-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPhagocyte Responses to Protozoan Infection and How Meets the Challenge
article has not abstract
Published in the journal: . PLoS Pathog 8(8): e32767. doi:10.1371/journal.ppat.1002794
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002794Summary
article has not abstract
Introduction
The intracellular protozoan Toxoplasma gondii is arguably the most successful parasite on the planet. It exploits an uncommonly wide host range that encompasses essentially all warm-blooded animals including both mammalian and avian species. Sexual reproduction in the intestine of the definitive host, the cat, results in fecal shedding of up to 108 highly infectious oocysts. The presence of felines from equatorial latitudes to sub-arctic regions of the globe ensures widespread distribution of the parasite. Moreover, unlike closely related apicomplexans such as the Plasmodia, passage through the definitive host is not obligatory to complete the life-cycle, because T. gondii can be transmitted from one intermediate host to the next through predation and carnivorism [1].
While Toxoplasma causes asymptomatic infection in most hosts, the parasite may emerge as an opportunistic infection under immunodeficient conditions such as in AIDS patients and during congenital infection. This danger underscores the importance of the encounter between T. gondii and the host immune system in determining the success of this particular host-parasite interaction. It is well understood that complete evasion of immunity (or for that matter passive failure to trigger immunity) results in rampant infection and host death, an outcome undesirable for both host and parasite. At the same time, we are learning in greater detail the mechanisms employed by the host immune system to destroy Toxoplasma. The parasite must obviously avoid this outcome of immunity to ensure persistence. The global success of T. gondii (over 109 asymptomatic infections in humans alone) suggests that the parasite employs sophisticated molecular strategies to balance evasion versus activation of the host immune response. As summarized in Figure 1, the multiple ways this unifying principle plays out is revealed in studies on infection in phagocytes of innate immunity, namely dendritic cells (DC), macrophages, and neutrophils. These cells are among the first to encounter and be infected by Toxoplasma after the parasite crosses the intestinal epithelium. The studies together form a platform from which we can further understand the complex relationship between microbial pathogens and cells of the innate immune system.
Fig. 1. Integrating phagocyte function with T. gondii infection. 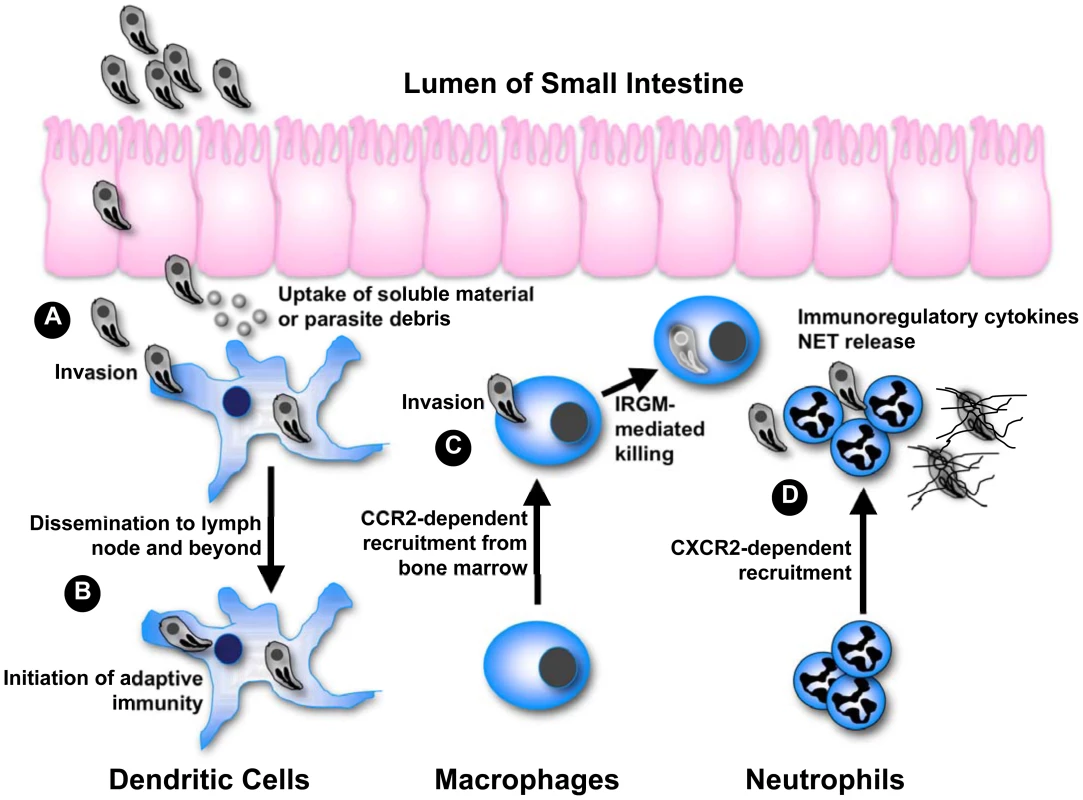
(A) After crossing the epithelium of the small intestine, tachyzoites invade DC and stimulate IL-12 production through the activity of GRA5. Uptake of extracellular parasite debris containing profilin also stimulates IL-12 production. Some parasite strains trigger activation of STAT3 and STAT6 through secretory kinase ROP16, simultaneously down-regulating IL-12 induction. (B) Infected DC migrate to the draining lymph node and beyond, thereby disseminating infection. Infected as well as antigen-bearing DC also initiate immunity in the lymph node. (C) Inflammatory macrophages are recruited from the bone marrow in dependence upon CCR2. Following IFN-γ-mediated induction of IRGM-family effector molecules, intracellular parasites are destroyed by disruption of the parasitophorous vacuole. Some parasite strains release an effector kinase called ROP18 that phosphorylates and inactivates host IRGM molecules. (D) CXCR2-dependent neutrophils are also part of the innate response to Toxoplasma, although their functional importance is debated. In vitro studies have shown that neutrophils release several proinflammatory cytokines important for DC activation and resistance to the parasite. Neutrophils also produce extracellular traps that can ensnare parasites possibly interfering with invasion. Dendritic Cells Are Sentinels and Trojan Horses
Early on it was recognized that DC were an early source of IL-12 driving protective Th1 responses to Toxoplasma. Further studies using an intraperitoneal infection model showed that ablation of CD11c+ DC results in failure to mount protective immunity and death during infection [2]. With the discovery of Toll-like receptors (TLR) and their ligands in the late 1990s, attention turned to the role of this system in sensing protozoan pathogens, in particular T. gondii [3]. Indeed, mice lacking MyD88, a central adaptor of TLR signaling, are extremely susceptible to infection. More specifically, it has been shown using cell-specific gene-deleted mouse strains that MyD88 expression in CD11c+ dendritic cells is required to resist Toxoplasma infection [4]. There is evidence for involvement of mouse TLR2, TLR4, TLR9, and TLR11 in the innate immune response to T. gondii [3]. Of these receptors, deletion of TLR11 has the most dramatic effect on loss of host resistance [5]. However, Tlr11−/− mice fail to recapitulate the extreme susceptibility phenotype of Myd88−/− mice. This has led to the suggestion that multiple TLR function together to provide optimal resistance, or alternatively that other untested TLR serve as the major MyD88-dependent receptor mediating protective immunity.
Toxoplasma profilin (TgPRF), an actin polymerizing molecule, is the ligand recognized by TLR11 [5]. In DC, TgPRF stimulates TLR11-dependent IL-12 production. Interestingly, it was recently found that this response occurs through phagocytic uptake of parasite material followed by TLR11 activation from within the endolysosome [6]. Surface-expressed glycosylphosphatidylinositol moieties purified from tachyzoites have also been found to mediate TLR2 and TLR4 activation [3], although the in vivo importance of this phenomenon is not clear. While the TLR11/TgPRF interaction is significant in the rodent response to Toxoplasma, the importance of TgPRF in human infection is uncertain since we do not express functional TLR11. In addition to TLR-dependent recognition of Toxoplasma, there is clear evidence for MyD88-independent resistance. This is because Myd88−/− mice develop strong (albeit delayed) Th1 responses during oral infection, and the same mouse strain develops protective immunity following intraperitoneal infection with attenuated parasites [7]. In this regard, it was recently shown that release of tachyzoite dense granule protein GRA5 into the host cytoplasm by intracellular parasites bypasses MyD88 to activate NFκB and stimulate IL-12 synthesis [8]. The relative roles that profilin and GRA5 assume during normal infection have not yet been determined. However, GRA5 IL-12 inducing properties are parasite strain-specific, in that only one lineage (Type II) of the three predominant strains found in Europe and North America possess this activity [8]. On the other hand, there is no evidence that profilin acts in a parasite-strain-dependent manner.
Another function of DC during the response to Toxoplasma is to serve as early reservoirs of infection [9], [10]. It has been suggested that parasites utilize DC in a “Trojan horse” strategy to disseminate throughout the host. Upon in vitro infection, DC acquire a hypermotility phenotype that is dependent upon host cell G-protein signaling triggered by the parasite. Intraperitoneal inoculation of tachyzoite-harboring DC spreads infection more rapidly than injection of extracellular parasites alone, suggesting that DC hypermotility promotes dissemination during in vivo infection, although whether a similar phenomenon occurs during oral infection is not clear [11]. Interestingly, hypermotility and in vivo dissemination of infected DC occur most efficiently with Type II Toxoplasma, the strain most frequently found in human infection [12].
Macrophages/Monocytes Are Potent Killers but They Are Vulnerable to Exploitation
Activated cells of the macrophage/monocyte lineage have long been understood to possess potent microbicidal activity against Toxoplasma and other intracellular pathogens. CCR2-dependent inflammatory monocytes activated by IFN-γ and expressing Ly6C have recently been identified as important effectors of resistance to Toxoplasma. After oral infection, these cells are recruited to the intestinal mucosa, where they appear to control infection through direct parasite killing (Figure 1) [13]. In addition to their microbicidal activity, it is possible that they are involved in triggering Th1 immunity since inflammatory monocytes, like DC, also express IL-12 and TNF-α [13].
Killing of Toxoplasma in mice depends to a great extent on an immunity-related GTPase (IRG) protein-based resistance system [14]. The IRG proteins are strongly induced by IFN-γ, and they are sequentially loaded onto the cytoplasmic face of the parasitophorous vacuole membrane (PVM) that harbors intracellular tachyzoites. Through mechanisms not well understood, this results in disintegration of the PVM and release of tachyzoites into the host cell cytoplasm where they cannot survive. However, the IRG resistance system is inconsistently expressed across species and is in fact absent in humans [14]. Other important mechanisms of killing presumably exist. In this regard, it is of interest that human monocyte-derived macrophages kill intracellular Toxoplasma in an IFN-γ-independent, CD40-dependent mechanism that appears to involve autophagy [15].
The well-known fact that Toxoplasma strains differ in mouse virulence and the ability to perform genetic crosses in cats have allowed investigators to use quantitative trait locus analysis to identify virulence-associated parasite loci [16], [17]. Possibly indicating the importance of rodents in the natural life-cycle of T. gondii, the Rop18 gene, encoding the secretory rhoptry protein kinase ROP18, was found to play a key role in inactivating IRG molecules, allowing Toxoplasma strains expressing active ROP18 to avoid killing by this resistance system [18], [19]. Along the same lines, ROP16 was identified as a parasite kinase injected into the host cytoplasm that activates signal transducer and activator of transcription (STAT) molecules through tyrosine phosphorylation [20]. Because both STAT3 and STAT6 are activated by ROP16, the effects of ROP16 on the host cell are complex and likely to involve both avoidance of killing and access to host cell nutrients [21], [22]. Furthermore, suppression of macrophage cytokines IL-12 and TNF-α are among the consequences of parasite-mediated STAT3 activation [23]. Recently, the parasite conoid-associated protein CAP-1 was identified using a forward genetics approach [24]. Deletion of this molecule increases the sensitivity of parasites to nitric-oxide-dependent killing, although how CAP-1 functions is not known. The widespread suppressive effects of Toxoplasma on macrophage responses has led to investigations on chromatin remodeling around promoters of IFN-γ and LPS responsive genes. There is evidence that T. gondii interferes with histone phosphorylation and acetylation at loci responsive to STAT-1 and TLR-mediated signaling [25], [26]. Because this phenomenon is strain independent, it seems unlikely to involve the polymorphic parasite kinases discovered through virulence locus identification. Regardless, targeting histone modification may be a way for the parasite to specifically affect the activity of large cohorts of host genes using a single mechanism.
Neutrophils: Knowns and Known Unknowns
The standard view of neutrophils is that they rapidly home to sites of infection, phagocytose pathogens, release anti-microbial granules, and undergo apoptosis. However, neutrophils can also release immunoregulatory cytokines and chemokines, suggesting that they may also participate in shaping immunity [27]. There is evidence that neutrophils are important in recruiting and activating dendritic cells in response to microbial pathogens including Toxoplasma [28]. Recently, these cells have been found to release chromatin and granule-associated neutrophil extracellular traps (NET) that ensnare and kill microbes. Originally described as a response to bacterial and fungal pathogens, new studies indicate that NET release also occurs in response to protozoan pathogens, including Toxoplasma [29]. Both human and mouse neutrophils undergo a vigorous parasite strain-independent NET response during tachyzoite co-culture, and entrapment within NET could in principle interfere with the ability of T. gondii to find safe harbor within host cells.
Although neutrophils are recruited in abundance in response to Toxoplasma and their depletion is associated with increased susceptibility to infection, their in vivo function is controversial. Accumulation of these cells in the intestinal mucosa following infection could be a response to bacteria that translocate from the lumen to the subepithelium during Toxoplasma infection rather than a host response to the parasite itself [30]. It has been argued that neutrophils have no role in protection, at least in mouse models of infection [31]. The reason for the uncertainty is that in vivo antibody-mediated depletion protocols long used to remove neutrophils are now understood to also eliminate inflammatory monocytes because of common expression of Gr-1 (Ly6C/G). In fact, a recent depletion study using a neutrophil-specific anti-Ly6G antibody failed to find a protective effect of these cells on resistance to Toxoplasma infection [31]. Nevertheless, mice lacking CXCR2 fail to recruit neutrophils during T. gondii infection and display an increase in susceptibility, albeit to a lesser extent than removal of Ly6C/G+ cells [32]. This particular study was carried out with CXCR2 knockout mice on a BALB/c genetic background, unlike antibody depletion studies that were performed on a C57BL/6 background. It is possible that the role of neutrophils during Toxoplasma infection varies depending on mouse strain, host species, or parasite lineage.
Conclusions
Toxoplasma and other tissue-invasive microbial pathogens encounter the innate immune system from the earliest stages of infection. Using genetically altered mice and parasites in combination, we are gaining fascinating insight into the molecular biology of the host-parasite interaction that plays out between Toxoplasma and the phagocytes of innate immunity. The overall theme emerging is that faced with a strong and multi-pronged host innate immune response, Toxoplasma does not retreat but actively engages cells of the innate defense system (Figure 1). Balancing stimulation of host defense with avoidance of immune elimination allows this extraordinary parasite to persist in its host and ensures widespread transmission throughout the vertebrate animal kingdom.
Zdroje
1. BoothroydJC (2009) Expansion of host range as a driving force in the evolution of Toxoplasma. Mem Inst Oswaldo Cruz 104 : 179–184.
2. LiuCH, FanYT, DiasA, EsperL, CornRA, et al. (2006) Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against toxoplasma gondii infection in mice. J Immunol 177 : 31–35.
3. GazzinelliRT, DenkersEY (2006) Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol 6 : 895–906.
4. HouB, BensonA, KuzmichL, DeFrancoAL, YarovinskyF (2011) Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc Natl Acad Sci U S A 108 : 278–283.
5. YarovinskyF, ZhangD, AndersonJF, BannenbergGL, SerhanCN, et al. (2005) TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308 : 1626–1629.
6. PiferR, BensonA, SturgeCR, YarovinskyF (2011) UNC93B1 is essential for TLR11 activation and IL-12 dependent host resistance to Toxoplasma Gondii. J Biol Chem 286 : 3307–3314.
7. SukhumavasiW, EganCE, WarrenAL, TaylorGA, FoxBA, et al. (2008) TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol 181 : 3464–3473.
8. RosowskiEE, LuD, JulienL, RoddaL, GaiserRA, et al. (2011) Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med 208 : 195–212.
9. BierlyAL, ShufeskyWJ, SukhumavasiW, MorelliA, DenkersEY (2008) Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J Immunol 181 : 8445–8491.
10. CourretN, DarcheS, SonigoP, MilonG, Buzoni-GatelD, et al. (2006) CD11c and CD11b expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107 : 309–316.
11. LambertH, VutovaPP, AdamsWC, LoreK, BarraganA (2009) The toxoplasma gondii-shuttling function of dendritic cells is linked to the parasite genotype. Infect Immun 77 : 1679–1688.
12. LambertH, HitzigerN, DellacasaI, SvenssonM, BarraganA (2006) Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol 8 : 1611–1623.
13. DunayIR, DamattaRA, FuxB, PrestiR, GrecoS, et al. (2008) Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen toxoplasma gondii. Immunity 29 : 306–317.
14. HowardJC, HunnJP, SteinfeldtT (2011) The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr Opin Microbiol 14 : 414–421.
15. AndradeRM, WessendarpM, GubbelsMJ, StriepenB, SubausteCS (2006) CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest 116 : 2366–2377.
16. TaylorS, BarraganA, SuC, FuxB, FentressSJ, et al. (2006) A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314 : 1776–1780.
17. SaeijJP, BoyleJP, CollerS, TaylorS, SibleyLD, et al. (2006) Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314 : 1780–1783.
18. FentressSJ, BehnkeMS, DunayIR, MashayekhiM, RommereimLM, et al. (2010) Phosphorylation of immunity-related GTPases by a toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8 : 484–495.
19. SteinfeldtT, Konen-WaismanS, TongL, PawlowskiN, LamkemeyerT, et al. (2010) Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent toxoplasma gondii. PLoS Biol 8: e1000576 doi:10.1371/journal.pbio.1000576.
20. SaeijJP, CollerS, BoyleJP, JeromeME, WhiteMW, et al. (2007) Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445 : 324–327.
21. ButcherBA, FoxBA, RommereimLM, KimSG, MaurerKJ, et al. (2011) Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog 7: e1002236 doi:10.1371/journal.ppat.1002236.
22. JensenKD, WangY, WojnoED, ShastriAJ, HuK, et al. (2011) Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9 : 472–483.
23. ButcherBA, KimL, PanopoulosA, WatowichSS, MurrayPJ, et al. (2005) Cutting edge: IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-a in host macrophages. J Immunol 174 : 3148–3152.
24. SkariahS, BednarczykRB, McIntyreMK, TaylorGA, MordueDG (2012) Discovery of a novel toxoplasma gondii conoid-associated protein important for parasite resistance to reactive nitrogen intermediates. J Immunol 188 : 3404–3415.
25. LangC, HildebrandtA, BrandF, OpitzL, DihaziH, et al. (2012) Impaired chromatin remodelling at STAT1-regulated promoters leads to global unresponsiveness of Toxoplasma gondii-Infected macrophages to IFN-gamma. PLoS Pathog 8: e1002483 doi:10.1371/journal.ppat.1002483.
26. LengJ, ButcherBA, EganCE, AbdallahDS, DenkersEY (2009) Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation. J Immunol 182 : 489–497.
27. NathanC (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6 : 173–182.
28. BennounaS, BlissSK, CurielTJ, DenkersEY (2003) Cross-talk in the innate immune system: neutrophils instruct early recruitment and activation of dendritic cells during microbial infection. J Immunol 171 : 6052–6058.
29. Abi AbdallahDS, LinC, BallCJ, KingMR, DuhamelGE, et al. (2012) Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect Immun 80 : 768–777.
30. HeimesaatMM, BereswillS, FischerA, FuchsD, StruckD, et al. (2006) Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol 177 : 8785–8795.
31. DunayIR, FuchsA, SibleyLD (2010) Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun 78 : 1564–1570.
32. Del RioL, BennounaS, SalinasJ, DenkersEY (2001) CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J Immunol 167 : 6503–6509.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Exon Level Transcriptomic Profiling of HIV-1-Infected CD4 T Cells Reveals Virus-Induced Genes and Host Environment Favorable for Viral Replication
- Five Mechanisms of Manipulation by Bacterial Effectors: A Ubiquitous Theme
- Nonhuman Primate Models for HIV Cure Research
- The Ebola Virus Glycoprotein Contributes to but Is Not Sufficient for Virulence
- Trichomonosis, a Common Curable STI, and Prostate Carcinogenesis—A Proposed Molecular Mechanism
- Host Defense and Tolerance: Unique Challenges in the Placenta
- CPAF: A Chlamydial Protease in Search of an Authentic Substrate
- Small Protease Sensitive Oligomers of PrP in Distinct Human Prions Determine Conversion Rate of PrP
- Invariant NKT Cells: Regulation and Function during Viral Infection
- Human Monoclonal Antibody HCV1 Effectively Prevents and Treats HCV Infection in Chimpanzees
- Chemokine Receptor Ccr1 Drives Neutrophil-Mediated Kidney Immunopathology and Mortality in Invasive Candidiasis
- Phagocyte Responses to Protozoan Infection and How Meets the Challenge
- Telomere Length Affects the Frequency and Mechanism of Antigenic Variation in
- A Biofilm-Induced Pathway for Matrix Glucan Delivery: Implications for Drug Resistance
- The HSV-1 Exonuclease, UL12, Stimulates Recombination by a Single Strand Annealing Mechanism
- Inhibition of Fatty Acid Synthase (Fas2) Induces Mitochondrial Cell Death in Serum
- Interferon-alpha Subtype 11 Activates NK Cells and Enables Control of Retroviral Infection
- Transposon-mediated Chromosomal Integration of Transgenes in the Parasitic Nematode and Establishment of Stable Transgenic Lines
- Structural and Biochemical Basis for Development of Influenza Virus Inhibitors Targeting the PA Endonuclease
- Upregulation of Retinal Dehydrogenase 2 in Alternatively Activated Macrophages during Retinoid-dependent Type-2 Immunity to Helminth Infection in Mice
- Measles Immune Suppression: Lessons from the Macaque Model
- Fungi and the Rise of Mammals
- Bacterial Cell Surface Heterogeneity: A Pathogen's Disguise
- Cytoplasmic Entry Induces Fetal Wastage by Disrupting Maternal Foxp3 Regulatory T Cell-Sustained Fetal Tolerance
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Invariant NKT Cells: Regulation and Function during Viral Infection
- Host Defense and Tolerance: Unique Challenges in the Placenta
- Nonhuman Primate Models for HIV Cure Research
- Exon Level Transcriptomic Profiling of HIV-1-Infected CD4 T Cells Reveals Virus-Induced Genes and Host Environment Favorable for Viral Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



