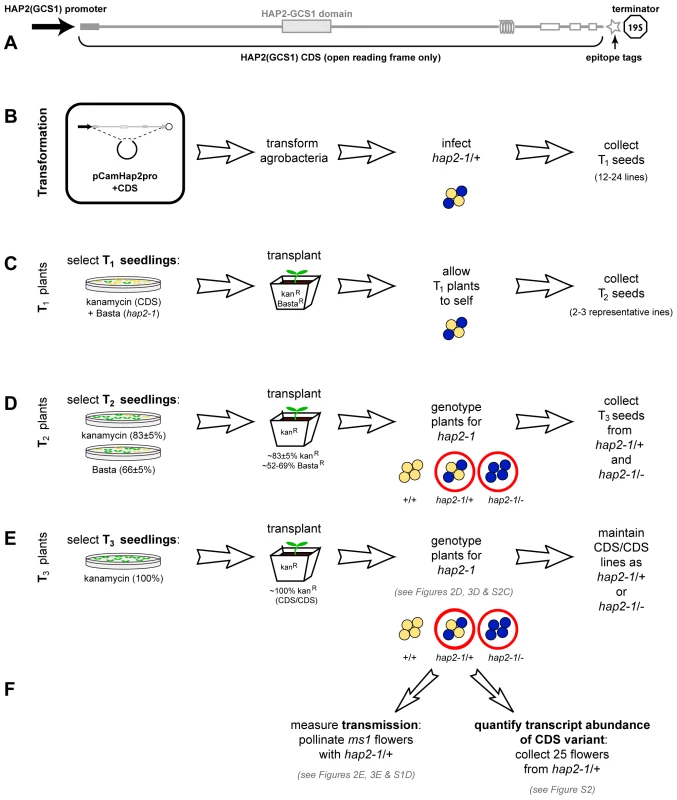
(A) Schematic of the AtN•AtC construct representing the 1.5 kb promoter, HAP2(GCS1) coding sequence with major domains, C-terminal V5C4 epitope tag, and 19S terminator and polyadenlyation sequence. Grey box represents the predicted signal sequence in exon 1, coils represent the predicted transmembrane domain. The HAP2-GCS1 domain (Protein Family ID: PF10699; InterPro ID, IPR018928) is the most conserved sequence among orthologs. (B–F) Method to obtain single-locus-insertion transgenic plants for complementation analysis. (B) hap2-1/+ plants were transformed. (C) Primary transformants (T1) plants were selected for both BastaR and kanR, indicating they carried hap2-1 and the CDS construct. (D) The progeny (T2) of self-fertilization of these individuals (T1) were assayed for segregation of the CDS construct and hap2-1 by scoring kanR and BastaR. Self-fertilization of hap2-1 resulted in 50% BastaR progeny [2],[4]. Complementation of the hap2-1 transmission defect by a single locus insertion of a CDS encoding functional HAP2(GCS1) increases this to ∼66% BastaR in the progeny of T1 plants. We screened progeny from 15–24 transformants and selected at least two lines whose progeny exhibited ∼66% BastaR. In cases where complementation failed and no lines resulted in >50% BastaR progeny, we chose two lines siring the highest percentage BastaR progeny for further analysis. We identified and selected transgenic lines with a single insertion of the CDS construct by analyzing kanR among progeny. (E) Lines homozygous for a CDS variant (CDS/CDS) were identified by scoring kanR in T3 progeny (100%). (F) Assays used to evaluate expression and complementation of each hap2-1/+, CDS/CDS line. The descendents of at least two individuals from each transgenic line were used to conduct each assay. Fig. 2. Analysis of HAP2(GCS1) deletions and interspecific chimeras. 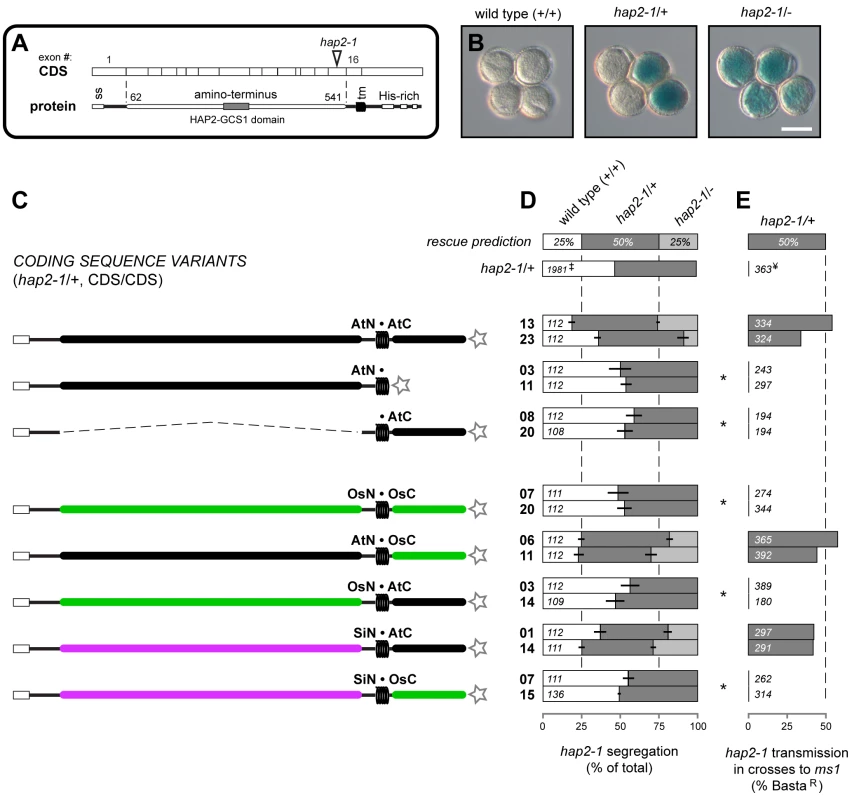
(A) Schematic of the HAP2(GCS1) CDS and protein. Exon boundaries (numbered above) are mapped to the open reading frame and major features including the signal sequence (ss), transmembrane domain (tm), Arabidopsis amino acid position numbers delineating the N-terminal region, and the T-DNA insertion for hap2-1 are shown. (B) Pollen tetrads from wild-type (+/+), hap2-1/+, or hap2-1/- plants. Scale bar = 50 µm. (C) Diagram of the HAP2(GCS1) variants tested. Star at the C- terminus represents the epitope tag. Genbank accession numbers: A. thaliana = DQ022375; S. irio = GU724984; O. sativa = AK072871. (D-E) Percentage of progeny inheriting hap2-1 from hap2-1/+, CDS/CDS plants following self-fertilization (D) or crosses of pollen to ms1 pistils (E). Expected frequency of progeny inheriting hap2-1 if variant is fully functional is shown in the top bar (“rescue prediction”), and previously published hap2-1 results are shown for comparison (‡, [2] and ¥, [4]). Data from two independent transgenic lines are shown for each HAP2(GCS1) variant. Bars represent the mean percentages from each transgenic line; horizontal lines denote the percentage range between descendents from two individuals per line. Total number of plants (D) or seedlings (E) scored per line is shown. Asterisks denote significant differences from expected complementation (p<10−5). We use quantitative molecular-genetic assays in Arabidopsis to characterize the major features of HAP2(GCS1). We find that both regions of the protein are essential for function, and that these regions are under different selective pressures: Primary sequence from a closely related plant, not a distant relative, can replace the Arabidopsis N-terminus. Thus, while HAP2(GCS1) does not define species-level interactions between gametes, function of the N-terminus may be constrained by co-evolution with partner proteins expressed by female gametes. On the other hand, the Arabidopsis HAP2(GCS1) C-terminus retains function when replaced with sequence from a distantly related plant or mutated sequences, as long as positive charge is retained. Thus, net charge over the C-terminus is the critical feature of this region. These experiments thus establish essential characteristics of an ancient protein required for gamete fusion.
Results
A Genetic Assay to Evaluate the In Vivo Function of HAP2(GCS1) Variants
hap2-1 blocks the ability of sperm to participate in fertilization, but does not affect female reproduction [4]. This allele was generated by insertion of a T-DNA carrying two marker genes to facilitate analysis of segregation and transmission: (i) resistance to the herbicide Basta (BastaR) and (ii) β-glucuronidase (GUS) driven by the pollen-specific LAT52 promoter (LAT52:GUS) [2],[4]. hap2-1 was identified in the quartet (qrt1-2) background, a mutation that maintains male meiotic products in tetrads [10]. This feature, combined with LAT52:GUS expression in pollen, allows one to distinguish heterozygous hap2-1 (hap2-1/+) pollen from wild-type (Figure 1B–1F and Figure 2B). Self-fertilization of hap2-1/+ results in 50% heterozygous (BastaR, two GUS+ to two GUS- pollen per tetrad) and 50% wild-type plants (Basta sensitive (BastaS), GUS- pollen); homozygous hap2-1 (hap2-1/-) plants are not recovered [2],[4], and thus hap2-1 transmission is distorted (not the expected 1∶2∶1 segregation of wild type: heterozygous: homozygous mutant). Furthermore, pollination of wild-type females with hap2-1/+ pollen yields no progeny with the hap2-1 allele, e.g. all progeny are sired by wild-type pollen produced by the heterozygous father. Thus, hap2-1 cannot be transmitted though the male germline.
We developed a system to test if coding sequence (CDS) variants of HAP2(GCS1) could restore male transmission of the hap2-1 allele (Figure 1). Transformation of hap2-1/+ mutants with a wild-type HAP2(GCS1) genomic clone [including 1.5 kb of HAP2(GCS1) promoter sequence] complemented the fertilization defect, and self-fertilization of hap2-1/+ mutants carrying this transgene produced hap2-1/- progeny (BastaR, four GUS+ pollen per tetrad) [4]. Pollen from these plants was also capable of transmitting hap2-1 to progeny when crossed to wild-type females, producing BastaR hap2-1/+ seedlings [4]. We transformed hap2-1/+ plants with a series of HAP2(GCS1) CDS variants under the control of the same 1.5 kb HAP2(GCS1) promoter sequence; each variant T-DNA construct carried a kanamycin resistance (KanR) gene. To track expression of CDS constructs and to differentiate between endogenous and introduced HAP2(GCS1), we included sequences encoding short epitope tags (V5 [11] and tetra-cysteine (CCGPCC) [12]; see Materials and Methods) at the 3′ end of the variants (Figure 1A).
To determine if HAP2(GCS1) variants were capable of mediating gamete fusion, we generated hap2-1/+ transgenic lines that were homozygous for the CDS variant (CDS/CDS, Figure 1). These plants produce two pollen genotypes whose ability to fertilize female gametes could be directly compared: (i) HAP2(GCS1), CDS (BastaS, GUS-, KanR) or (ii) hap2-1, CDS (BastaR, GUS+, KanR). As with the genomic construct [4], if the CDS variant encodes fully functional HAP2(GCS1), the ability to fertilize wild-type females, and thus transmit hap2-1, should be restored to hap2-1 sperm.
Introduction of either the native CDS (data not shown), or an epitope tagged version rescued fertility of hap2-1 (Figure 2C–2E, top row, and Figure S1). In both transgenic lines analyzed, hap2-1/- plants were recovered following self-fertilization, and segregation was restored to ∼1∶2∶1 (25% wild-type, 50% hap2-1/+, 25% hap2-1/-, Figure 2D). When these lines were used to pollinate male sterile1 (ms1) females, hap2-1 was inherited (Figure 2E and Figure S1), indicating complete or nearly complete complementation of hap2-1 by the epitope-tagged, native Arabidopsis HAP2(GCS1) CDS. These control experiments demonstrated that the addition of C-terminal epitope tags did not disrupt the function of the HAP2(GCS1) CDS and that expressing the HAP2(GCS1) CDS from the HAP2(GCS1) promoter resulted in expression of functional HAP2(GCS1) protein.
Regional HAP2(GCS1) Deletions Are Not Functional
We first asked if the regions N - or C-terminal to the HAP2(GCS1) transmembrane domain were essential for HAP2(GCS1) function. The amino acids encoded by exons 2-15 (amino acid residues 62–541, Figure 2A) were deleted, retaining exon 1 and its signal peptide to ensure that the protein product was properly directed to the secretory pathway (•AtC, Figure 2C). In a second construct, we directly fused epitope tags to the end of the transmembrane domain to test if the C-terminus was essential (AtN•, Figure 2C). None of the 24 primary transformants established for either variant segregated >50% BastaR seedlings, produced hap2-1/- plants, or restored normal segregation among the progeny of self-fertilization (Figure 2D and Figure S1). Further, hap2-1/+ pollen did not produce BastaR progeny when crossed to ms1 (Figure 2E and Figure S1).
Epitope tag sequences were detected in floral mRNA extracted from AtN• or •AtC lines, and the abundance of HAP2(GCS1) mRNA was higher in these flowers than in hap2-1/+ flowers (Figure S2), suggesting that failure of •AtC or AtN• variants to rescue hap2-1 was not due to lack of construct expression. Thus, the two regions of HAP2(GCS1) that lie on either side of the transmembrane domain are essential for function.
Exchange of the Arabidopsis Amino Terminus with Sequence from a Closely Related Species Produces a Functional Protein
We next asked if replacement of the major regions of Arabidopsis HAP2(GCS1) with sequences from plant orthologs rescued hap2-1. We chose rice (Oryza sativa, Os) as a representative monocot sequence; monocots and dicots diverged at least 200 million years ago [13]. OsHAP2(GCS1) is 59% identical with Arabidopsis in the N-terminal region and 37% identical at the C-terminus [4] (Figure S3). Expression of the OsHAP2(GCS1) CDS from the AtHAP2(GCS1) promoter failed to rescue the Arabidopsis hap2-1 fertilization defect (not shown). To ensure that the rice protein was properly expressed and localized in Arabidopsis sperm, we replaced Arabidopsis exons 2-15 and the Arabidopsis C-terminus with the orthologous rice sequences to maintain the Arabidopsis signal sequence and transmembrane domain (OsN•OsC, Figure 2C and Figure S1). This exchange also failed to rescue the hap2-1 defect (Figure 2D and 2E and Figure S1).
In contrast, a chimera consisting of the Arabidopsis N-terminal region and the rice C-terminal region was fully functional (AtN•OsC, Figure 2C–2E). Surprisingly, the reciprocal variant made by exchanging Arabidopsis exons 2–15 with the more conserved rice N-terminal sequence, was not functional (OsN•AtC, Figure 2C–2E and Figure S1). However, a similar chimera made with sequence from Sisymbrium irio (Sisymbrium, Si, 89% identical N-terminus, Figure S3), a closely related member of the same family as Arabidopsis (Brassicaceae [14]), did complement hap2-1 (SiN•AtC, Figure 2C–2E and Figure S1). This result suggests that the failure of OsN•AtC to rescue hap2-1 was a consequence of primary sequence divergence. Thus, conservation of primary amino acid sequence is essential for proper function of the N-terminus, but not the C-terminus. A Sisymbrium N-terminus / rice C-terminus chimera was not functional (SiN•OsC, Figure 2C–2E and Figure S1) even though each of these regions can function when paired with the complementary Arabidopsis sequence. This result suggests that the Sisymbrium N-terminus and the rice C-terminus have reduced function compared to their Arabidopsis counterparts, and that this hybrid CDS produces a non-functional protein.
Positive Charge Is the Principal Functional Feature of the HAP2(GCS1) C-Terminus
The ability of the AtN•OsC chimera to rescue hap2-1 implies that the greater sequence diversity in the C-terminus compared to the N-terminus [3]–[4],[7] may be a consequence of evolutionary drift rather than the influence of positive selection, which can also produce such primary sequence diversity [1]. The drift hypothesis is further supported by the observation that the C-terminal enrichment in histidine residues is so far limited to flowering plants [3]–[4], [7]–[8]. Thus, localized positive charge at the C-terminus of HAP2(GCS1) may be functionally more important than primary sequence.
We dissected the Arabidopsis HAP2(GCS1) C-terminus to determine what features were required for function. Alignments of flowering plant C-termini representing dicots and monocots revealed a run of 13 conserved amino acids immediately after the predicted transmembrane domain followed by three histidine-rich domains. The longest histidine-rich stretch is adjacent to the transmembrane domain, and has an average pI of 12.5 (Figure 3A). A variant composed of the complete amino terminus plus the conserved 13 amino acids was not functional (AtN•+13, Figure 3B–3E and Figure S1). However, extending the C-terminus to include the first histidine-rich domain (H1) resulted in a fully functional variant (AtN•AtC mut Δ, Figure 3D and 3E and Figure S1). Replacement of all histidines in this truncated version with other polar, charged amino acids (arginine and lysine) also resulted in a variant that complemented hap2-1 (AtN•AtC mut +Δ, pI = 11.4, Figure 3B–3E and Figure S1). In contrast, replacing histidines with nonpolar residues (glycine and alanine) significantly impaired the function of HAP2(GCS1) (AtN•AtC mut øΔ, pI = 12.2, Figure 3B–3E and Figure S1). When this neutralized domain was extended to include the downstream histidine-rich domains H2 and H3, function was restored (AtN•AtC mut ø, pI = 11.1, the same as the endogenous sequence; Figure 3B–3E and Figure S1). Thus, a hypomorph of HAP2(GCS1) can be made by neutralizing the C-terminus with nonpolar residues.
Fig. 3. Positive charge in the C-terminal region is required for HAP2(GCS1) function. 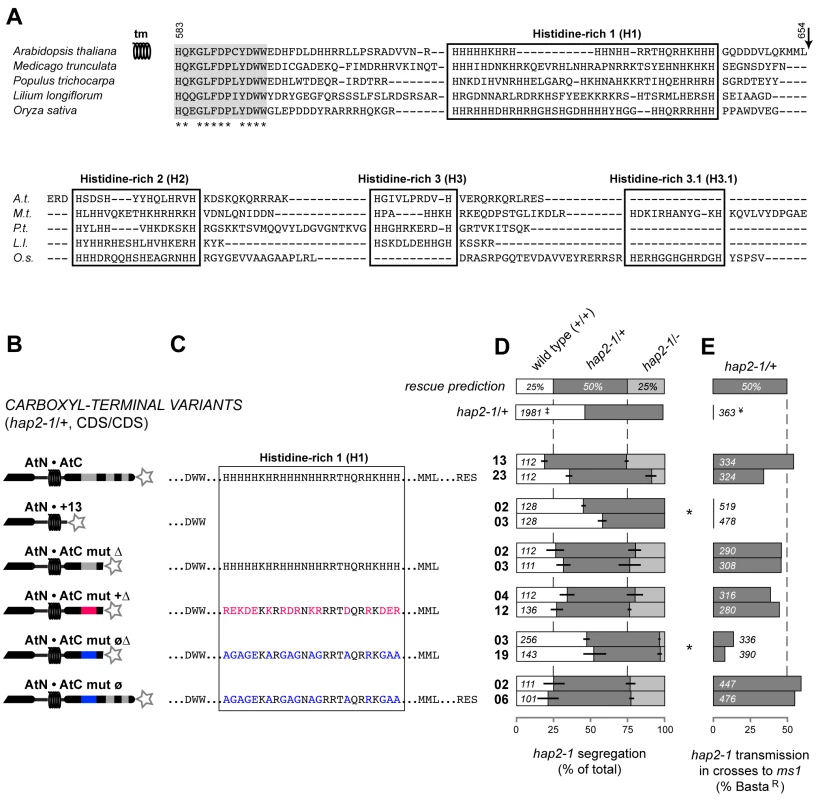
(A) Alignment of HAP2(GCS1) C-termini from selected angiosperms for which complete coding sequences are available (Genbank accessions dicots: A. thaliana = DQ022375, M. trunculata = AC146573, P. trichocarpa = XP_002298057; monocots: L. longiflorum = BAE71142, O. sativa = AK072871). Thirteen conserved amino acids after the transmembrane domain (grey) and histidine-rich domains (boxed) are highlighted. Arrow marks the point where a partial C-terminal truncation was made (AtN•AtC mut Δ, and variants). Numbers correspond to key residues in the Arabidopsis primary sequence. (B) Diagram of the C-terminal HAP2(GCS1) variants tested. Star at the C-terminus of the diagrams represents the epitope tag. (C) Sequences of the H1 domain in each C-terminal variant. (D–E) are presented as in Figure 2. We observed the following trend among the C-terminal variants tested. Complete removal of the C-terminus (AtN•) or histidine-rich region (AtN•+13) abrogates HAP2(GCS1) function. C-termini consisting of the endogenous H1 or positively charged H1 variants are fully functional. However, substituting nonpolar residues for histidine in H1 domain generates a minimally functional protein, yielding only 2–4% hap2-1/- progeny from self-fertilization and 9–15% transmission of the hap2-1 allele in crosses to ms1 females (Figure 3E and Figure S1).
The AtN•AtC mut øΔ Hypomorph Disrupts Sperm Function in Fertilization
We further characterized rare AtN•AtC mut øΔ, hap2-1/- plants to understand the effect of the nonpolar C-terminus on HAP2(GCS1) function. Two sperm were present in AtN•AtC mut øΔ, hap2-1 pollen grains (97–99%, n>500 per line), indicating gametophyte development was normal. Furthermore, AtN•AtC mut øΔ, hap2-1 pollen tubes were able to target ovules and deliver sperm, as judged by counting the number of ovules that received LAT52:GUS activity 7.5 hours after manual self-pollination (Figure 4A and 4B). Female function was not affected in AtN•AtC mut øΔ, hap2-1/- lines, and full seed-set was obtained when pistils were pollinated with qrt1-2 pollen (Figure 4C). However, when AtN•AtC mut øΔ, hap2-1/- plants were allowed to self-fertilize, only four to seven seeds formed in each silique (∼10%, assuming an average of 50 seeds per normal silique; Figure 4C).
Fig. 4. AtN•AtC mut øΔ is a hypomorph of HAP2(GCS1) that affects only male fertility. 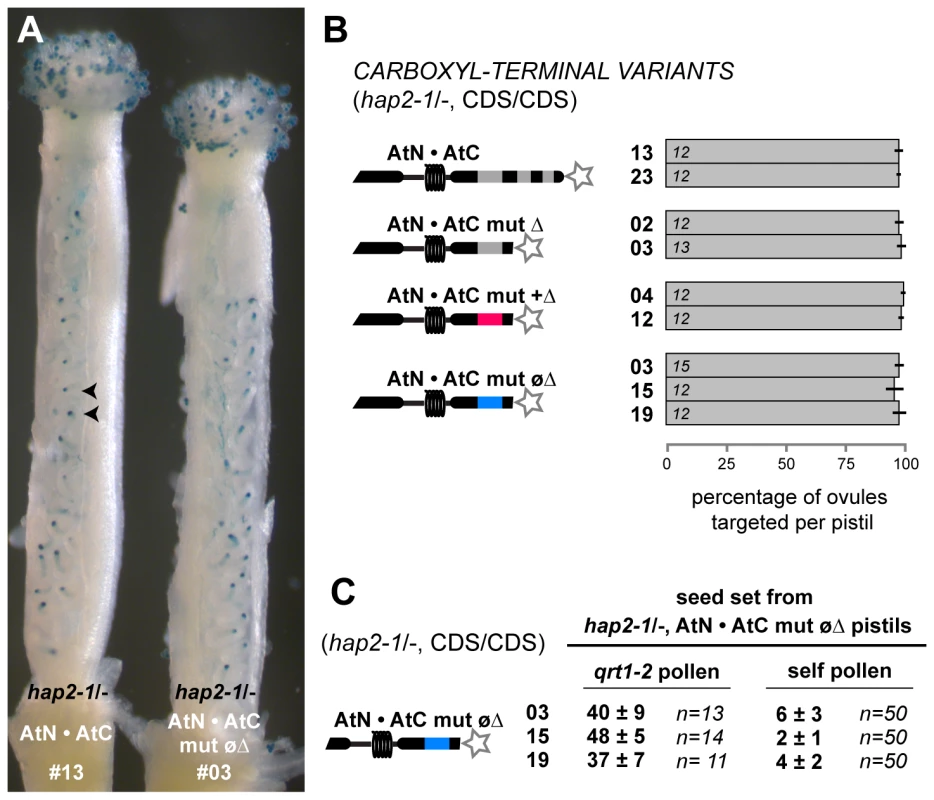
(A) Representative pistils of self-pollinated plants homozygous for hap2-1 and the HAP2(GCS1) variant (hap2-1/-, CDS/CDS). Ovules targeted by pollen tubes are indicated by the blue dots (GUS staining, arrowheads). Image shows pistils from hap2-1/-, AtN•AtC line #13 (left) and hap2-1/-, AtN•AtC mut øΔ #03 (right). (B) Percentage of ovules targeted per pistil. Number of pistils scored per line is shown. (C) Seed set per silique when hap2-1/-, AtN•AtC mut øΔ pistils are crossed with qrt1-2 pollen (left) or manually self-pollinated (right). Number of siliques scored is indicated. The finding that AtN•AtC mut øΔ, hap2-1 sperm were being released in nearly all ovules, yet seed formation was dramatically reduced, suggested that the HAP2(GCS1) hypomorph specifically disrupted fertilization. Analysis of embryo and endosperm development four days after self-pollination of AtN•AtC mut øΔ, hap2-1/- plants revealed normal development in 2–8% of ovules (Figure 5A and 5B), consistent with the number of normal seeds observed after self-fertilization (Figure 4C). We also found that 18–50% of ovules remained unfertilized (Figure 5A and 5B), consistent with lack of HAP2(GCS1) function. In addition, we observed a significant number of ovules that contained either an embryo or endosperm, but not both products of double fertilization. Single fertilization events were not observed when qrt1-2 pollen was used to pollinate AtN•AtC mut øΔ, hap2-1/- pistils (Figure 5B). Analysis of ovule development in ms1 pistils pollinated with AtN•AtC mut øΔ, hap2-1 pollen two days after pollination also yielded significant numbers of unfertilized and singly fertilized ovules (Figure 5C and 5D). When ms1 pistils were pollinated with qrt1-2, hap2-1/+, or hap2-1/- carrying the functional AtN•AtC CDS, however, no single fertilization events were observed. About 25% of the ovules in pistils pollinated with hap2-1/+ pollen remain unfertilized (Figure 5D), as previously reported [4].
Fig. 5. AtN•AtC mut øΔ, hap2-1 sperm are defective at double fertilization. 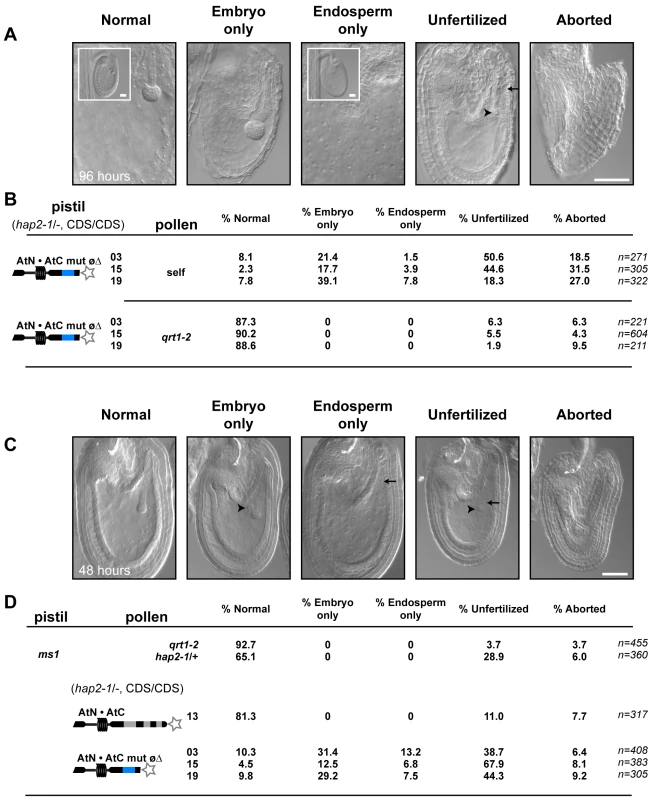
(A) Examples of the products of fertilization by hap2-1, AtN•AtC mut øΔ sperm 96 hours after self-pollination. Scale bar = 50 µm. (B) Frequency of observed fertilization events 96-hours after pollination with self (top) or qrt1-2 (bottom) pollen. Number of ovules scored is indicated. (C) Examples of ms1 ovules fertilized by hap2-1, AtN•AtC mut øΔ sperm 48 hours after pollination. (D) Frequency of observed fertilization events 48-hours after pollination of ms1 pistils with pollen from the indicated line. Number of ovules scored is indicated. These data suggest that neutralizing HAP2(GCS1) C-terminal charge crippled function. In the majority of cases, neither sperm was capable of fusing with the egg or central cell (unfertilized, Figure 5). However, in a significant number of ovules, HAP2(GCS1) function dropped below a critical threshold in only one of the two sperm, producing single fertilization events (embryo-only, endosperm-only, Figure 5). These results are consistent with the conclusion that AtN•AtC mut øΔ represents a hypomorph of HAP2(GCS1).
Discussion
In vivo analysis of HAP2(GCS1) variants has defined two regions that play distinct roles during HAP2(GSC1)-mediated double fertilization. The protein can be divided into N - and C-terminal regions based on the position of the transmembrane domain. Both regions are essential for function, but different evolutionary constraints are driving their roles in fertilization.
We propose that an extracellular orientation of the N-terminus allows this region to regulate gamete fusion by its interaction with factors on the egg or central cell. This organization is consistent with Hidden Markof Modeling (www.cbs.dtu.dk/services/TMHMM-2.0/); with the invariant conservation of cysteine residues within the HAP2-GCS1 domain that are predicted to participate in disulfide bonding in an extracellular environment; and with the successful use of N-terminal epitopes to produce antibodies that block Plasmodium reproduction [15]. The conserved HAP2-GCS1 domain could, for example, interact with another membrane-bound protein on female gametes facilitating the juxtaposition of the two plasma membranes. Our analysis of interspecific HAP2(GCS1) chimeras is consistent with an extracellular orientation of the N-terminus. Replacement of the Arabidopsis N-terminus with that of a closely related species (Sisymbrium, 89% identical) generated a HAP2(GCS1) variant capable of mediating fusion with Arabidopsis female gametes, but a variant generated with a distantly related sequence failed (rice, 59% identical). These data are consistent with the hypothesis that the egg and central cell express a protein that interacts with HAP2(GCS1) to mediate fusion, and that this protein:protein interaction fails beyond a certain level of sequence divergence in the HAP2(GCS1) N-terminal domain.
Our data also show that HAP2(GCS1) does not contribute to a species level barrier to hybridization. Wind and/or animals indiscriminately pollinate many flowering plant species, so it is important to consider mechanisms that limit hybridization. In some organisms, protein:protein interactions essential for complementary gamete binding and fusion are rapidly co-evolving to enhance reproductive isolation of one species from another [1],[16]. We observe that the N-terminus of Sisymbrium, but not rice HAP2(GCS1), can mediate fertilization with Arabidopsis female gametes. Arabidopsis and Sisymbrium are in the Brassica family, but belong to distinct tribes [14]. Thus, our data suggest that Arabidopsis female gametes can distinguish between the N-terminal sequences of HAP2(GCS1) from Arabidopsis and distantly related rice, but cannot discriminate Arabidopsis from closely related Sisymbrium. The recent finding that pollen tubes are attracted to ovules by small proteins with species-specific activity [17] supports a model that barriers prior to gamete-gamete interaction account for species-level discrimination in flowering plants, potentially leaving the proteins involved in gamete-gamete interactions to evolve without diversifying selection.
Positive charge, not primary amino acid sequence, is the C-terminal characteristic conserved among HAP2(GCS1) orthologs and our data show that positive charge is required for function. Unlike the protein:protein interactions proposed for the N-terminus, the intracellular C-terminus may be functioning through electrostatic interactions with negatively charged molecules (e.g. the inner face of the plasma membrane) that favor membrane fusion. Positively charged domains located on the intracellular domain of fusion-associated small transmembrane (FAST) proteins have been implicated in fusion of host cells by non-enveloped viruses [18].
Flowering plant C-termini are enriched in histidine whereas other positively charged amino acids (arginine and lysine) are prevalent in other orthologs [4], [7]–[8], suggesting that selection for one class of charged amino acids over another has shaped the evolution of HAP2(GCS1) in different eukaryotes. These three positively charged amino acids were functionally interchangeable in our Arabidopsis experiments. In nature, however, differences in the composition of the C-terminal domain may have been selected to meet the unique demands of the reproductive systems that use HAP2(GCS1). Under physiologic pH (e.g. pH 5–7), histidine exists in either a protonated or neutral form (pKa = 6.08) whereas lysine (pKa = 10.5) and arginine (pKa = 12.0) are always protonated. Perhaps the difference in sperm delivery mechanisms between flowering plants and other eukaryotes selected for the bimodal charge state of histidine. Flowering plant sperm develop within the pollen cytoplasm and are delivered to the ovule by a pollen tube. hap2-1 pollen tubes have a reduced ability to target ovules compared to wild type, suggesting that in flowering plants, HAP2(GCS1) may have a role in pollen tube guidance that is distinct from its essential role in gamete fusion [4]. Future experiments will test these hypotheses by determining if HAP2(GCS1) variants with modified C-termini can complement the pollen tube guidance defect observed when hap2-1 pollen tubes compete with wild-type pollen tubes for access to ovules. Pollen tubes burst upon arrival at the ovule, exposing sperm to the extracellular environment. It will be interesting to determine if this change in environment results in a drop in sperm pH that activates HAP2(GCS1) function.
hap2-1 sperm expressing a HAP2(GCS1) variant with a neutralized C-terminus (AtN•AtC mut øΔ) had significantly reduced fertility. Double fertilization occurred in only ∼7% of the ovules we analyzed, while many ovules remained unfertilized (∼40%). A large portion of the ovules contained products of single fertilization events (∼23% embryo-only, 8% endosperm-only) that fail to complete seed development. These results highlight a unique advantage of flowering plants for the study of gamete fusion: the outcomes of two distinct fertilization events, both requiring HAP2(GCS1) function, can be observed independently. This situation provides a sensitive means to detect reduced fusion efficiency. We consistently observed fertilization of only one female gamete when two hap2-1 sperm expressing the AtN•AtC mut øΔ HAP2(GCS1) variant were delivered to an ovule, specifically detecting more single fertilizations with the egg (embryo-only) than the central cell (endosperm-only). This suggests that sperm:central cell fusion may require more HAP2(GCS1) activity or that central cell fusion is particularly sensitive to the C-terminal charge of HAP2(GCS1).
All evidence to date indicates that HAP2(GCS1) has an essential role in fertilization [2]-[4],[7],[9],[15], but its exact function remains unknown. Observations made in Chlamydomonas suggest it is required for gamete fusion because gamete attraction and binding/juxtaposition of membranes are normal in HAP2(GCS1) loss-of-function minus gametes, yet membranes fail to fuse [7]. One hypothesis is that HAP2(GCS1) directly catalyzes membrane fusion [19]. While HAP2(GCS1) does not share primary sequence with known fusogenic proteins, it shares features with the FAST proteins of non-enveloped viruses. FAST proteins have a single transmembrane domain, a conserved, extracellular N-terminus and a variable C-terminus that is positively charged [18]. A cell expressing FAST proteins can fuse with a non-expressing neighboring cell [18],[20], so like HAP2(GCS1), the requirement for FAST proteins in fusion is asymmetric. Thus, by virtue of their common attributes, HAP2(GCS1) and FAST proteins may use a similar mechanism to catalyze membrane fusion.
We have mapped the key domains of HAP2(GCS1) and propose a model in which the N-terminus functions by interacting with female gamete-expressed proteins and the C-terminus is required to interact with the plasma membrane through its positive charge. By analogy to known fusogenic proteins, we propose that these interactions bring gamete membranes into close proximity, destabilize the phospholipid bilayer, and generate membrane structures favoring their fusion [19], [21]–[23]. Future studies designed to directly assess the ability of HAP2(GCS1) to catalyze membrane fusion will be required to test this model and to elucidate the biochemical function of this ancient reproductive protein.
Materials and Methods
Maintenance of Plants
All seeds were plated onto solid Murashige and Skoog (MS) medium (MP Biomedicals LLC, Solon, OH, USA) supplemented with 0.5% sucrose containing 25 µg/mL glufosinate ammonium (Basta; Sigma Aldrich/Riedel-de Haën, St. Louis, MO, USA) and/or 50 µg/mL kanamycin sulfate (Sigma-Aldrich, St. Louis, MO, USA). Seedlings were transplanted to sterile 2MIX potting medium (Conrad Fafard Incorporated, Agawam, MA, USA) and grown at 20°C, 16 day/8 night hour light cycle in a GCW30 walk-in Arabidopsis chamber (Environmental Growth Changes, Chagrin Falls, OH, USA) at 50–60% humidity. Plants were bottom-watered with 0.5X 15-5-15 (N-P-K) Peters Professional fertilizer (The Scotts Company, Marysville, OH, USA) as needed.
Plasmid Constructs
Chimeric constructs were made using a modified Arabidopsis HAP2(GCS1) CDS. Mutations were made in the CDS to eliminate the endogenous EcoRI site at position 71 (‘A’ of the initiating methionine codon of A thaliana is position 1), to create a second BamH I site at position 1618 that complements the endogenous BamH I site at 178, and to create a Bmt I site at position 1740. Each directed mutation was made using the QuickChange mutagenesis protocol (Stratagene/Agilent Technologies, Santa Clara, CA, USA). Additional changes and sequence swaps were introduced through linker primers that contain both appropriate restriction sites and new sequences.
All CDS variants were subcloned into a custom vector based on the pTAT backbone [24] that contains sequence encoding tandem V5 [11] and tetra-cysteine (CCGPCC) [12] epitope tags downstream of the multiple cloning site. The tagged CDS was then moved into pCamHap2, a variant of pCambia2300 (Genbank: AF234315; [25], containing ∼1.5 kb of the endogenous Arabidopsis HAP2(GCS1) promoter [4] and 19S terminator flanking a modified multiple cloning site (Figure 1).
Transformation of hap2-1/+ and Selection of Transgenic Lines
Each recombinant pCamHap2 T-DNA plasmid was transformed into Agrobacterium strain GV3101 [26] and resultant colonies were expanded for floral dipping [27]. T1 plants were selected on MS plates containing both Basta and kanamyacin, but subsequent generations were selected on either Basta or kanamyacin (Figure 1). Fifteen to twenty-four T1 plants were screened, and at least 2 lines were selected for further analysis with ∼66% BastaR (expected full rescue in T2 plants). In cases where complementation failed and no lines resulted in >50% BastaR, lines with the highest percentage BastaR were analyzed. Transgenic lines with a single insertion of the CDS construct were selected based on kanR data (expect 75–83% kanR).
GUS Staining
Stage 12 flowers or pistils dissected 7.5 hour after manual pollination were fixed and stained for GUS according to previously published methods [2],[4].
Quantitative Reverse Transcription Polymerase Chain Reaction
Stage 12–14 flowers were collected, frozen in liquid nitrogen, and stored at −80°C until needed. RNA was isolated from 25–50 flowers per line using Qiagen RNA Mini columns (Qiagen Corporation, Valencia, CA, USA), including the optional on-column DNase treatment. Complementary first strand DNA was synthesized from 1 µg of total RNA with random hexamers and poly dT primers using the TaqMan kit (Applied Biosystems, Foster City, CA, USA). One fortieth of each reverse transcription reaction was used per 25 µL quantitative real-time PCR (qPCR) replicate. qPCR was completed on a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using Platinum Taq SYBR Green Mix with ROX (Invitrogen Corporation, Carlsbad, CA, USA). Amplification was quantified over 40 cycles of 95°C, 0∶15 denaturation and 60°C, 2∶30 extension, and amplicons were evaluated with a standard dissociation step. Primers for the HAP2(GCS1) transmembrane domain (F = 5′ - TCCAACAAATGCTCGAGTTTC; R = 5′ - ATTGGGAAGAGAGCGAGGAG; 101 bp), H3.3 (At1g19890; F = 5′ - ATTGCCTTTCCAACGACTTG; R = 5′ - AACAACCCCACCAAGTATGC; 120 bp) and the exogenous V5C4 dual epitope (F = 5′ - CCTAACCCTCTCCTCGGTCT; R = 5′ - TCCCTTATCGGGAAACTACTCA) were used at a final concentration of 100 nM.
Ct values of triplicate reactions were averaged per sample, normalized to sperm-expressed histone H3.3 transcript [28]–[29], and compared to the expression levels in hap2-1/+ flowers (transmembrane primers) or presented without fold-change ratios (V5C4 epitope primers). This difference in data presentation is due to the nature of the targets of each primer set: The transmembrane primers (TM) amplify the same sequence from both endogenous HAP2(GCS1) and CDS transcripts. This primer set thus allows us to quantify how much more HAP2(GCS1) variant mRNA is present compared to hap2-1/+. The epitope tag primers (V5C4) only recognize the CDS transgene (Figure S2D), thus only a normalized value may be presented against a similar amplification from plants that lack this tag.
Embryo Sac Analysis by Chloral Hydrate Clearing
Pistils were dissected 48 (ms1 crosses) or 96 hours (self-pollinated pistils) after manual pollination, and prepared for analysis by chloral hydrate clearing [30].
Statistics
The two-tailed Student's t-test was used to evaluate differences in transmission of hap2-1. Significance was assigned based on p-values <10−5.
Supporting Information
Zdroje
1. SwansonWJ
VacquierVD
2002 The rapid evolution of reproductive proteins. Nat Rev Genet 3 137 144
2. JohnsonMA
von BesserK
ZhouQ
SmithE
AuxG
2004 Arabidopsis hapless mutations define essential gametophytic functions. Genetics 168 971 982
3. MoriT
KuroiwaH
HigashiyamaT
KuroiwaT
2006 GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 8 64 71
4. von BesserK
FrankAC
JohnsonMA
PreussD
2006 Arabidopsis HAP2(GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133 4761 4769
5. SwansonR
EdlundAF
PreussD
2004 Species specificity in pollen-pistil interactions. Annu Rev Genet 38 793 818
6. BergerF
HamamuraY
IngouffM
HigashiyamaT
2008 Double fertilization - caught in the act. Trends Plant Sci 13 437 443
7. LiuY
TewariR
NingJ
BlagboroughAM
GarbomS
2008 The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22 1051 1068
8. SteeleRE
DanaCE
2009 Evolutionary history of the HAP2/GCS1 gene and sexual reproduction in metazoans. PLoS ONE 4 e7680 doi:10.1371/journal.pone.0007680
9. HiraiM
AraiM
MoriT
MiyagishimaSY
KawaiS
2008 Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr Biol 18 607 613
10. PreussD
RheeSY
DavisRW
1994 Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 264 1458 1460
11. SouthernJA
YoungDF
HeaneyF
BaumgartnerWK
RandallRE
1991 Identification of an epitope on the P and V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. J Gen Virol 72 (Pt7) 1551 1557
12. AdamsSR
CampbellRE
GrossLA
MartinBR
WalkupGK
2002 New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J Am Chem Soc 124 6063 6076
13. WolfeKH
GouyM
YangYW
SharpPM
LiWH
1989 Date of the monocot-dicot divergence estimated from chloroplast DNA sequence data. Proc Natl Acad Sci U S A 86 6201 6205
14. BaileyCD
KochMA
MayerM
MummenhoffK
O'KaneSLJr
2006 Toward a global phylogeny of the Brassicaceae. Mol Biol Evol 23 2142 2160
15. BlagboroughAM
SindenRE
2009 Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine 20 5187 5194
16. HaygoodR
2004 Sexual conflict and protein polymorphism. Evolution Int J Org Evolution 58 1414 1423
17. OkudaS
TsutsuiH
ShiinaK
SprunckS
TakeuchiH
2009 Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458 357 361
18. ShmulevitzM
DuncanR
2000 A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J 19 902 912
19. WongJL
JohnsonMA
2010 Is HAP2-GCS1 an ancestral gamete fusogen? Trends Cell Biol Jan 15. [Epub ahead of print]
20. CorcoranJA
SalsmanJ
de AntuenoR
TouhamiA
JerichoMH
2006 The p14 fusion-associated small transmembrane (FAST) protein effects membrane fusion from a subset of membrane microdomains. J Biol Chem 281 31778 31789
21. BasanezG
2002 Membrane fusion: the process and its energy suppliers. Cell Mol Life Sci 59 1478 1490
22. ChernomordikLV
KozlovMM
2008 Mechanics of membrane fusion. Nat Struct Mol Biol 15 675 683
23. WicknerW
SchekmanR
2008 Membrane fusion. Nat Struct Mol Biol 15 658 664
24. NagaharaH
Vocero-AkbaniAM
SnyderEL
HoA
LathamDG
1998 Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med 4 1449 1452
25. HajdukiewiczP
SvabZ
MaligaP
1994 The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25 989 994
26. KonczC
SchellJ
1986 The Promoter of Tl-DNA Gene 5 Controls the Tissue-Specific Expression of Chimeric Genes Carried by a Novel Type of Agrobacterium Binary Vector. Molecular & General Genetics 204 383 396
27. CloughSJ
BentAF
1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
28. IngouffM
HamamuraY
GourguesM
HigashiyamaT
BergerF
2007 Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol 17 1032 1037
29. OkadaT
EndoM
SinghMB
BhallaPL
2005 Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J 44 557 568
30. YadegariR
DepaivaGR
LauxT
KoltunowAM
ApuyaN
1994 Cell-Differentiation and Morphogenesis Are Uncoupled in Arabidopsis raspberry Embryos. Plant Cell 6 1713 1729
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 3
-
Všechny články tohoto čísla
- Parental Genome Dosage Imbalance Deregulates Imprinting in
- Identification and Functional Analysis of the Vision-Specific BBS3 (ARL6) Long Isoform
- HAP2(GCS1)-Dependent Gamete Fusion Requires a Positively Charged Carboxy-Terminal Domain
- Initial Genomics of the Human Nucleolus
- Role of RecA and the SOS Response in Thymineless Death in
- PPS, a Large Multidomain Protein, Functions with Sex-Lethal to Regulate Alternative Splicing in
- Mislocalization of XPF-ERCC1 Nuclease Contributes to Reduced DNA Repair in XP-F Patients
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Human Population Differentiation Is Strongly Correlated with Local Recombination Rate
- Local-Scale Patterns of Genetic Variability, Outcrossing, and Spatial Structure in Natural Stands of
- Arginylation-Dependent Neural Crest Cell Migration Is Essential for Mouse Development
- HP1 Recruitment in the Absence of Argonaute Proteins in
- MiR-218 Inhibits Invasion and Metastasis of Gastric Cancer by Targeting the Robo1 Receptor
- Bias and Evolution of the Mutationally Accessible Phenotypic Space in a Developmental System
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Rapid Assessment of Genetic Ancestry in Populations of Unknown Origin by Genome-Wide Genotyping of Pooled Samples
- Regulation of Lifespan, Metabolism, and Stress Responses by the SH2B Protein, Lnk
- KRAB–Zinc Finger Proteins and KAP1 Can Mediate Long-Range Transcriptional Repression through Heterochromatin Spreading
- Identification of the Regulatory Logic Controlling Pathoadaptation by the SsrA-SsrB Two-Component System
- Drosophila Xpd Regulates Cdk7 Localization, Mitotic Kinase Activity, Spindle Dynamics, and Chromosome Segregation
- Multiple Signals Converge on a Differentiation MAPK Pathway
- In the Tradition of Science: An Interview with Victor Ambros
- Association of the Polymorphism His615Arg with Melanin Content in East Asian Populations: Further Evidence of Convergent Evolution of Skin Pigmentation
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Derepression of the Plant Chromovirus Induces Germline Transposition in Regenerated Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



