-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe MCM-Binding Protein ETG1 Aids Sister Chromatid Cohesion Required for Postreplicative Homologous Recombination Repair
The DNA replication process represents a source of DNA stress that causes potentially spontaneous genome damage. This effect might be strengthened by mutations in crucial replication factors, requiring the activation of DNA damage checkpoints to enable DNA repair before anaphase onset. Here, we demonstrate that depletion of the evolutionarily conserved minichromosome maintenance helicase-binding protein ETG1 of Arabidopsis thaliana resulted in a stringent late G2 cell cycle arrest. This arrest correlated with a partial loss of sister chromatid cohesion. The lack-of-cohesion phenotype was intensified in plants without functional CTF18, a replication fork factor needed for cohesion establishment. The synergistic effect of the etg1 and ctf18 mutants on sister chromatid cohesion strengthened the impact on plant growth of the replication stress caused by ETG1 deficiency because of inefficient DNA repair. We conclude that the ETG1 replication factor is required for efficient cohesion and that cohesion establishment is essential for proper development of plants suffering from endogenous DNA stress. Cohesion defects observed upon knockdown of its human counterpart suggest an equally important developmental role for the orthologous mammalian ETG1 protein.
Published in the journal: . PLoS Genet 6(1): e32767. doi:10.1371/journal.pgen.1000817
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000817Summary
The DNA replication process represents a source of DNA stress that causes potentially spontaneous genome damage. This effect might be strengthened by mutations in crucial replication factors, requiring the activation of DNA damage checkpoints to enable DNA repair before anaphase onset. Here, we demonstrate that depletion of the evolutionarily conserved minichromosome maintenance helicase-binding protein ETG1 of Arabidopsis thaliana resulted in a stringent late G2 cell cycle arrest. This arrest correlated with a partial loss of sister chromatid cohesion. The lack-of-cohesion phenotype was intensified in plants without functional CTF18, a replication fork factor needed for cohesion establishment. The synergistic effect of the etg1 and ctf18 mutants on sister chromatid cohesion strengthened the impact on plant growth of the replication stress caused by ETG1 deficiency because of inefficient DNA repair. We conclude that the ETG1 replication factor is required for efficient cohesion and that cohesion establishment is essential for proper development of plants suffering from endogenous DNA stress. Cohesion defects observed upon knockdown of its human counterpart suggest an equally important developmental role for the orthologous mammalian ETG1 protein.
Introduction
For one single cell to generate two cells, numerous events must be coordinated, in particular, faithful DNA replication and partitioning of the sister chromatids to each of the daughter cells. It is of utmost importance to ensure the error-free duplication of the replicated DNA during each cell cycle, preventing the transmission of potentially harmful mutations to the daughter cells, which otherwise might result in developmental defects or even cancer. DNA damage and replication errors might originate from DNA stress provoked by either exogenous (such as γ-irradiation and UV-B light) or endogenous (such as metabolic byproducts) sources. The latter include the replication process itself that necessitates the cooperation of many different proteins in a highly complex manner. Errors arisen during replication are preferentially repaired through homologous recombination between the replicated sister chromatids that lay in close proximity thanks to cohesion. This sister chromatid cohesion is mediated by cohesin that consists of four subunits in budding yeast (Saccharomyces cerevisiae): two structural maintenance of chromosome (SMC) proteins, designated SMC1 and SMC3, and two non-SMC subunits, designated SCC1 (also known as Mcd1/Rad21) and SCC3 [1]–[5]. SMC1 and SMC3 are self-folded by antiparallel coiled-coil interactions, creating a rod-shaped molecule with an ATP-binding “head” at one end and a “hinge” domain at the other. The two SMC subunits associate with each other through their hinge domains, producing a V-shaped dimer [6],[7]. Cohesin forms a tripartite ring in which the open-V structure of the SMC heterodimer is closed by the simultaneous binding of the N - and C-terminal regions of SCC1 to the head domains of SMC3 and SMC1, respectively [8].
Cohesin is deposited on unreplicated chromatin in a reaction requiring ATP hydrolysis by the SMC heads and the cohesin-loading complex SCC2/SCC4 [9]–[11]. Whereas cohesion loading occurs well before S phase, the process of cohesion establishment is intimately connected with DNA replication. In budding yeast, cohesion depends on an acetyltransferase, designated Eco1/Ctf7, that acetylates two lysine residues on the ATPase head domain of SMC3 [12]–[14]. Eco1/Ctf7 interacts physically and genetically with the proliferating cell nuclear antigen (PCNA) and the replication factor C (RFC) and has been found to travel along the DNA with replication forks [15]–[17], suggesting that replication fork progression and sister chromatid cohesion are coupled events. This model is supported by the observation that cohesion defects are caused by mutations in replisome components, e.g., the DNA polymerase α-binding protein Ctf4 [18]–[19], the Chl1 helicase [20], and RFC components, such as Ctf18 [21]. In budding yeast and humans, Ctf18 associates with Rfc2, Rfc3, Rfc4, and Rfc5 to form the RFCCtf18 complex, which, in turn, couples with two additional subunits, Dcc1 and Ctf8, creating a heptameric complex with the PCNA that has a loading and unloading activity and plays a role in sister chromatid cohesion [19]–[23].
E2F transcription factors control the expression of many genes involved in DNA replication and DNA repair. By studying Arabidopsis thaliana E2F target genes, we have previously identified the E2F TARGET GENE 1 (ETG1) as a novel evolutionarily conserved replisome factor. ETG1 binds with the minichromosome maintenance (MCM) complex and is crucial for efficient DNA replication [24]. Similarly to ETG1, the human orthologous MCM-BP protein binds to replication origins as part of the MCM complex [25]. Plants lacking the ETG1 gene have serrated leaves because of cell cycle inhibition triggered by the DNA replication checkpoints, as shown by the transcriptional induction of DNA stress and checkpoint genes [24]. Here we demonstrate that the ETG1 protein plays an additional role in the establishment of sister chromatid cohesion. Cohesion along chromosome arms was impaired in ETG1-deficient cells. Strikingly, the growth inhibition and the DNA stress phenotype of etg1 mutant plants were strongly enhanced in cohesion mutant backgrounds. Decreased DNA repair kinetics illustrate that the lack of cohesion accounts for severe growth defects in plants suffering from endogenous DNA stress, emphasizing the importance of cohesion for correct plant development. Finally, we show that the knockdown of the human MCM-BP results in a loss of cohesion phenotype as well, suggesting an equally important developmental role for the orthologous mammalian ETG1 proteins.
Results
Upregulation of mitosis-specific genes in etg1 mutants
ETG1-deficient plants suffer from endogenous DNA stress and display a transient cell cycle arrest [24]. To gain more insight into this defective cell cycle, we examined transcript levels of 22,750 genes by using Affymetrix ATH1 GeneChip arrays. Triplicate batches of proliferating first leaves of 9-day-old wild-type and two independent etg1-1 and etg1-2 mutant plants were harvested for total RNA preparation. Statistical analysis identified a total of 219 genes differentially expressed between wild-type and etg1 plants at a P-value <0.01, among which 89% upregulated and 11% downregulated and displaying a 1.3 - to 14.8-fold change in expression. Strikingly, of the 195 upregulated genes, 103 (53%) showed an expression peak during mitosis (Table S1 and Table S2; Figure S1). Transcription of genes expressed specifically during mitosis is regulated by a common upstream cis-acting element (ynCAACGG), designated mitosis-specific activator (MSA). In total, 82 upregulated genes in etg1 plants possessed an MSA element within the first 1 kb region upstream of the translation start, which is significantly more than expected by chance (P-value <0.001) and indicative for an arrest in late G2 or mitosis. This hypothesis was corroborated by an overall transcriptional induction of G2 and M-phase expressed genes in etg1 knockout plants, as demonstrated by plotting the average signal log ratios (SLRs) between the expression levels in wild-type and etg1 mutant plants of 9,910 genes previously defined as cell cycle regulated [26] (Figure 1A).
Fig. 1. Upregulation of G2/M-specific genes in ETG1-deficient plants. 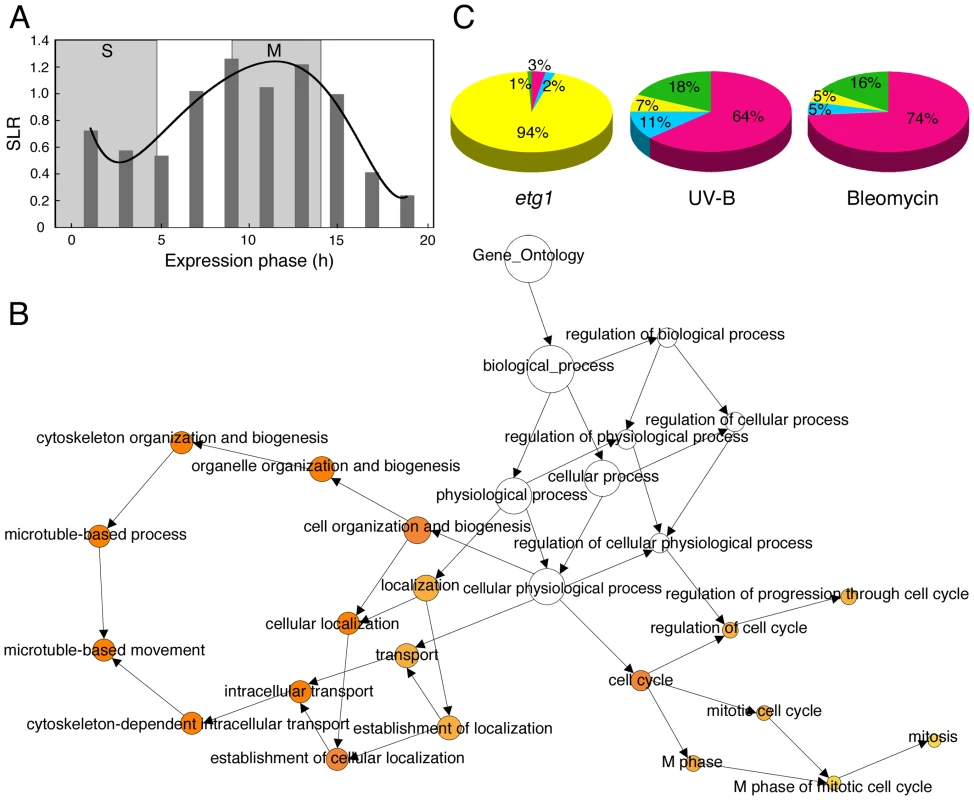
(A) Plot of average signal log ratios (SLRs; logarithms to the base 2) between wild-type and etg1 mutant plants for 9,910 genes during the 22-h cell cycle of Arabidopsis [26]. Genes were sorted into 10 bins representing the 10 time points of measurements, based on their maximal expression during the cell cycle. Cells were synchronized by a release from an aphidicolin arrest. Synchronized cells completed S phase in 5 h and went through mitosis from 9 to 14 h. The solid line represents the best-fitted curve. (B) GO analysis of the 196 upregulated genes in the first leaves of etg1 mutant plants. The yellow-to-orange color of the circles correspond to the level of significance of the overrepresented GO category of ≤0.01 according to a multiple t test with false discovery rate–corrected P value. The size of the circle is proportional to the number of genes in the category. (C) Comparison of the distribution of cell cycle phase-dependent upregulated genes in etg1 plants and plants treated with UV-B- or bleomycin. Microarray data sets of UV-B, and bleomycin treatment were imported from [28] and [29], respectively. S (red), G2 (blue), M (yellow), and G1 (green) phase-specific gene expression patterns were defined by Menges et al. [26]. To identify the biological processes that might be affected in the etg1 mutant plants, the up - and downregulated genes were analyzed for gene ontology (GO) enrichment [27]. Among the upregulated genes, regulation of progression through cell cycle, mitotic cell cycle, and microtubule-based movement genes were significantly overrepresented (Figure 1B), indicating that lack of ETG1 resulted in mitotic defects. This result was unexpected because ETG1 is an E2F-dependent target gene expressed during the S phase and its gene product is required for DNA replication. Additionally, ETG1-deficient plants had been found to interact synergistically with the wee1 and atr1 replication checkpoint mutants and to suffer from DNA replication stress [24], which is anticipated to arrest the cell cycle during S or early G2. Indeed, when the etg1 microarray data set was compared with that of plants exposed to UV-B light [28] or to the radiomimetic drug bleomycin [29], the distribution of the significantly upregulated cell cycle phase markers was different. In the latter cases, the set of modified genes was clearly enriched for S-phase genes (Figure 1C), suggesting that the cell cycle arrest in the etg1 mutants occurs downstream of that one triggered by UV-B or bleomycin.
ETG1 is required for sister chromatid arm cohesion
Sister chromatid cohesion is essential for the appropriate distribution of chromosomes into the daughter cells upon cell division. Several observations indicate that cohesion establishment is coupled with DNA replication [18]–[21]. Therefore, we analyzed by fluorescent in situ hybridization (FISH) whether the cell cycle arrest in etg1 mutant plants might be linked with a loss of cohesion. The two adjacent overlapping bacterial artificial chromosomes (BACs) T2P11 and T7N9 that label the upper mid-arm position of chromosome 1 were used for FISH analysis on flow-sorted 4C leaf nuclei to compare the frequency of sister arm alignment between the wild-type and etg1-1. The number of FISH signals indicates whether sister chromatids are linked at the position tested: one (pairing of both homologs) or two FISH signals mark the positional alignment of sister chromatids at the corresponding region and three or four signals the sister chromatid separation at one or both homologous chromosomes, respectively (Figure 2B–2D). When compared to the wild-type nuclei, the etg1-1 nuclei showed a significant increase (P-value <0.001) in sister chromatid separation (Figure 2A and 2E). Positional sister chromatid separation occurred in 28.1% of the homologs in 4C wild-type leaf nuclei, whereas etg1-1 leaf nuclei displayed 42.7% sister chromatid separation per homolog (Figure 2A). By contrast, FISH applied to the 178-bp centromere-specific sequence (pAL) revealed 4–12 signals in both the wild-type and the etg1-1 mutant 4C nuclei (Figure 2A and 2F). These findings suggest that ETG1 is required for correct establishment of sister chromatid cohesion along chromosome arms, but not at centromeres.
Fig. 2. Requirement of ETG1 for establishment of sister chromatid arm cohesion. 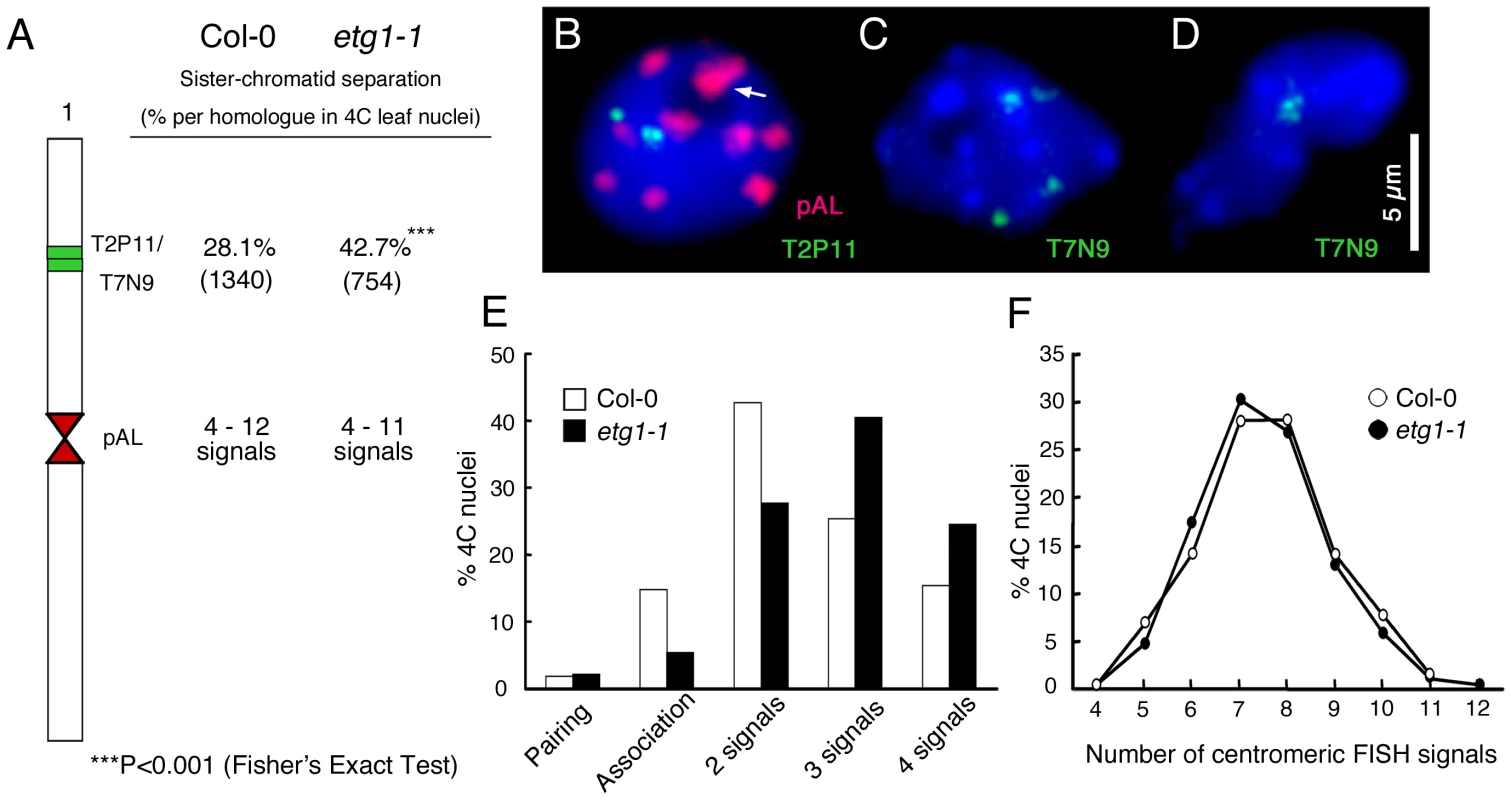
(A) Percentage of positional separation frequencies per homologous chromosome (number of investigated nuclei in parentheses) analyzed in wild-type (Col-0) and etg1-1 mutant plants after FISH with the labeled T2P11 or T7N9 BACs from chromosome 1. The FISH probe pAL detects the centromeric 178-bp repeats. (B–D) Structural arrangement of FISH signal positions in 4C nuclei counterstained with DAPI. (B) Positional alignment (T2P11) at both chromosome 1 homologs. Two of 10 centromeric signals associated (arrow). (C) Positional sister chromatid separation at both homologs. (D) Positional association of both homologs. (E) Percentage of sister chromatid alignment/separation frequencies analyzed in wild-type (Col-0) and etg1-1. (F) Identical frequencies of centromere-specific FISH signals in wild-type and etg1-1 nuclei. Arabidopsis CTF18 is required for sister chromatid cohesion
The ETG1 gene had originally been identified by microarray analysis as a transcript induced in plants that ectopically expressed the heterodimeric E2Fa-DPa transcription factor [30] and had been demonstrated to be directly controlled by the E2Fa and E2Fb transcription factors [24]. Interestingly, putative orthologous genes encoding proteins involved in cohesion establishment during the replication process were also transcriptionally upregulated in E2Fa-DPa-overexpressing plants, such as ECO1 (also known as CTF7) (At4g31400), CHL1 (At1g79890), and CTF18 (At1g04730) (Figure S2). In budding yeast, Ctf18 localizes at the replication fork and is a critical factor for establishment of cohesion [16],[19]. Putative orthologous proteins were found in human, mouse, Arabidopsis, rice, fruitfly, worm, and fission yeast (Figure 3A; Figure S3). Transcriptional activation of the Arabidopsis CTF18 gene in E2Fa-DPa-overexpressing plants was confirmed by quantitative real-time PCR analysis (Figure 3B). The spatial expression pattern of CTF18 was analyzed in more than six independent transgenic lines expressing the β-glucuronidase (GUS) reporter gene under control of the CTF18 promoter. In 7-day-old seedlings, the levels of CTF18 expression were high at the shoot apical and root meristems (Figure 3C and 3D), corresponding with an anticipated role for CTF18 during cell cycle progression.
Fig. 3. Importance of Arabidopsis CTF18 for sister chromatid cohesion. 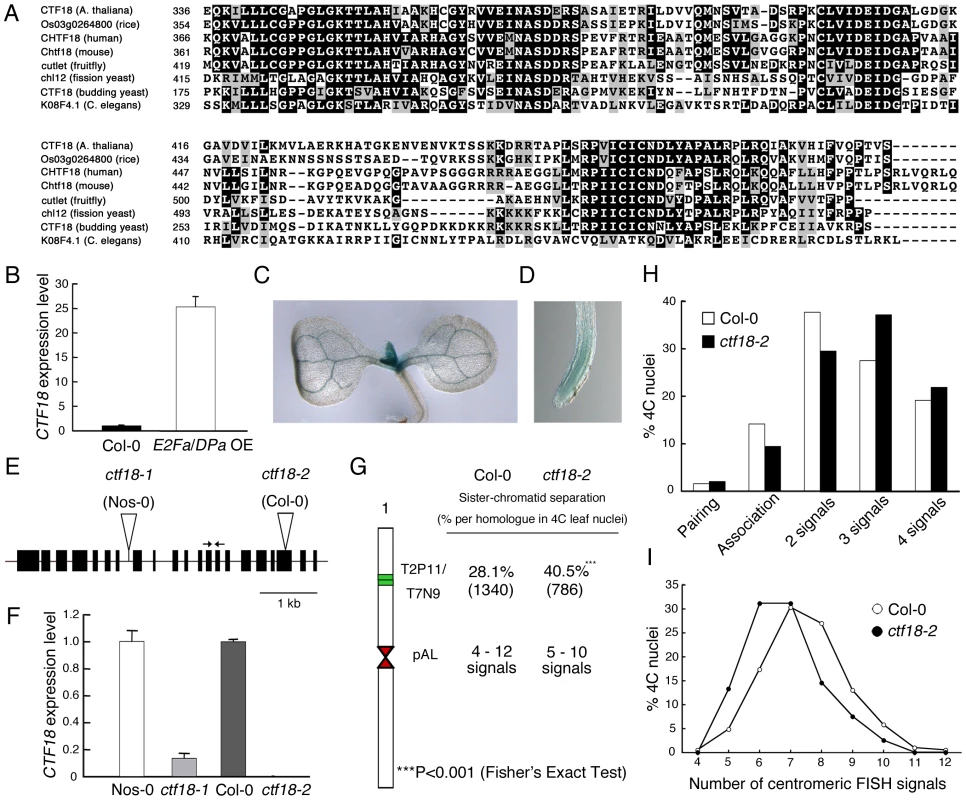
(A) Alignment of the AAA domain of CTF18 with its orthologous proteins: CHTF18 (NP_071375; human), Chtf18 (NP_663384; mouse), cutlet (NP_787969; fruitfly), Os03g0264800 (rice), CTF18 (NP_013795; budding yeast), chl12 (NP_595200; fission yeast), and K08F4.1 (NP_501841; C. elegans). (B) Real-time RT-PCR analysis of CTF18 expression in wild-type (WT; Col-0) and E2Fa-DPa-overexpressing (E2Fa/DPaOE) plants. Total RNA prepared from 6-day-old plants was amplified by RT-PCR. All values were normalized against the expression level of the ACTIN2 gene. (C,D) Histochemical localization of the GUS activity in transgenic 7-day-old shoot (C) and root (D) carrying the CTF18 promoter. (E) Exon (boxes) and intron (lines) structure of CTF18. White triangles and arrows indicate T-DNA insertion sites and primer positions used for real-time RT-PCR analysis, respectively. (F) Real-time RT-PCR analysis of CTF18 expression in wild-type (Nos-0 and Col-0), ctf18-1, and ctf18-2 plants. Total RNA prepared from 9-day-old plants was amplified by RT-PCR. All values were normalized against the expression level of the ACTIN2 gene. (G) Positional sister arm separation frequency detection by FISH detecting ∼100 kb mid-arm segments (BACs T2P11 and T7N9) and centromeric signal number detected by the 178-bp pAL probe. (H) Percentage of sister chromatid alignment/separation frequencies analyzed in wild-type (Col-0) and ctf18-2 after FISH with labeled BACs T2P11 and T7N9 from chromosome 1. (I) Similar frequencies of centromere-specific FISH signals in wild-type and ctf18-2 nuclei. To investigate the role of the plant CTF18 in the establishment of sister chromatid cohesion, we investigated the loss-of-function effect of CTF18 in T-DNA insertion mutant nuclei. In plants with the T-DNA inserted into the 7th intron (ctf18-1; ecotype Nossen-0 [Nos-0]), the CTF18 transcript level was reduced by 85% compared to that in control plants, whereas in plants with the T-DNA inserted into the 19th exon (ctf18-2; ecotype Columbia-0 [Col-0]) (Figure 3E), no transcripts were detectable (Figure 3F). FISH analysis with the BAC clones T2P11 and T7N9 on ctf18-2 leaf nuclei revealed sister chromatid separation in 40.5% of homologous chromosomes versus 28.1% in the wild type (P-value <0.001; Figure 3G and 3H), demonstrating that CTF18 contributes to sister chromatid cohesion. These observations reveal that the function of the CTF18 protein is highly conserved in eukaryotes. Again, as observed for the etg1-1 mutants, the frequency of centromeric signals did not change significantly (Figure 3I).
Sister chromatid cohesion is crucial for development of ETG1-deficient plants
To analyze the genetic interaction between etg1 and other sister chromatid cohesion mutants, double mutants were constructed for etg1 and ctf18. Single mutants were viable and developed normally (Figure 4A–4D). By contrast, etg1-1 ctf18-1 double mutants had distinctly smaller leaves (Figure 4E), a phenotype observed for etg1-2 ctf18-1 and etg1-2 ctf18-2 as well (Figure S4). When the first leaf pair from each mature plant was compared, the leaf blade area of etg1-1 ctf18-1 double mutants was strongly reduced, whereas those of the etg1-1 and ctf18-1 single mutants was nearly identical to those of the control lines (Figure 4F). Furthermore, the total abaxial pavement cell number per leaf was considerably lower in etg1-1 ctf18-1 mutants than that in control plants (Figure 4G), whereas their cell area was similar to that of the etg1-1 single mutant (Figure 4H), indicating a stringent cell division arrest in the double mutant. The growth defect was also observed in etg1-1 ctf18-1 roots, with a significantly reduced root growth rate (Figure 4I and 4J) and a significantly lower number of dividing cells in the root meristem (metaphase, anaphase, and telophase) in the etg1-1 ctf18-1 mutant than observed in the single mutant and wild-type plants (Figure 4K).
Fig. 4. Inhibition of plant growth by loss of sister chromatid cohesion. 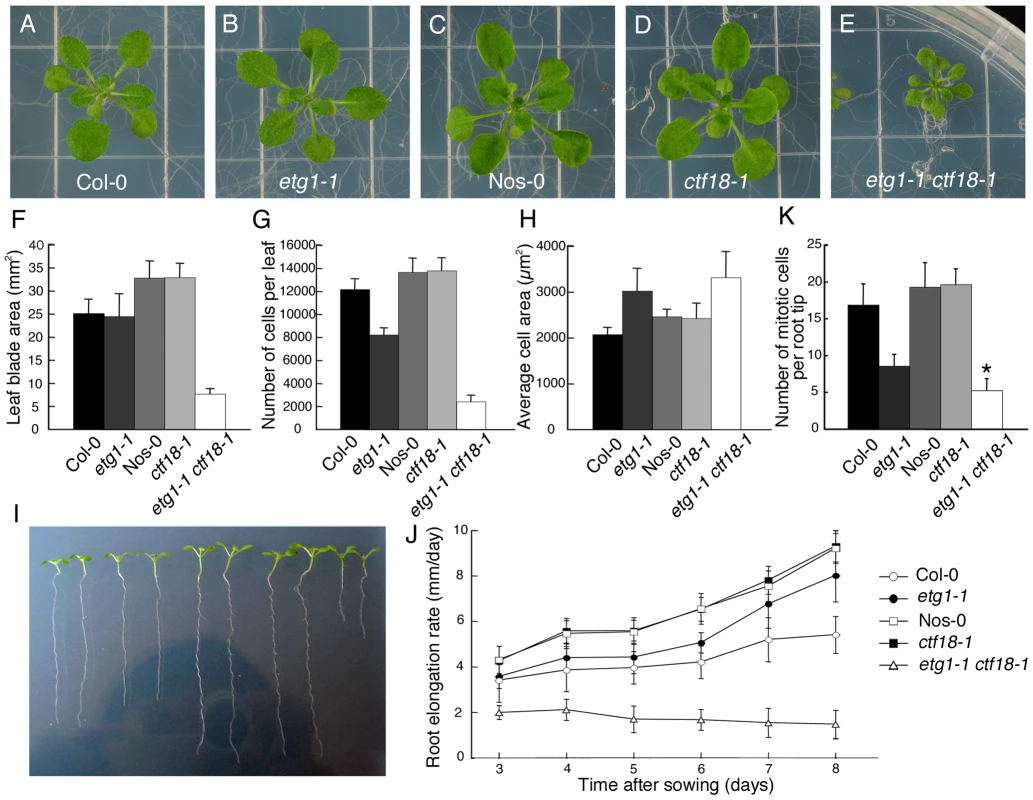
(A–E) Seedling phenotypes of 21-day-old wild-type (Col-0) (A), etg1-1 (B), wild-type (Nos-0) (C), ctf18-1 (D), and etg1-1 ctf18-1 (E) grown on MS plates. (F-H) Leaf growth of the first leaf pair of wild-type (Col-0), etg1-1, wild-type (Nos-0), ctf18-1, and etg1-1 ctf18-1 plants. Leaf blade area (F), epidermal cell number (G), and average epidermal cell size (H) on the abaxial side of the leaf. Data represent average ± SD (n = 5). (I) Root phenotype of 8-day-old wild-type (Col-0), etg1-1, wild-type (Nos-0), ctf18-1, and etg1-1 ctf18-1 plants grown on MS plates (two plants each). (J) Kinematic root growth analysis of the root elongation rate of wild-type (Col-0), etg1-1, wild-type (Nos-0), ctf18-1, and etg1-1 ctf18-1 plants. Plants were grown on MS agar plates. Data represent average ± SD (n = 20). (K) Number of mitotic cells per root tip of 7-day-old wild-type (Col-0), etg1-1, wild-type (Nos-0), ctf18-1, and etg1-1 ctf18-1 seedlings. Data represent average ± SD (n = 20 to 30). Asterisk marks statistically significant differences by Student's t-test (P-value <0.05). FISH analysis revealed that the cohesion phenotype observed in the etg1-2 and ctf18-2 single mutants was aggravated in the double mutant, displaying sister arm separation in up to 54.7% of homologous chromosomes (Figure 5A). Moreover, in contrast to the single mutants, centromere cohesion was clearly impaired in the double mutant, as indicated by the increase in number of centromeric signals per nucleus in etg1-2 ctf18-2. Up to 22 signals could be observed (compared to up to maximum 12 signals in wild-type nuclei), indicating a strong defect in centromere cohesion (Figure 5A–5C).
Fig. 5. Synergistic effect of etg1 and ctf18 on sister chromatid cohesion in 4C leaf nuclei. 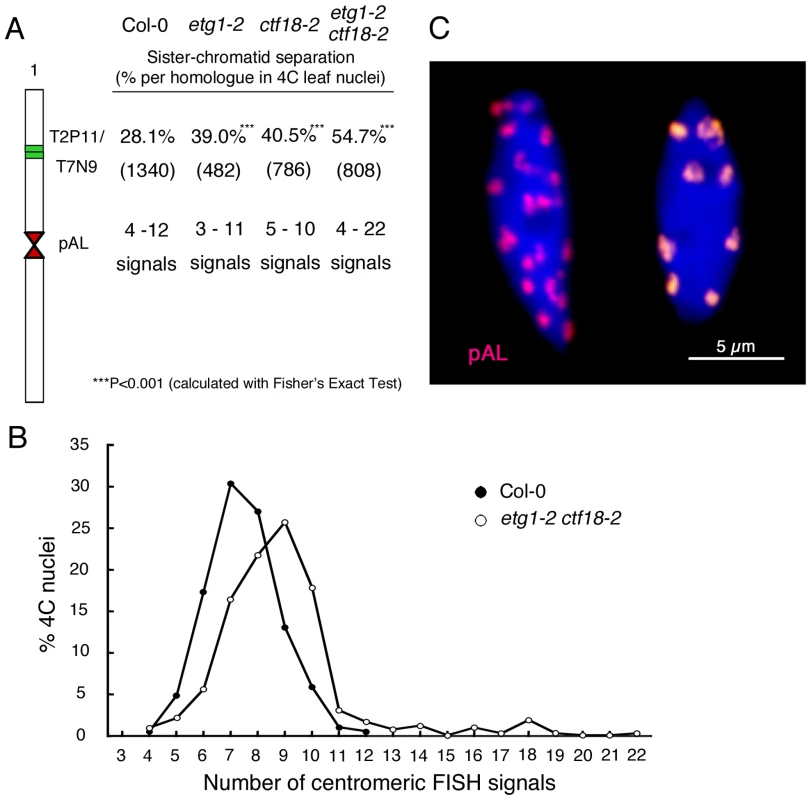
(A) Percentage of positional separation frequencies (number of investigated nuclei in parentheses) analyzed in wild-type (Col-0) and etg1-2 ctf18-2 mutant plants after FISH with the labeled T2P11 BAC from chromosome 1. The FISH probe pAL detected the centromeric 178-bp repeats. More than the expected 20 signals from separated sister centromeres in 4C nuclei might be caused by signal splitting due to pAL repeat elongation along the centromeres. (B) Frequency of centromeric signals in the etg1 ctf18 double mutant compared to wild-type nuclei. (C) Sister centromere separation in an etg1 ctf18 nucleus (left) compared to aligned sister centromeres in a wild-type nucleus (right). Due to occasional centromere fusion, the average signal number in the wild-type is 8 instead of 10. Arabidopsis cohesins contain four alternative SCC1 homologs (SYN1 to SYN4) with different functions during somatic and meiotic cell cycles (reviewed in [31]). The impact of etg1 on plant growth and sister chromatid cohesion was confirmed by crosses between etg1 and the syn4 cohesin mutant, also known as rad21.3 [32]. SYN4 represents an Arabidopsis α-kleisin protein required for cohesion along chromosome arms and at centromeres [33]. As observed for the etg1 ctf18 plants, the etg1 syn4 double mutant plants had smaller leaves than the control plants, correlating with a significantly reduced cell number (Figure S5). The synergistic effects of the etg1 and the cohesion mutants ctf18 and syn4 on cell division strengthen the hypothesis that ETG1 is required for sister chromatid cohesion and illustrate the importance of sister chromatid cohesion for correct development of plants lacking ETG1.
Sister chromatid cohesion is required for DNA repair of etg1 mutants
Previously, we reported that ETG1-deficient plants suffer from replication stress, resulting in transcriptional induction of DNA repair genes and activation of the DNA replication checkpoint [24]. To assess the level of DNA damage in cohesion mutants, we compared the expression levels of the marker genes coding for poly(ADP-ribose) polymerase 2 (PARP2), breast cancer (BRCA), and B-type cyclin 1 (CYCB1;1) by real-time reverse-transcription (RT)-PCR in wild-type versus etg1-1, ctf18-1, and etg1-1 ctf18-1 mutant plants. γ-irradiation, UV light, and radiomimetic agents (such as bleomycin) are known to induce PARP2, BRCA, and CYCB1;1 expression [29],[34]. The expression level of these DNA stress genes was significantly upregulated in the etg1-1 mutant, confirming previous data [24]. By contrast, the cohesion mutant ctf18-1 showed no signs of DNA stress (Figure 6A). Interestingly, expression of the PARP2, BRCA, and CYCB1;1 genes was hyper-induced in etg1-1 ctf18-1 double mutant plants (Figure 6A), indicating severe DNA stress. To examine the level of DNA damage, 8-day-old seedlings were assayed by comet assay. In agreement with previous analyses, etg1-1, but not ctf18-1, mutants, exhibited significant DNA damage in comparison with control plants, whereas the DNA damage level of the etg1-1 ctf18-1 double mutant seedlings was much higher than that observed in the single etg1-1 mutants (Figure 6B and 6C).
Fig. 6. Inefficient DNA repair upon loss of sister chromatid cohesion. 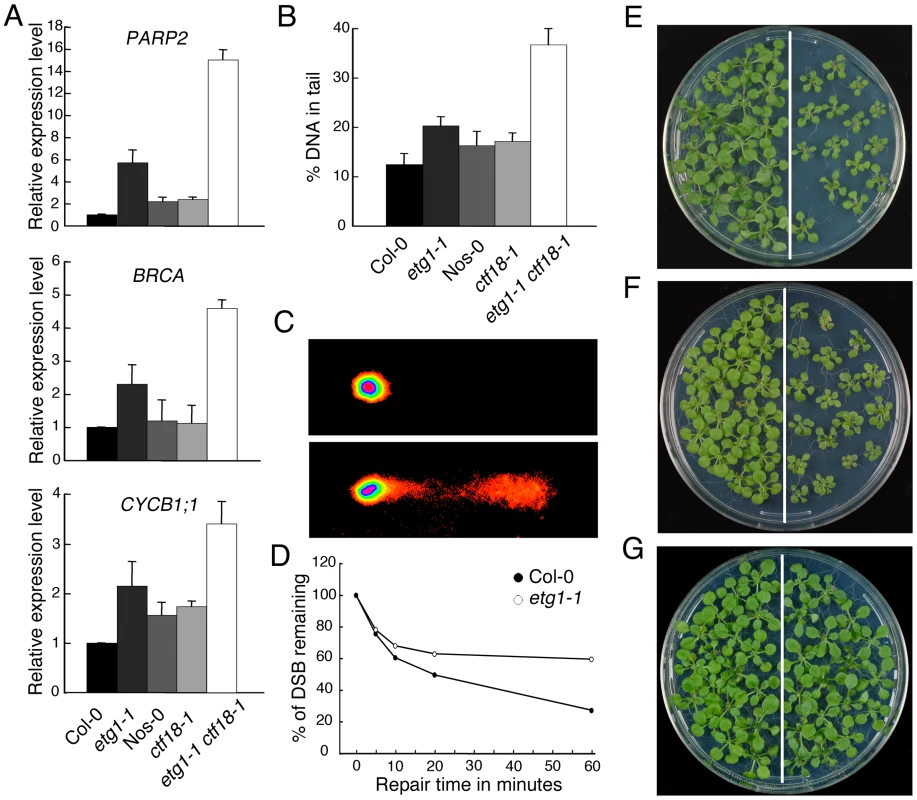
(A) Real-time RT-PCR analysis of DNA stress-inducible genes PARP2, BRCA, and CYCB1;1 in wild-type (Col-0), etg1-1, wild-type (Nos-0), ctf18-1, and etg1-1 ctf18-1 plants. Total RNA prepared from 8-day-old seedlings was amplified by RT-PCR. All values were normalized against the expression level of the ACTIN2 gene. (B) Statistical analysis of a comet assay. The average %-values of DNA in tails of nuclei of 7-day-old wild-type (Col-0), etg1-1, wild-type (Nos-0), ctf18-1, and etg1-1 ctf18-1 seedlings. Error bars indicate SD. (C) Examples of comets from plant nuclei with undamaged (top) or damaged (bottom) DNA. (D) Kinetics of DSB repair in wild-type versus etg1-1 mutant plants. Fractions of remaining DSB were calculated for 0, 5, 10, 20, and 60 min recovery time after treatment with 50 µg/ml bleomycin for 1 h. Maximum damage was normalized as 100% at t = 0. (E–G) Wild-type (Col-0, left) and etg1-1 (right) plants were grown on medium holding 50 ppm methyl methane sulfonate (E), 3 µg/ml mitomycin C (F), or no supplement (G). Plants were photographed 3 weeks after sowing. In Arabidopsis, the S phase-established cohesion is seemingly a prerequisite for double-strand break (DSB)-dependently enforced cohesion that, in turn, is required for homologous recombination repair between sister chromatids [35]. Therefore, the DSB repair, kinetics of etg1-1 and wild-type plants were compared during the recovery from bleomycin treatment by calculating the extent of the remaining DNA damage from the percentage of DNA in the comet tails. Whereas in wild-type seedlings almost 80% of all DSBs was repaired within 1 h, in etg1-1 plants, it was significantly delayed (Figure 6D). Moreover, the etg1-1 mutants displayed increased sensitivity to methyl methane sulfonate (a monofunctional alkylating agent) and mitomycin C (a multifunctional DNA cross-linking agent), both triggering DSBs indirectly by interfering of DNA excision repair with DNA replication (Figures 6E–6G). In replicating cells, such DSBs are preferentially repaired through homologous recombination that needs an intact sister chromatid in physical proximity. These data substantiate a role for ETG1 in sister chromatid cohesion and support the idea that cohesion might be important for homologous recombination repair.
Knockdown of the human ETG1 results in defective chromatid cohesion
A role for MCM-BP, the human ETG1 homolog, in sister chromatid cohesion was assessed with the RNA interference technique by means of pooled short interference RNAs (siRNAs). In human embryonic kidney 293T (HEK-293T) cells transfected with siRNAs that targeted MCM-BP, the MCM-BP expression was much lower than that of untransfected or transfected control cells (Figure 7A). Protein gel blotting with a specific antibody confirmed that the decrease in MCM-BP transcription was accompanied by a reduced MCM-BP protein abundance (Figure 7B). No change in protein level was seen for MCM4, MCM6, and MCM7, which are the most conserved and core subunits of the MCM complex, demonstrating that the siRNAs targeted specifically MCM-BP without affecting the abundance of other proteins of the MCM complex. As seen in mitotic spreads (Figure 7C–7F), depletion of MCM-BP strongly affected sister chromatid cohesion, resulting in a higher proportion of nuclei with completely separated sister chromatids (38% versus 2% or 3% in untransfected or transfected control cells, respectively). Thus, as observed for its plant counterpart, MCM-BP seems to be important for sister chromatid cohesion.
Fig. 7. Induction of cohesion defects by downregulation of human MCM-BP. 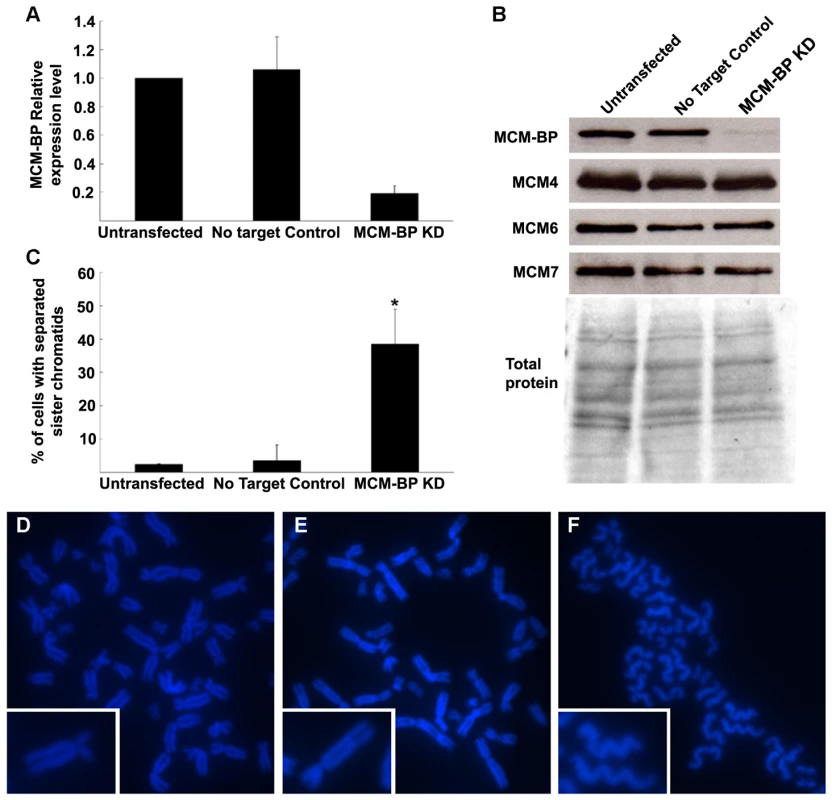
(A) MCM-BP transcript levels in siRNA transfected HEK-293T cells versus untransfected and transfected control cells. Samples were harvested 48 h after transfection. All values were normalized against the expression level of the TBP and UBC genes. (B) MCM-BP, MCM4, MCM6, and MCM7 protein levels in samples as described in (A). (C) Quantification of nuclei showing totally separated sister chromatids (n>100 per condition, obtained from two independent transfection experiments). The asterisk marks a significant difference by Student's t-test (P-value <0.05). (C–E) Representative images of mitotic spread of untransformed (D), control transfected (E), and MCM-BP siRNA-transfected HEK-293T cells (F). Insets show higher magnification images of single sister chromatid pairs. Discussion
ETG1 is a replisome component involved in establishment of sister chromatid cohesion
Previously, we have demonstrated that the ETG1 protein binds to replisome components, is required for efficient DNA replication, and its absence causes DNA replication stress [24]. Here, we showed that ETG1 plays an additional role in sister chromatid cohesion. The effects of ETG1 deficiency on cohesion might result from impaired DNA replication, rather than vice versa, because no DNA stress was observed in ctf18 mutants displaying a loss in sister chromatid cohesion similar to that of etg1 knockout plants. Cohesion was lost at mid-arm positions, but not at the centromeres. Only when the etg1 mutation was introgressed into the ctf18 mutant background, additional loss of sister centromere cohesion was observed. Cohesin binding at centromeric DNA is particularly important because centromeres are directly exposed to spindle pulling forces during mitosis and must resist sister chromatid segregation until all chromosomes are bipolarly attached to the spindle. Enhanced cohesion at centromeres compared to chromosome arms has been observed in yeasts and mammals [5]. In wild-type nuclei of Arabidopsis, cohesion along the arms is less consistent than at the centromeres, suggesting that also in plants centromere cohesion is enforced [36],[37]. The preferential release of sister arm cohesion after ETG1 depletion might be explained by a lower cohesin concentration at the arms than at the centromeres. Alternatively, additional proteins that are not affected by the loss of ETG1 might be needed for centromere cohesion. In yeasts and mammals, the shugoshin (SGO) protein is essential for protection of centromere cohesion [38]. The recruitment of SGO to centromeres in these species depends on the checkpoint protein BUB1 and on HP1α [39]–[41]. Interestingly, the putative Arabidopsis ortholog of BUB1 is transcriptionally upregulated in ETG1-deficient plants. This finding suggests that the cohesion defect in etg1 activates the spindle checkpoint to prevent centromeres from segregating.
Several observations suggest that the cohesion establishment is coupled to the replication process and occurs in close vicinity to the replisome. Cohesion is initiated by the acetyltransferase ECO1/Ctf7 that travels along the DNA together with replication forks [16]. Furthermore, in yeasts, the depletion or mutation of several nonessential components of the replisome, such as the replication factor Ctf18, causes cohesion defects [19]–[21]. Knockout mutation of the Arabidopsis CTF18 homolog impairs sister chromatid cohesion as well. Through its association with MCM proteins, ETG1 is very likely a component of the replisome and plays a role in establishing cohesion during the replication process rather than in cohesin loading. This model is supported by co-regulated expression of ETG1 and other genes involved in cohesion establishment, such as ECO1/CTF7, CHL1, and CTF18 that are expressed during S phase and, interestingly, are transcriptionally upregulated in E2Fa-DPa-overexpressing plants [30]. A role in cohesion establishment is also suggested by the synergetic effect between etg1 and ctf18 on sister chromatid cohesion. Additionally, the human MCM-BP was found to associate preferentially with chromatin during G1/S and S, corresponding with the timing of cohesion establishment, whereas cohesion loading occurred immediately after formation of the nuclear envelope in telophase [25],[42]. Taken together, these results imply that ETG1 functions during S phase for cohesion establishment and that it represents a novel important link between DNA replication and sister chromatid cohesion. As ETG1 is part of the MCM complex, it is not unlikely that the MCM subunits contribute to cohesion establishment as well, however, but until now, no such role has been reported for MCMs in yeasts and mammals.
Impaired sister chromatid cohesion enhances pre-mitotic arrest after DNA damage
Cohesion is essential after replication to allow homologous recombination repair of DNA DSBs that might have arisen by genotoxic impact directly or indirectly by interference of excision repair with replication-mediated gaps. In fission yeast (Schizosaccharomyces pombe), the cohesion component SCC1/RAD21 was first identified in genetic screens for mutants that are hypersensitive to DNA damage [43]. Also SYN2/RAD21.1-deficient plants are hypersensitive to ionizing radiation [32] and SMC5/6 complex proteins, as well as the S phase-dependent cohesin component SYN1, are needed to enhance sister chromatid alignment required for homologous recombination after induction of DSBs by X-irradiation [35]. Although CTF18 is necessary for sister chromatid cohesion, the corresponding knockout plants are viable and develop normally, without any significant effect on cell cycle progression. These data indicate that mild chromosome cohesion defects (up to 40% of sister chromatid separation) are not that critical for cell cycle progression and plant development. However, the more pronounced cohesion deficiency in the etg1 ctf18 double mutant has a strong impact on plant growth: leaf size is severely reduced and root growth is inhibited as a consequence of restricted cell division. This phenotype correlates with an increased loss of sister chromatid cohesion and a strong increase in the expression of DNA damage response genes. While in the etg1 single mutants the developmental disturbance caused by endogenous DNA stress is weak, in the etg1 ctf18 double mutant, the synergetic effect on development indicates that cohesion of the sister chromatids is highly important for correct DNA repair. We assume that the etg1 ctf18 double mutants are less efficient in correct DNA repair by homologous recombination because of the reduced sister arm cohesion, reminiscent of the smc5/6 mutants that are hypersensitive to genotoxins and defective in homologous recombination [35]. This model is supported by slower DSB repair kinetics in ETG1-deficient seedlings than in wild-type plants and by their increased sensitivity to genotoxins. The presumed disturbance of homologous recombination repair in etg1 ctf18 plants might enforce a more stringent checkpoint than that in the single etg1 mutants, thus inhibiting growth by strongly reduced cell division. These results underscore the importance of cohesion for the development of DNA damage-suffering plants. In mammals and yeasts, the spindle checkpoint arrests cells in mitosis upon incorrect mitotic spindle attachment to the chromosomes [44]. Depletion of the origin recognition complex (Orc2) protein of budding yeast delays progression through mitosis because of impaired sister chromatid cohesion and activation of both the DNA damage and the Mad2 spindle checkpoints [45]. Whether the induction of spindle checkpoint or rather of DNA damage checkpoint in the single etg1 and in etg1 ctf18 double mutants contributes to the observed G2/M arrest remains to be tested.
Except for mediating DNA repair, cohesion is essential for diverse biological processes, including chromosome segregation and gene expression [46]. The importance of cohesion is illustrated by the observations that defects in cohesion factors associate with human genetic disorders, including colorectal cancer and developmental diseases, such as the Cornelia de Lange syndrome [47],[48]. Studies from a number of organisms have shown that defects in sister chromatid cohesion lead to chromosome mis-segregation, with aneuploidy, a hallmark of cancer progression, as a consequence [48],[49]. Similarly to its plant counterpart, the depletion of the human MCM-BP protein caused chromatid cohesion defects, suggesting that aberrant MCM-BP expression might result in chromosome instability that increases the organism's risk of neoplastic transformation.
Materials and Methods
Plant growth conditions and plasmid construction
Arabidopsis thaliana (L.) Heyhn. (ecotypes Columbia-0 [Col-0] and Nossen-0 [Nos-0]) plants were grown under long-day conditions (16 h/8 h light/darkness) at 22°C on half-strength Murashige and Skoog (MS) agar plates [50]. The ctf18-1 (13-0845-1) and ctf18-2 (SALK_126071) alleles were retrieved from the Salk Institute Genomic Analysis Laboratory engine (http://signal.salk.edu/cgi-bin/tdnaexpress) and the seeds were acquired from the RIKEN BioResource Centre and Arabidopsis Biological Research Center, respectively. To screen for homozygous insertion alleles, the following primer pairs were designed: 5′-TCACATTGCAGCTAAGCATTG-3′ and 5′-GCTAACGTGTACCGGAGACAG-3′ for ctf18-1, and 5′-ACAACTGGCGGGTTTGGTCATG-3′ and 5′-TTCTAACGGGTCTCTTCACAGC-3′ for ctf18-2. The CTF18 promoter sequence was amplified from Arabidopsis genomic DNA by PCR with the 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTATGAGTTCATAGCTGACTCATCC-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCCTCCTCCGGCAATGGGATATCG-3′ primers. The PCR fragment was cloned into the pDONR201 entry vector by BP recombination reaction and subsequently transferred into the pKGWFS7 destination vector [51] by LR recombination reaction, resulting in a transcriptional fusion between the CTF18 promoter and the enhanced green fluorescent protein and GUS (eGFP::GUS) gene. The construct was transferred into the Agrobacterium tumefaciens C58C1RifR strain harboring plasmid pMP90. The obtained Agrobacterium strains were used to generate stably transformed Arabidopsis with the floral dip transformation method [52]. Transgenic plants were grown on kanamycin-containing medium and later transferred to soil. The etg1-1, etg1-2, and atrad21.3/syn4 mutants and the E2Fa/DPa-overexpressing plants have been described previously [24],[32],[53].
Phenotypic analysis
Plants were germinated and grown in round 12-cm Petri dishes filled with 100 ml of half-strength MS medium (Duchefa, Haarlem, The Netherlands) and 0.8% plant tissue culture agar (Lab M, Bury, UK). Three-week-old plants were harvested, cleared overnight in 100% ethanol, and subsequently stored in lactic acid for microscopy. The leaf primordia were observed under a microscope fitted with differential interference contrast optics (DMLB; Leica, Wetzlar, Germany). The total (blade) area of the first leaves of each seedling was determined from drawing-tube images with the public domain image analysis program ImageJ (version 1.30v; http://rsb.info.nih.gov/ij/). The primordia were digitized directly with a charge-coupled device camera mounted on a binocular (Stemi SV11; Zeiss, Jena, Germany), connected to a personal computer fitted with a frame-grabber board LG3 (Scion Corp., Frederick, MD, US2). Cell density was determined from scanned drawing-tube images of outlines of at least 30 cells of the abaxial epidermis located 25% and 75% from the distance between the tip and the base of the leaf primordium, halfway between the midrib and the leaf margin. The following parameters were determined: total area of all cells in the drawing, total number of cells, and number of guard cells. From these data, the average cell area was calculated and the total number of cells per leaf estimated by dividing the leaf area by the average cell area (averaged between the apical and basal positions).
Quantitative PCR analysis
RNA was extracted from Arabidopsis tissues with RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). First-stranded cDNA was prepared from total RNA with the Superscript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. For quantitative PCR, a LightCycler 480 SYBR Green I Master (Roche Diagnostics, Brussels, Belgium) was used with 100 nM primers and 0.1 µg of RT reaction product. Reactions were run and analyzed on the LightCycler 480 Real-Time PCR System (Roche Diagnostics) according to the manufacturer's instructions. Quantitative reactions were done in triplicate and averaged. Primers used were 5′-GGCTCCTCTTAACCCAAAGGC-3′ and 5′-CACACCATCACCAGAATCCAGC-3′ for ACTIN2, 5′-TAATGACGCTTCTGGCAGTG-3′ and 5′-CATGGTAGTGGAGCTGCAAA-3′ for CTF18, 5′-ATGGCGTTCTGCTCCTCTGC-3′ and 5′-GGTGCTGTTTTCCCCACACC-3′ for PARP2, 5′-TGTTCCCTCTTTCAGCGATTTGATG-3′ and 5′-GGCCTCTGAGTCCATTCAAACA-3′ for BRCA, 5′-CTCAAAATCCCACGCTTCTTGTGG-3′ and 5′-CACGTCTACTACCTTTGGTTTCCC-3′ for CYCB1;1, 5′-GCTAGCTCCATGGGACAGAG-3′ and 5′-CCCCAAACTCCAAATGTCAC-3′ for NQK1, 5′ - TAGGAGCAGCAATGCATCAG-3′ and 5′-CTGCAATGTCAAGCCCTCTT-3′ for PLEIADE, 5′-TCTGCGGCTCTACGGTTACT-3′ and 5′-CTCTAGCCAATGACGCAACA-3′ for AURORA2, 5′-GTGGCAAGCCTTCTTCACTC-3′ and 5′-TCCTTTTCCCTGACATTTGC-3′ for MYB3R4, 5′-TTGATTGCTAATCCACAGATGG-3′ and 5′-AAGCGTGTCGACTTTGTGAA-3′ for MAD2, and 5′ - CCTAGGATCTCATCATTACTCTACACC-3′ and 5′ - CCATGTATCCTCGTACGGAGTTCC -3′ for CDKA;1.
Histochemical GUS measurements
Histochemical GUS assays were carried out according to standard protocols [54]. The young seedlings were incubated in 80% acetone for 2 h at 4°C. After the material had been washed in phosphate buffer, it was immersed in the enzymatic reaction mixture (1 mg/mL of 5-bromo-4-chloro-3-indolyl β-d-glucuronide, 2 mM ferricyanide, and 0.5 mM of ferrocyanide in 100 mM phosphate buffer, pH 7.4). The reaction was carried out overnight at 37°C in the dark. The material was cleared with chlorolactophenol (chloral hydrate/phenol/lactic acid 2∶1∶1) and observed under a light microscope or a stereoscope.
Root growth analysis
For root growth experiments, seedlings were grown in square plates in vertical position in half-strength MS medium containing 10 g/L plant tissue culture agar. Root growth was marked every 24 h on plates that were photographed and was measured with ImageJ software by calculating the distance between successive marks along the root axis.
Determination of the mitotic index
Roots were fixed in a solution of formaldehyde, ethanol, and acetic acid (2∶17∶1) for 12 h at 4°C, washed twice in water, and mounted under cover slips. The samples were crushed, snap-frozen with liquid nitrogen to remove the cover slip, and mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) containing 1 µg/ml 4′,6-diamidino-2-phenylindole (DAPI). The roots were analyzed for mitotic stages with an Axiovert fluorescence microscope (Zeiss).
Microarray and GO analysis
For the microarray experiment, RNA was extracted from 9-day-old Arabidopsis leaf primordia with the RNeasy Plant Mini Kit (Qiagen). The microarray experiment was done by the VIB MicroArrays Facility (Leuven, Belgium; http://www.microarrays.be/) with the ATH1 GeneChip array (Affymetrix, Santa Clara, CA, USA) of 23,800 probe sets designed for Arabidopsis. The experimental design comprised three replicates of each genotype, with one replicate corresponding to one RNA extraction from an independent pool of plants. Raw data obtained by microarray were analyzed as described [55]. To determine significantly overrepresented GO categories among up - and down-regulated genes, we used the BiNGO plugin for Cytoscape (http://www.psb.ugent.be/cbd/papers/BiNGO/) [27]. Promoter motif enrichment was calculated with the hypergeometric distribution based on MSA motif instances as reported [56].
FISH analysis, microscopic evaluation, image processing, and statistics
Preparation of nuclei, probe labeling, and fluorescent in situ hybridization (FISH) were as described [57]. FISH signals were analyzed with an epifluorescence microscope Axiophot (Zeiss) with a 100x/1.45 α-plan-fluar objective and a 3-chip color camera (DXC-950P; Sony, Tokyo, Japan). The microscope was integrated into a Digital Optical 3D Microscope system (Schwertner GbR, Jena, Germany) to check signal separation/distances along x-, y-, and z-axes. Images were captured separately for each fluorochrome with appropriate excitation and emission filters. The images were merged with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA, USA). FISH signals indicating positional sister chromatid separation were compared against those of the Col-0 wild-type by the one-sided Fisher's exact test.
Comet assay for DNA damage measurement
DNA damage was detected by comet using a CometAssay kit (Trevigen, Gaithersburg, MD, USA). Samples were prepared as described [58]. The percentage of DNA in each comet tail was evaluated with Comet Score software (http://www.autocomet.com). DNA damage was calculated by averaging the values for the percentage of DNA in tails from three individual slides, scoring 80 comets per slide. The percentage of the remaining damage after a given post-treatment recovery time is defined as: % of DSB remaining = (mean % tail-DNA (tx) - mean % tail-DNA (control)) / (mean % tail-DNA (t0) - mean % tail-DNA (control))×100.
HEK-293T cell culture and transfection
HEK-293T cell cultures were grown in 5 ml of complete medium (Dulbecco's modified Eagle medium with 10% fetal calf serum; Invitrogen) at 37°C and 5% CO2. siRNAs were transfected into HEK-293T cells grown in 6-well plates according to the manufacturer's instructions (DharmaFECT, Thermo Fisher Scientific, Waltham, MA, USA). Final concentrations of each siRNA were 30 nM. The following siRNA sequences were used: human MCM-BP (C10ORF119) (SMARTpool; J-014474-09, J-014474-10, J-014474-11, and J-014474-12) and control (SMARTpool non-targeting pool).
Protein gel blotting
Protein extracts were prepared from 2-day-old transfected HEK293T cells. Protein gel blotting was carried out according to standard procedures with primary anti-MCM4 (ab4459), anti-MCM6 (ab4458), and anti-MCF7 (ab52489) antibodies (Abcam, Cambridge, UK) at a dilution of 1∶2,000, 1∶2,000, and 1∶10,000, respectively. The MCM-BP antibody [25] was used at a 1∶1,000 dilution and a horseradish peroxidase–conjugated donkey anti-rabbit (GE-Healthcare) diluted 1∶10,000 as a secondary antibody. Proteins were detected with Western Lightning Plus-ECL luminol reagent (Perkin Elmer, Massachusetts, USA) according to the manufacturer's instructions.
Chromosome spreads and DAPI staining
Sub-confluent HEK cells were treated for 48 h after transfection with KaryoMAX colcemid (Invitrogen) to enrich for mitotic chromosomes. The complete medium was replaced by 2 ml of medium at a final concentration of KaryoMAX of 0.6 µg/ml. Cells were incubated at 37°C with 5% CO2 for 5 h before harvesting, trypsinized, pelleted (110 g for 5 min), and resuspended in 1 ml of a hypotonic solution of KCl at a final concentration of 60 mM for 30 min at room temperature. After incubation, HEK cells were twice pelleted (110 g for 5 min) and resuspended in freshly made methanol:glacial acetic acid (3∶1) added drop-wise. Two or 3 drops of suspended cells were applied to precleaned smear glass slides (Menzel-Gläzer, Braunschweig, Germany) and chromosomes were counterstained with VectaShield (Vector Laboratories, Burlingame, CA, USA) containing DAPI. A minimum of 200 mitotic spreads were imaged for each control or siRNA-treated cell population with the DAPI channel of a BX61 Olympus epifluorescence microscope equipped with a 100×/1.30 UPlan FLN objective coupled to a U-C MAD 3 imaging system with a Cell∧M imaging software (Olympus, Tokyo, Japan).
Quantitative PCR analysis of MCM-BP knocked-down cells
HEK-293T cells were collected 48 h after transfection with a rubber policeman. RNA was extracted with an RNeasy animal Mini Kit (Qiagen) and cDNA was prepared with the cDNA Synthesis System according to manufacturer's instructions (Roche Diagnostics, Indianapolis, USA). For quantitative PCR, a LightCycler 480 SYBR Green I Master (Roche Diagnostics) was used with 100 nM primers and 0.1 µg of reverse transcription reaction product. Reactions were run and analyzed on the LightCycler 480 RealTime PCR System according to manufacturer's instructions (Roche Diagnostics). All quantifications were normalized to the TATA Binding Protein (TBP) and Ubiquitin C (UBC) expression levels. Quantitative reactions were done in triplicate and averaged. Primers used were 5′ACTCTCCACGAAATACCACTTTG3′ and 5′GTAGGATGTTGAGGGACTGACTCG3′ for MCM-BP, 5′CGGCTGTTTAACTTCGCTTC3′ and 5′CACACGCCAAGAAACAGTGA3′ for TBP, and 5′ATTTGGGTCGCGGTTCTTG3′ and 5′TGCCTTGACATTCTCGATGGT3′ for UBC.
Supporting Information
Zdroje
1. GuacciV
KoshlandD
StrunnikovA
1997 A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 91 47 57
2. MichaelisC
CioskR
NasmythK
1997 Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91 35 45
3. LosadaA
HiranoM
HiranoT
1998 Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev 12 1986 1997
4. NasmythK
HaeringCH
2005 The structure and function of SMC and kleisin complexes. Annu Rev Biochem 74 595 648
5. PetersJ-M
TedeschiA
SchmitzJ
2008 The cohesin complex and its roles in chromosome biology. Genes Dev 22 3089 3114
6. MelbyTE
CiampaglioCN
BriscoeG
EricksonHP
1998 The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J Cell Biol 142 1595 1604
7. AndersonDE
LosadaA
EricksonHP
HiranoT
2002 Condensin and cohesin display different arm conformations with characteristic hinge angles. J Cell Biol 156 419 424
8. HaeringCH
LöweJ
HochwagenA
NasmythK
2002 Molecular architecture of SMC proteins and the yeast cohesin complex. Mol Cell 9 773 788
9. CioskR
ShirayamaM
ShevchenkoA
TanakaT
TothA
2000 Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol Cell 5 243 254
10. ArumugamP
GruberS
TanakaK
HaeringCH
MechtlerK
2003 ATP hydrolysis is required for cohesin's association with chromosomes. Curr Biol 13 1941 1953
11. WeitzerS
LehaneC
UhlmannF
2003 A model for ATP hydrolysis-dependent binding of cohesin to DNA. Curr Biol 13 1930 1940
12. ÜnalE
Heidinger-PauliJM
KimW
GuacciV
OnnI
2008 A molecular determinant for the establishment of sister chromatid cohesion. Science 321 566 569
13. BenShaharTR
HeegerS
LehaneC
EastP
FlynnH
2008 Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321 563 566
14. ZhangJ
ShiX
LiY
KimB.-J
JiaJ
2008 Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell 31 143 151
15. KennaMA
SkibbensRV
2003 Mechanical link between cohesion establishment and DNA replication: Ctf7p/Eco1p, a cohesion establishment factor, associates with three different replication factor C complexes. Mol Cell Biol 23 2999 3007
16. LengronneA
MclntyreJ
KatouY
KanohY
HopfnerK-P
2006 Establishment of sister chromatid cohesion at the S. cerevisiae replication fork. Mol Cell 23 787 799
17. MoldovanG-L
PfanderB
JentschS
2006 PCNA controls establishment of sister chromatid cohesion during S phase. Mol Cell 23 723 732
18. MilesJ
FormosaT
1992 Evidence that POB1, a Saccharomyces cerevisiae protein that binds to DNA polymerase α acts in DNA metabolism in vivo. Mol Cell Biol 12 57245735
19. HannaJS
KrollES
LundbladV
SpencerFA
2001 Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol Cell Biol 21 3144 3158
20. PetronczkiM
ChwallaB
SiomosMF
YokobayashiS
HelmhartW
2004 Sister-chromatid cohesion mediated by the alternative RFCCtf18/Dcc1/Ctf8 the helicase Chl1 and the polymeraseαassociated protein Ctf4 is essential for chromatid disjunction during meiosis II. J Cell Sci 117 3547 3559
21. MayerML
PotI
ChangM
XuH
AneliunasV
2004 Identification of protein complexes required for efficient sister chromatid cohesion. Mol Biol Cell 15 1736 1745
22. NaikiT
KondoT
NakadaD
MatsumotoK
SugimotoK
2001 Chl12 (Ctf18) forms a novel replication factor C-related complex and functions redundantly with Rad24 in the DNA replication checkpoint pathway. Mol Cell Biol 21 5838 5845
23. BylundGO
BurgersPMJ
2005 Replication protein A-directed unloading of PCNA by the Ctf18 cohesion establishment complex. Mol Cell Biol 25 5445 5455
24. TakahashiN
LammensT
BoudolfV
MaesS
YoshizumiT
2008 The DNA replication checkpoint aids survival of plants deficient in the novel replisome factor ETG1. EMBO J 27 1840 1851
25. SakweAM
NguyenT
AthanasopoulosV
ShireK
FrappierL
2007 Identification and characterization of a novel component of the human minichromosome maintenance complex. Mol Cell Biol 27 3044 3055
26. MengesM
HennigL
GruissemW
MurrayJAH
2003 Genome-wide gene expression in an Arabidopsis cell suspension. Plant Mol Biol 53 423 442
27. MaereS
HeymansK
KuiperM
2005 BiNGO: a Cytoscape plugin to assess overrepresentation of Gene Ontology categories in Biological Networks. Bioinformatics 21 3448 3449
28. KilianJ
WhiteheadD
HorakJ
WankeD
WeinlS
2007 The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50 347 363
29. MolinierJ
OakeleyEJ
NiederhauserO
KovalchukI
HohnB
2005 Dynamic response of plant genome to ultraviolet radiation and other genotoxic stresses. Mutat Res 571 235 247
30. VandepoeleK
VliegheK
FlorquinK
HennigL
BeemsterGTS
2005 Genome-wide identification of potential plant E2F target genes. Plant Physiol 139 316 328
31. SchubertV
2009 SMC proteins and their multiple functions in higher plants. Cytogenet Genome Res 124 202 214
32. da CostaNunesJA
BhattAM
O'SheaS
WestCE
BrayCM
2006 Characterization of the three Arabidopsis thaliana RAD21 cohesins reveals differential responses to ionizing radiation. J Exp Bot 57 971 983
33. SchubertV
WeiβlederA
AliH
FuchsJ
LermontovaI
2009 Cohesin gene defects may impair sister chromatid alignment and genome stability in Arabidopsis thaliana. Chromosoma 118 591 605
34. CulliganKM
RobertsonCE
ForemanJ
DoernerP
BrittAB
2006 ATR and ATM play both distinct and additive roles in response to ionizing radiation. Plant J 48 947 961
35. WatanabeK
PacherP
DukowicS
SchubertV
PuchtaH
SchubertI
2009 The STRUCTURAL MAINTENANCE OF CHROMOSOMES 5/6 complex promotes sister chromatid alignment and homologous recombination after DNA damage in Arabidopsis thaliana. Plant Cell 21 2688 2699
36. SchubertV
KlatteM
PecinkaA
MeisterA
JasencakovaZ
2006 Sister chromatids are often incompletely aligned in meristematic and endopolyploid interphase nuclei of Arabidopsis thaliana. Genetics 172 467 475
37. SchubertV
KimYM
BerrA
FuchsJ
MeisterA
2007 Random homologous pairing and incomplete sister chromatid alignment are common in angiosperm interphase nuclei. Mol Genet Genomics 278 167 176
38. McGuinnessBE
HirotaT
KudoNR
PetersJ-M
NasmythK
2005 Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol 3 e86 doi:10.1371/journal.pbio.0030086
39. TangZ
SunY
HarleySE
ZouH
YuH
2004 Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc Natl Acad Sci USA 101 18012 18017
40. KitajimaTS
HaufS
OhsugiM
YamamotoT
WatanabeY
2005 Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting shugoshin localization. Curr Biol 15 353 359
41. YamagishiY
SakunoT
ShimuraM
WatanabeY
2008 Heterochromatin links to centromeric protection by recruiting shugoshin. Nature 455 251 255
42. GerlichD
KochB
DupeuxF
PetersJ-M
EllenbergJ
2006 Live-cell imaging reveals a stable cohesin-chromatin interaction after but not before DNA replication. Curr Biol 16 1571 1578
43. BirkenbihlRP
SubramaniS
1992 Cloning and characterization of rad21 an essential gene of Schizosaccharomyces pombe involved in DNA double-strand-break repair. Nucleic Acids Res 20 6605 6611
44. BurkeDJ
StukenbergPT
2008 Linking kinetochore-microtubule binding to the spindle checkpoint. Dev Cell 14 474 479
45. ShimadaK
GasserSM
2007 The origin recognition complex functions in sister-chromatid cohesion in Saccharomyces cerevisiae. Cell 128 85 99
46. WatrinE
PetersJ-M
2006 Cohesin and DNA damage repair. Exp Cell Res 312 2687 2693
47. DorsettD
2007 Roles of the sister chromatid cohesion apparatus in gene expression, development, and human syndromes. Chromosoma 116 113
48. BarberTD
McManusK
YuenKWY
ReisM
ParmigianiG
2008 Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci USA 105 3443 3448
49. SkibbensRV
2008 Cell biology of cancer: BRCA1 and sister chromatid pairing reactions? Cell Cycle 7 449 452
50. ValvekensD
Van MontaguM
Van LijsebettensM
1988 Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA 85 5536 5540
51. KarimiM
InzéD
DepickerA
2002 GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7
52. CloughSJ
BentAF
1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
53. De VeylderL
BeeckmanT
BeemsterGTS
de Almeida EnglerJ
OrmeneseS
2002 Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. EMBO J 21 1360 1368
54. BeeckmanT
EnglerG
1994 An easy technique for the clearing of histochemically stained plant tissue. Plant Mol Biol Rep 12 37 42
55. LammensT
BoudolfV
KheibarshekanL
ZalmasLP
GaamoucheT
2008 Atypical E2F activity restrains APC/CCCS52A2 function obligatory for endocycle onset. Proc Natl Acad Sci USA 105 14721 14726
56. VandepoeleK
CasneufT
Van de PeerY
2006 Identification of novel regulatory modules in dicotyledonous plants using expression data and comparative genomics. Genome Biol 7 R103.1 R103.15
57. SchubertV
KimYM
SchubertI
2008 Arabidopsis sister chromatids often show complete alignment or separation along a 1.2Mb euchromatic region but no cohesion “hot spots”. Chromosoma 117 261 266
58. WangC
LiuZ
2006 Arabidopsis ribonucleotide reductases are critical for cell cycle progression, DNA damage repair, and plant development. Plant Cell 18 350 365
Štítky
Genetika Reprodukční medicína
Článek Activation of Mutant Enzyme Function by Proteasome Inhibitors and Treatments that Induce Hsp70Článek Maternal Ethanol Consumption Alters the Epigenotype and the Phenotype of Offspring in a Mouse ModelČlánek Genetic Dissection of Differential Signaling Threshold Requirements for the Wnt/β-Catenin PathwayČlánek Distinct Type of Transmission Barrier Revealed by Study of Multiple Prion Determinants of Rnq1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 1
-
Všechny články tohoto čísla
- Irradiation-Induced Genome Fragmentation Triggers Transposition of a Single Resident Insertion Sequence
- A Major Role of the RecFOR Pathway in DNA Double-Strand-Break Repair through ESDSA in
- Kidney Development in the Absence of and Requires
- Modeling of Environmental Effects in Genome-Wide Association Studies Identifies and as Novel Loci Influencing Serum Cholesterol Levels
- Inverse Correlation between Promoter Strength and Excision Activity in Class 1 Integrons
- Activation of Mutant Enzyme Function by Proteasome Inhibitors and Treatments that Induce Hsp70
- Postnatal Survival of Mice with Maternal Duplication of Distal Chromosome 7 Induced by a / Imprinting Control Region Lacking Insulator Function
- The Werner Syndrome Protein Functions Upstream of ATR and ATM in Response to DNA Replication Inhibition and Double-Strand DNA Breaks
- Maternal Ethanol Consumption Alters the Epigenotype and the Phenotype of Offspring in a Mouse Model
- Understanding Gene Sequence Variation in the Context of Transcription Regulation in Yeast
- miR-30 Regulates Mitochondrial Fission through Targeting p53 and the Dynamin-Related Protein-1 Pathway
- Elevated Levels of the Polo Kinase Cdc5 Override the Mec1/ATR Checkpoint in Budding Yeast by Acting at Different Steps of the Signaling Pathway
- Alternative Epigenetic Chromatin States of Polycomb Target Genes
- Co-Orientation of Replication and Transcription Preserves Genome Integrity
- A Comprehensive Map of Insulator Elements for the Genome
- Environmental and Genetic Determinants of Colony Morphology in Yeast
- U87MG Decoded: The Genomic Sequence of a Cytogenetically Aberrant Human Cancer Cell Line
- The MCM-Binding Protein ETG1 Aids Sister Chromatid Cohesion Required for Postreplicative Homologous Recombination Repair
- Genetic Dissection of Differential Signaling Threshold Requirements for the Wnt/β-Catenin Pathway
- Differential Localization and Independent Acquisition of the H3K9me2 and H3K9me3 Chromatin Modifications in the Adult Germ Line
- Genetic Crossovers Are Predicted Accurately by the Computed Human Recombination Map
- Collaborative Action of Brca1 and CtIP in Elimination of Covalent Modifications from Double-Strand Breaks to Facilitate Subsequent Break Repair
- Distinct Type of Transmission Barrier Revealed by Study of Multiple Prion Determinants of Rnq1
- Genome-Wide Association Study Identifies as a Novel Susceptibility Gene for Osteoporosis
- and Regulate Reproductive Habit in Rice
- Nonsense-Mediated Decay Enables Intron Gain in
- Altered Gene Expression and DNA Damage in Peripheral Blood Cells from Friedreich's Ataxia Patients: Cellular Model of Pathology
- The Systemic Imprint of Growth and Its Uses in Ecological (Meta)Genomics
- The Gift of Observation: An Interview with Mary Lyon
- Genotype and Gene Expression Associations with Immune Function in
- The Elongator Complex Regulates Neuronal α-tubulin Acetylation
- Rising from the Ashes: DNA Repair in
- Mis-Spliced Transcripts of Nicotinic Acetylcholine Receptor α6 Are Associated with Field Evolved Spinosad Resistance in (L.)
- BRIT1/MCPH1 Is Essential for Mitotic and Meiotic Recombination DNA Repair and Maintaining Genomic Stability in Mice
- Non-Coding Changes Cause Sex-Specific Wing Size Differences between Closely Related Species of
- Evidence for Pervasive Adaptive Protein Evolution in Wild Mice
- Evolutionary Mirages: Selection on Binding Site Composition Creates the Illusion of Conserved Grammars in Enhancers
- VEZF1 Elements Mediate Protection from DNA Methylation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Major Role of the RecFOR Pathway in DNA Double-Strand-Break Repair through ESDSA in
- Kidney Development in the Absence of and Requires
- The Werner Syndrome Protein Functions Upstream of ATR and ATM in Response to DNA Replication Inhibition and Double-Strand DNA Breaks
- Alternative Epigenetic Chromatin States of Polycomb Target Genes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



