-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociation between Adult Height and Risk of Colorectal, Lung, and Prostate Cancer: Results from Meta-analyses of Prospective Studies and Mendelian Randomization Analyses
In a Mendelian randomisation study Pierce and colleagues show a genetic association between adult height and increased risk of colorectal and lung cancer.
Published in the journal: . PLoS Med 13(9): e32767. doi:10.1371/journal.pmed.1002118
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002118Summary
In a Mendelian randomisation study Pierce and colleagues show a genetic association between adult height and increased risk of colorectal and lung cancer.
Introduction
Numerous studies have examined the relation between height and cancer; however, results have been inconsistent. The inconsistencies in observational studies could be due to factors that affect validity, including confounding, selection bias, reverse causation, and measurement error. Therefore, no consensus has been reached on whether height is a risk factor for colorectal, lung, or prostate cancer, three of the most common cancers affecting men and women. Furthermore, meta-analyses of prospective cohort studies, summarizing the association between height and colorectal, lung, or prostate cancers, have not been conducted.
Mendelian randomization is based on the principle that an individual’s genotype is randomized at conception [1] and utilizes genetic variants as instrumental variables for phenotypic exposures. This approach circumvents threats to validity found in conventional observational epidemiologic studies and potentially allows for causal inferences regarding the relation between exposure and disease [1–3]. Mendelian randomization analyses make the following assumptions regarding the genetic variants used in instrumental variables: (1) the genetic variants are associated with the exposure, (2) the genetic variants affect the outcome only via the exposure (also known as the “exclusion restriction”), and (3) the genetic variants are not associated with any confounders of the exposure-outcome association [4]. A recent genome-wide association study (GWAS) identified nearly 700 variants—reflecting 423 loci—that were associated with adult height in individuals of European descent [5]. These variants explain approximately 16% of height variance and have the potential to serve as strong instruments in Mendelian randomization analyses.
To comprehensively evaluate the association between height and risk of colorectal, lung, and prostate cancers, we conducted a systematic review and meta-analysis of previous prospective studies. Additionally, we carried out Mendelian randomization analyses utilizing GWAS summary statistics from the Genetic Associations and Mechanisms in Oncology (GAME-ON) and Genetic Investigation of Anthropometric Traits (GIANT) consortia studies of individuals of European descent.
Methods
Systematic Review and Meta-analysis
We searched PubMed, Embase, and Web of Science on January 3, 2016, for prospective studies (i.e., prospective cohort, nested case-control, and case-cohort studies) using the following search terms: lung neoplasms; lung cancer; colorectal neoplasms; colorectal cancer; prostate neoplasms; prostate cancer; lung, prostate, colorectal, colon, rectum, rectal or cancer; and body height, height, stature, body size, anthropometrics, or anthropometry. After restricting the results to English language and humans, a total of 15,691 publications were found.
Approximately 2,900 publications were duplicates. Following the title and abstract review, additional exclusions for outcomes other than cancer resulted in a total of 325 studies for full-text review. One Chinese study, for which all relevant tables and methods were written in English, was additionally included [6]. Full-text review revealed height examined in relation to lung cancer in 11 studies [6–16], to colorectal cancer in 24 studies [6,9–31], and to prostate cancer in 27 studies [6,9–11,16,30,32–52]; these studies were included in the meta-analysis. We also conducted a meta-analysis for a subset of prostate cancer studies that also reported estimates for aggressive prostate cancer (defined as Gleason score ≥ 7, metastatic spread, or regional/distant stage) [40,41,43–52]. Given that several studies examined multiple cancer sites, the final set of studies for each cancer type included in the meta-analysis is not exclusive. Fig 1 summarizes the search results and exclusions for this systematic review and the final set of studies used in the meta-analysis (detailed diagram according to cancer site provided in S5 Fig). Details regarding each study included in the meta-analysis are provided in S1–S4 Tables. We recently published a large-scale meta-analysis of prospective studies of the association of height with breast cancer (more than 113,000 incident breast cancers) [53]; we present the meta-analysis results for breast cancer from our previous study for comparison.
Fig. 1. PRISMA flow chart for studies included in the meta-analysis. 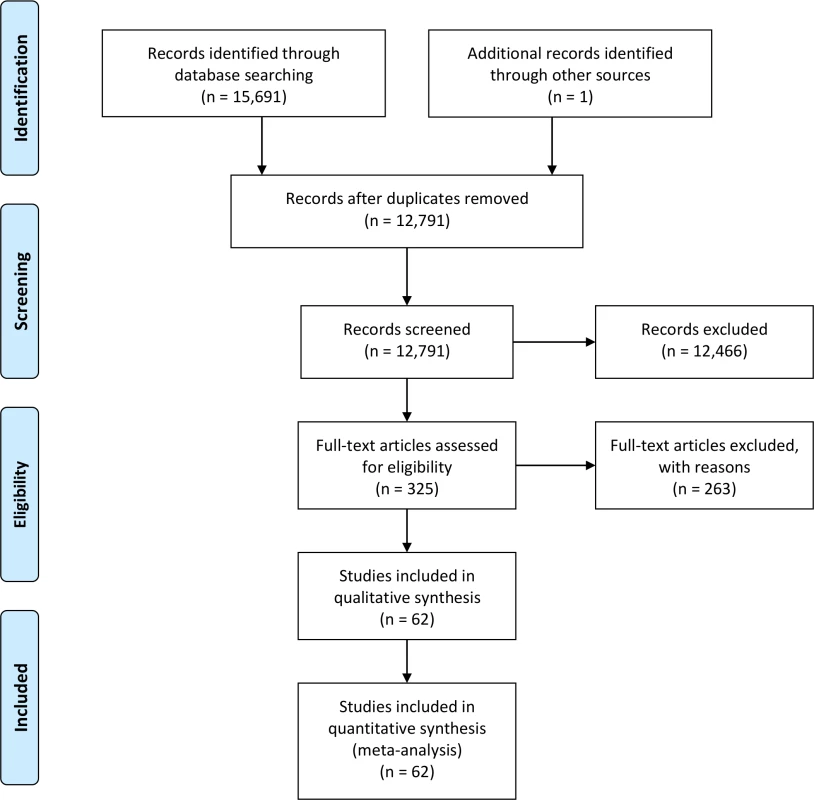
All study estimates were scaled for a 10-cm increase in height in relation to cancer, which, in general, approximated the average interquartile range in height across all studies. For studies that reported categorical estimates of height (i.e., quantiles), a continuous estimate and standard error was estimated using the method proposed by Greenland and Longnecker [54] using R package “dosresmeta” (R version 3.1.2). Scores were generated for each quantile for studies reporting categorical estimates and were equivalent to either the mean or median; scores for the uppermost open-ended quantiles were generated using the method presented by Il’yasova et al. [55].
Study-specific estimates were summarized using inverse-variance-weighted (IVW) fixed - and random-effects meta-analyses for all three cancers (colorectal, lung, and prostate) separately. Fixed-effects models assume no heterogeneity between the study estimates, whereas random-effects models take heterogeneity of the study estimates into consideration. Cochran’s test for homogeneity was applied in the analysis, with p < 0.05 indicating heterogeneity among the studies. Summary odds ratios (ORs) and 95% CIs for the random-effects meta-analyses were estimated using restricted maximum likelihood. Funnel plots were generated for each cancer to examine publication bias and were formally assessed using Begg’s and Egger’s statistical tests (S1–S4 Figs); no violations were observed. Meta-analysis was conducted using Stata version 12.1.
Mendelian Randomization Analysis Using Summary Statistics
Results from our recent Mendelian randomization analysis of adult height and breast cancer have been published [53], and thus the current analysis focuses on cancers of the colorectum, lung, and prostate. The analysis was conducted to estimate the effect of height (X) on the risk of cancer (Y) using genetic variants (g), where the causal estimate is equal to Yg/Xg [56]. For the association between genetic variants and height (Xg), we utilized available summary statistics from the GIANT GWAS. Summary statistics for the association between genetic variants and each of the three cancers (Yg) are from the GAME-ON consortium.
Nearly 700 genetic variants from 423 loci were identified as reaching genome-wide significance (p < 5 × 10−8) in the GIANT consortium study, explaining 16% of phenotypic variation in height [5]. In our study, we selected 423 uncorrelated variants, representing these loci, to construct the instrumental variables in our Mendelian randomization analysis (S5 Table).
GAME-ON data were utilized to provide regression coefficients and standard errors for each single nucleotide polymorphism (SNP) in relation to cancer (Yg). GAME-ON is network of genetic epidemiology consortia that contains GWAS data from the Colorectal Transdisciplinary Study (CORECT); Discovery, Biology, and Risk of Inherited Variants in Breast Cancer (DRIVE); Elucidating Loci Involved in Prostate Cancer Susceptibility (ELLIPSE); Follow-up of Ovarian Cancer Genetic Association and Interaction Studies (FOCI); and Transdisciplinary Research in Cancer of the Lung (TRICL). For the current study, we used data from CORECT, ELLIPSE, and TRICL. The GAME-ON consortium primarily consists of studies of individuals of European descent. SNPs with poor imputation quality (r2 < 0.3) were excluded (CORECT used IMPUTE 2.0 info score < 0.7 as the cutoff) [57]. The association between adult height and breast cancer risk was evaluated in our previous study using 168 height-associated variants [53]. For the analysis presented here, we updated the Mendelian randomization summary estimate for breast cancer using data from DRIVE for the 423 newly identified height-associated variants.
Not all SNPs with information regarding Xg (from the GIANT consortium) had corresponding information regarding Yg (from GAME-ON); therefore, these SNPs were excluded from the instrumental variables for colorectal cancer (n = 77) and prostate cancer (n = 4). No SNPs were excluded from the instrument for lung cancer. Thus, there were fewer instrumental variables for the colorectal and prostate cancer studies than for the lung cancer study. Using both summary statistics for Yg and Xg, an IVW meta-analysis was conducted to estimate the effect of genetically determined height on the risk of each cancer using the method of Burgess et al. [56]:
where Xg is the beta estimate for the association between the SNP and height (from GIANT), Yg is the beta estimate for the association between the SNP and cancer (from GAME-ON), and σYg is the standard error for Yg. Corresponding ORs and 95% CIs were calculated using β^IVW and se(β^IVW). All ORs and 95% CIs were subsequently standardized per 10 cm. SAS version 9.4 was utilized for the Mendelian randomization analysis using summarized data.Sensitivity Analyses
We conducted several sensitivity analyses to assess whether the association of height with cancer varied for different subgroups. Using data from a previous GIANT height GWAS that presented sex-stratified summary statistics for 168 independent SNPs explaining approximately 10% of variation in height [58], we conducted Mendelian randomization sensitivity analyses for colorectal and lung cancers for males and females separately. Additionally, in order to account for potential pleiotropy of the SNPs utilized in the genetic instrument (potentially violating the exclusion restriction assumption for a valid instrumental variable), we excluded SNPs with suggested pleiotropic effects associated with various diseases and traits (e.g., pulmonary function, phospholipid levels, cardiovascular disease, and cancer) [5]. Additionally we used a data-driven approach to explore violations of pleiotropy using Egger regression [59]. For the meta-analysis, we conducted additional analyses stratified by cancer of the colon versus rectum and self-reported height versus measured height. Given that height is associated with lung size and lung function [60]—which could lead to efficient nicotine uptake [61] and possibly increased likelihood of smoking initiation [62] among taller individuals—we also stratified our meta-analysis results by studies that adjusted for potential confounding by smoking. Finally, we conducted sensitivity meta-analyses for colorectal, prostate, and lung cancers, including only those studies of populations of European descent.
Results
Meta-analysis
For colorectal cancer (Fig 2), we observed an approximately 12% increased risk (95% CI 1.10, 1.15) for a 10-cm increase in adult height (p < 0.001); no important differences were observed when stratified by sex. A 7% increase (95% CI 5%, 10%) in the risk of prostate cancer (p < 0.001; Fig 3A), and a 5% increase (95% CI −2%, 13%) in the risk of aggressive prostate cancer, was observed for a 10-cm increase in height (p = 0.031; Fig 3B). A 10-cm increase in height was associated with a 7% increase in lung cancer risk in males (relative risk [RR] = 1.07, 95% CI 1.04, 1.10; Fig 4) and a 2% increase in females (RR = 1.02, 95% CI 0.99, 1.05; Fig 4).
Fig. 2. Forest plot for prospective studies of adult height and colorectal cancer, stratified by sex. 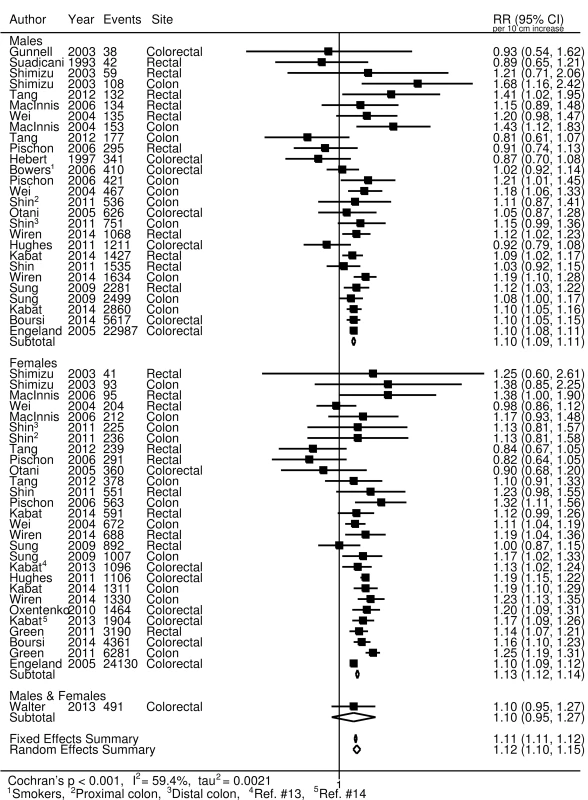
RR, relative risk. Fig. 3. Forest plot for prospective studies of adult height and prostate cancer. 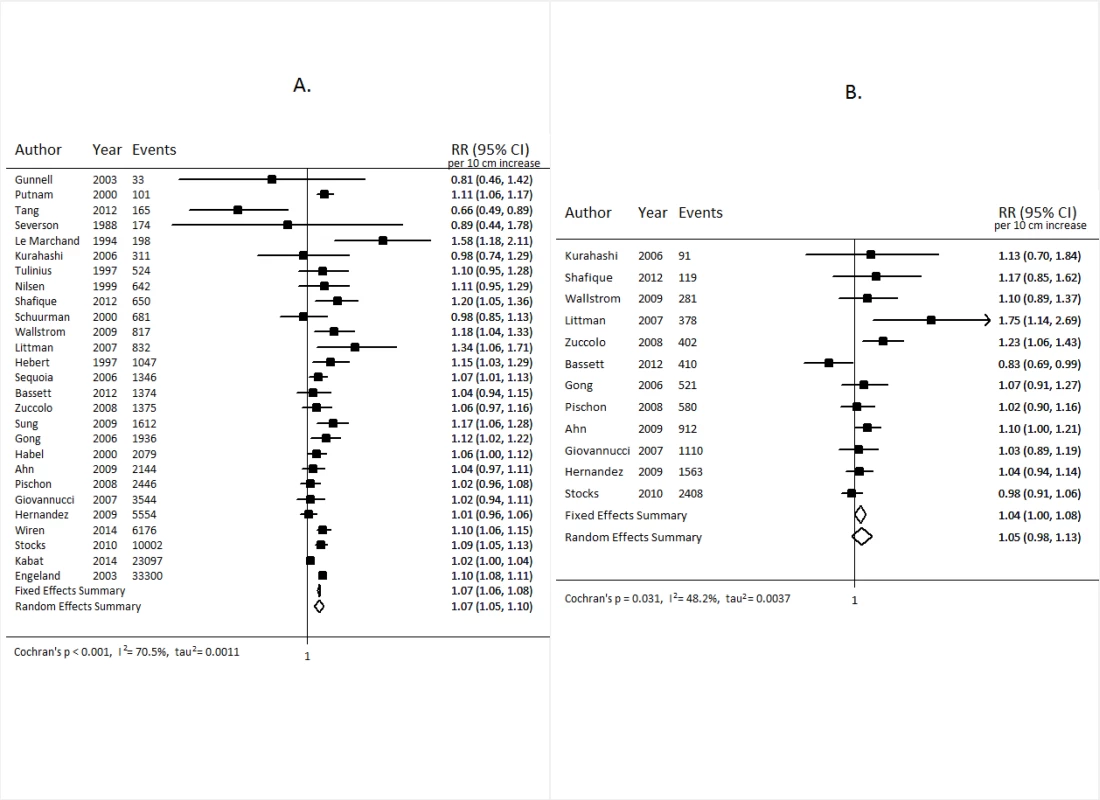
Overall prostate cancer (A); aggressive prostate cancer (B). RR, relative risk. Fig. 4. Forest plot of prospective studies of adult height and lung cancer, stratified by sex. 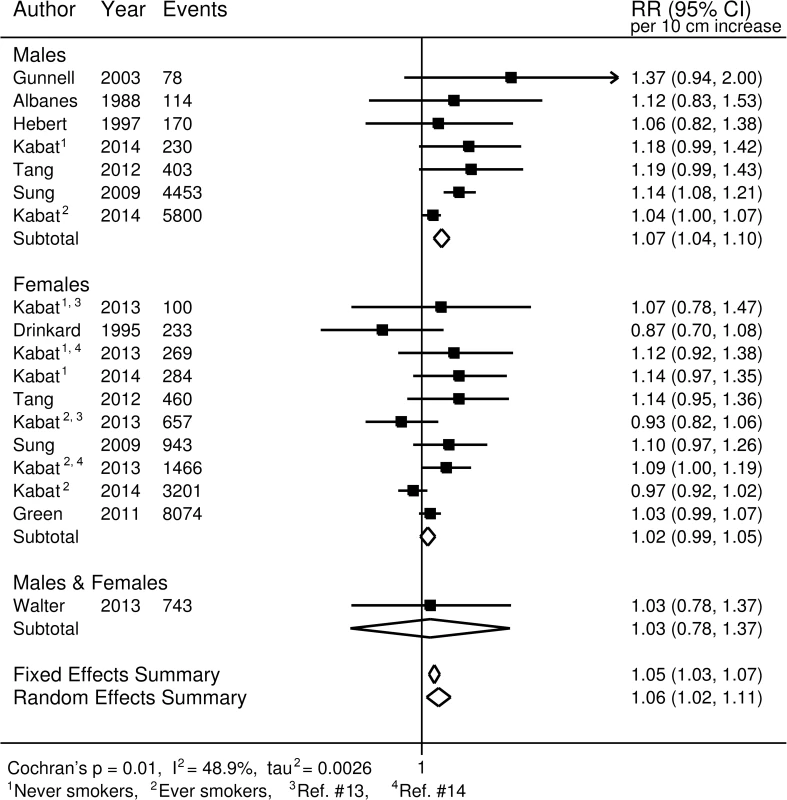
RR, relative risk. In general, the results from our sensitivity analyses, stratifying the studies based on several different factors, were not substantially different from those of the main analysis. After stratification by cancer site, our meta-analysis random-effects summary estimate for rectal cancer was lower (RR = 1.10, 95% CI 1.06, 1.13) than that for colon cancer (RR = 1.17, 95% CI 1.14, 1.19). No apparent differences were observed for lung cancer between studies that adjusted for smoking (RRsmoking-adjusted = 1.06, 95% CI 1.00, 1.11) and those that did not (RRnot-adjusted = 1.08, 95% CI 0.98, 1.21). Similarly, no differences were observed for smoking-adjusted studies for colorectal cancer (RRsmoking-adjusted = 1.11, 95% CI 1.08, 1.15; RRnot-adjusted = 1.13, 95% CI 1.09, 1.17). For prostate cancer, a modest difference was observed (RRsmoking-adjusted = 1.05, 95% CI 0.98, 1.12; RRnot-adjusted = 1.10, 95% CI 1.08, 1.11; homogeneity p = 0.181), but the difference was not statistically significant. Studies using self-reported height and studies using measured height yielded very similar summary estimates for prostate cancer (RRself-reported = 1.07, 95% CI 1.00, 1.14; RRmeasured = 1.09, 95% CI 1.07, 1.11) and colorectal cancer (RRself-reported = 1.13, 95% CI 1.08, 1.18; RRmeasured = 1.12, 95% CI 1.09, 1.14). However, some modest differences were observed for lung cancer (RRself-reported = 1.02, 95% CI 0.98, 1.07; RRmeasured = 1.10, 95% CI 1.04, 1.17; homogeneity p = 0.062). Additionally, the random-effects summary estimates for colorectal, prostate, and lung cancers did not differ substantially when we restricted the studies to only those conducted among populations of European descent (S6 Table).
Mendelian Randomization Analysis
Results from the Mendelian randomization analyses are presented in Table 1. A nearly 60% increased risk of colorectal cancer was observed per 10-cm increase in genetically predicted height (OR = 1.58, 95% CI 1.14, 2.18, p = 0.006); males and females had slightly different estimates per 10-cm increase in height, with females having a slightly higher risk than males (S7 Table). A modest association with genetically predicted height was observed for lung cancer (OR = 1.10, 95% CI 1.00, 1.22, p = 0.053). The association appears to be stronger for adenocarcinoma of the lung (OR = 1.16, 95% CI 1.00, 1.35, p = 0.056) than for squamous cell carcinoma of the lung (OR = 1.06, 95% CI 0.90, 1.25, p = 0.463), and the homogeneity p-value was not statistically significant (p = 0.427). However, the estimates for males and females according to subtype were nearly identical (S7 Table). No statistically significant association with genetically predicted height was seen for prostate cancer (overall prostate cancer, OR = 1.03, 95% CI 0.92, 1.15, p = 0.642; aggressive prostate cancer, OR = 0.98, 95% CI 0.84, 1.15, p = 0.822). The results for breast cancer showed an approximately 20% increase in risk per 10-cm increase in genetically predicted height (OR = 1.19, 95% CI 1.07, 1.33, p = 0.001), which is very similar to our previous estimate using a smaller set of height-associated genetic variants (OR = 1.21, 95% CI 1.05, 1.39, p = 0.008) [53].
Tab. 1. Odds ratios and 95% confidence intervals estimated from Mendelian randomization analyses compared to the summary estimates (Figs 2–4) from published prospective studies for the association between adult height and cancers of the breast, colorectum, prostate, and lung. 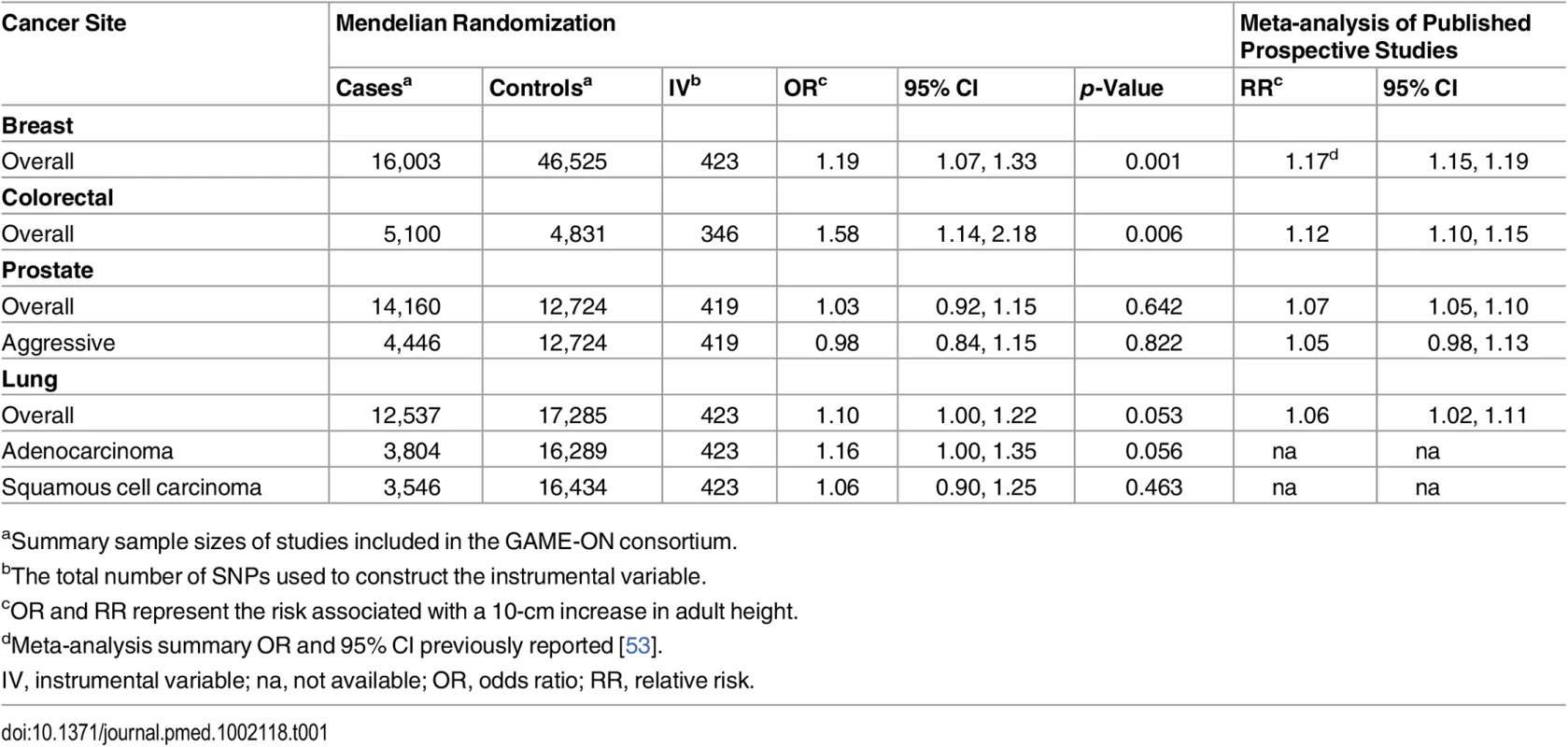
aSummary sample sizes of studies included in the GAME-ON consortium. We did not observe any substantial differences in the Mendelian randomization estimates after excluding genetic variants with suggested pleiotropic effects from the instrument (S8 Table). Furthermore, the impact of pleiotropy may be negligible, given that the average pleiotropic effect was small and the intercept from the data-driven Egger regression was not statistically significant (S9 Table).
Discussion
In this meta-analysis involving 62 published prospective studies, we observed a statistically significant increased risk for colorectal, prostate, and lung cancers associated with higher adult height. Our Mendelian randomization analyses confirmed these increased risks for colorectal and lung cancers but not for prostate cancer, indicating perhaps that the increased risk reported from previous prospective cohort studies for prostate cancer may be due to biases. To our knowledge, this is the first meta-analysis performed to summarize results from prospective cohort studies regarding the association of adult height and these three major cancers that affect the lives of men and women throughout the world. To our knowledge, this is also the first Mendelian randomization analysis performed to evaluate the association between adult height and risk of lung cancer. Our study, using an instrument comprising 423 height-related SNPs and explaining approximately 16% of phenotypic variation, provides strong evidence for a possible causal association between adult height and risk of breast, colorectal, and lung cancers. It suggests that certain genetic factors and biological pathways affecting adult height may affect the risk of these major cancers.
One potential reason taller individuals may be at higher risk is the increased opportunity for a cell to mutate given that taller people have more cells. However, multiple interrelated biological pathways have also been implicated in the association between height and cancer. One pathway is the insulin-like growth factor (IGF) pathway, which is known to promote cell proliferation and inhibit apoptosis [63]; several genetic variants of the IGF pathway have been identified as being related to height. Growth hormone is also known to stimulate IGF-1 expression, which plays an important role in determining adult height via regulation of bone growth [64]. Thus, attained height may represent cumulative exposure to IGF-1 throughout important periods of growth and development (i.e., in utero, childhood, adolescence) [65]. High levels of IGF-1 have also been shown to be positively associated with colorectal, lung, and prostate cancers [66–68]. Other lifestyle risk factors for colorectal cancer, such as increased caloric intake [69], being overweight [70], and having a sedentary lifestyle [71], have demonstrated higher levels of IGF-1 via increased insulin production and subsequent inhibition of IGF-binding protein synthesis [70]. Therefore, it is possible that high IGF-1 may be one of the underlying biological mechanisms mediating the association of height and colorectal cancer.
It is unlikely that the IGF-1 pathway alone would explain entirely the observed increased colorectal and lung cancer risk associated with adult height. Other pathways have also been revealed recently to influence adult height, including transforming growth factor beta (TGF-β) [72] and Hedgehog [73,74]. A number of loci (SMAD3, MTOR, GLI2, LAMA5) involved in these pathways were related to height in a recent GWAS [5]. Some of these pathways have also been linked to the pathogenesis of colorectal and lung cancers.
The results are similar when comparing the summary estimates from the meta-analysis and the associations from the Mendelian randomization analysis for both breast and lung cancers. For colorectal cancer, however, the association from our Mendelian randomization analysis was substantially stronger than that estimated from the meta-analysis of prospective cohort studies. This difference is likely due to sampling errors, given the small sample size of our Mendelian randomization analysis performed for colorectal cancer. Indeed, in a recent Mendelian randomization study with a larger sample size (more than 10,000 cases and 10,000 controls) than our study, a 10-cm increase in height was associated with a 7% (95% CI 1%, 14%) increased risk of colorectal cancer, and the risk was higher among females (OR = 1.09, 95% CI 1.01, 1.19) than males (OR = 1.05, 95% CI 0.96, 1.15) [75]. Although the magnitudes of the associations were different, a similar pattern—where females had a slightly higher risk than males—was also observed in our analysis (S7 Table). The consistency of the association in these two studies regarding adult height and increased colorectal cancer risk provides further support for a possible causal association.
We report modest increases in the risk of lung cancer with height in both the meta-analysis of prospective studies and the Mendelian randomization analysis. The majority of lung cancers are caused by smoking, and smoking may reduce IGF-1 levels [66]. Future investigations using individual-level data may consider stratification by smoking status to assess whether or not the association of height and lung cancer may be modified by tobacco smoking. For prostate cancer, we observed a null association with height in the Mendelian randomization analysis, providing no support for the modestly elevated risk observed in previous prospective cohort studies between adult height and the risk of prostate cancer. Our results are supported by a recently published Mendelian randomization study utilizing individual-level data that reported a null association between adult height and prostate cancer risk [76]. Our study should have 80% statistical power to detect a 9% increased risk in all prostate cancer associated with adult height, and an 11% increased risk in aggressive prostate cancer [77]. Therefore, we could not exclude the possibility of a weak association of adult height with prostate cancer.
There are several strengths to this study. The comprehensive literature search identified 62 studies related to adult height and the cancers evaluated in our study. Additionally, comparing the results from the Mendelian randomization analysis with the meta-analysis summary estimates could indicate whether or not the results from observational studies are biased, assuming the Mendelian randomization analyses represent an effect that may be closer to the true effect. Furthermore, we believe that the genetic variants used in our Mendelian randomization analysis serve as an adequate instrument for height for several reasons. One of the assumptions for a valid instrument is that the genetic marker is associated with the exposure phenotype of interest [3]. In our analysis, we utilized multiple genetic variants explaining approximately 16% of variation in height. It has also been demonstrated that using multiple SNPs helps to strengthen the genetic instrument and improve the precision of the estimate [56], and reduces bias stemming from potential violations of the other Mendelian randomization assumptions [4]. Additionally, all of the variants utilized were uncorrelated, and those that were not genotyped directly were imputed with high accuracy (r2 ≥ 0.3 for ELLIPSE and TRICL, and info score ≥ 0.7 for CORECT) [57]. We also explored the influence of pleiotropy by excluding variants (n = 36) with suggested pleiotropic effects on several different biological outcomes, including pulmonary function, sex hormone binding globulin levels, and age at menarche. Pleiotropic effects would violate the exclusion restriction assumption for a valid instrument, which states that the instrument affects the outcome only through the exposure phenotype of interest [3,4]. However, as our results from the sensitivity analyses indicate, removing the pleiotropic SNPs from the instrument did not appreciably alter the results. Furthermore, the average pleiotropic effect, estimated using a data-driven approach via Egger regression [59], was not statistically significant.
Our study also has several limitations. First, the meta-analyses for the three different cancers are subject to the limitations of the original epidemiologic studies, which could include measurement errors, confounding, selection bias, and random error. Additionally, concerns regarding causal inference from Mendelian randomization studies remain, given the strict criteria required for a valid instrument. In particular, we could not entirely exclude the possible influence of pleiotropic effects on our results since some of the SNPs used in our study might be associated with certain unknown traits that may be related to cancer risk. However, such an influence, if it exists, should be very small, since an instrumental variable constructed using more than 400 height-associated SNPs should be much more strongly associated with adult height than with any other traits. The sample sizes for the analysis of colorectal cancer and for the subgroup analyses of prostate and lung cancers are relatively small, and the relationship between height and these cancers warrants further study using larger sample sizes. Given that most studies showed a linear association between adult height and cancer risk, we assumed a linear association in this analysis, which helped in harmonizing estimates and allowed us to conduct a comprehensive meta-analysis including all relevant studies. A formal test of the shape of the association may be needed in future studies.
In summary, this large-scale meta-analysis of prospective studies and Mendelian randomization analysis provide strong evidence for a possible causal association between adult height and the risk of colorectal and lung cancers. Our study suggests that certain biological pathways affecting adult height may also be involved in the etiology of both colorectal and lung cancers.
Supporting Information
Zdroje
1. Palmer TM, Sterne JAC, Harbord RM, Lawlor DA, Sheehan NA, Meng S, et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epidemiol. 2011;173 : 1392–1403. doi: 10.1093/aje/kwr026 21555716
2. Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91 : 444–455.
3. Glymour MM, Tchetgen Tchetgen EJ, Robins JM. Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol. 2012;175 : 332–339. doi: 10.1093/aje/kwr323 22247045
4. VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P. Methodological challenges in Mendelian randomization. Epidemiology. 2014;25 : 427–435. doi: 10.1097/EDE.0000000000000081 24681576
5. Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46 : 1173–1186. doi: 10.1038/ng.3097 25282103
6. Tang R, Zheng W, Li H, Gao Y, Shu X, Xiang Y. Prospective cohort study of body height and cancer incidence among adult men and women in Shanghai. Tumor. 2012;32 : 992–1000.
7. Albanes D, Jones DY, Schatzkin A, Micozzi MS, Taylor PR. Adult stature and risk of cancer. Cancer Res. 1988;48 : 1658–1662. 3345534
8. Drinkard C, Sellers T, Potter J, Zheng W, Bostick R, Nelson C, et al. Association of body-mass index and body-fat distribution with risk of lung-cancer in older women. Am J Epidemiol. 1995;142 : 600–607. 7653468
9. Hebert PR, Ajani U, Cook NR, Lee IM, Chan KS, Hennekens CH. Adult height and incidence of cancer in male physicians (United States). Cancer Causes Control. 1997;8 : 591–597. 9242474
10. Gunnell D, May M, Ben-Shlomo Y, Yarnell J, Smith GD. Height, leg length, and cancer: the Caerphilly Study. Nutr Cancer. 2003;47 : 34–39. doi: 10.1207/s15327914nc4701_4 14769535
11. Sung J, Song Y-M, Lawlor DA, Smith GD, Ebrahim S. Height and site-specific cancer risk: a cohort study of a Korean adult population. Am J Epidemiol. 2009;170 : 53–64. doi: 10.1093/aje/kwp088 19403842
12. Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12 : 785–794. doi: 10.1016/S1470-2045(11)70154-1 21782509
13. Kabat GC, Heo M, Kamensky V, Miller AB, Rohan TE. Adult height in relation to risk of cancer in a cohort of Canadian women. Int J Cancer. 2013;132 : 1125–1132. doi: 10.1002/ijc.27704 22753236
14. Kabat GC, Anderson ML, Heo M, Hosgood HD 3rd, Kamensky V, Bea JW, et al. Adult stature and risk of cancer at different anatomic sites in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22 : 1353–1363. doi: 10.1158/1055-9965.EPI-13-0305 23887996
15. Walter RB, Brasky TM, Buckley SA, Potter JD, White E. Height as an explanatory factor for sex differences in human cancer. J Natl Cancer Inst. 2013;105 : 860–868. doi: 10.1093/jnci/djt102 23708052
16. Kabat GC, Kim MY, Hollenbeck AR, Rohan TE. Attained height, sex, and risk of cancer at different anatomic sites in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2014;25 : 1697–1706. doi: 10.1007/s10552-014-0476-1 25307804
17. Suadicani P, Hein HO, Gyntelberg F. Height, weight, and risk of colorectal cancer. An 18-year follow-up in a cohort of 5249 men. Scand J Gastroenterol. 1993;28 : 285–288. 8446855
18. Shimizu N, Nagata C, Shimizu H, Kametani M, Takeyama N, Ohnuma T, et al. Height, weight, and alcohol consumption in relation to the risk of colorectal cancer in Japan: a prospective study. Br J Cancer. 2003;88 : 1038–1043. doi: 10.1038/sj.bjc.6600845 12671701
19. MacInnis RJ, English DR, Hopper JL, Haydon AM, Gertig DM, Giles GG. Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2004;13 : 553–559. 15066919
20. Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108 : 433–442. doi: 10.1002/ijc.11540 14648711
21. Engeland A, Tretli S, Austad G, Bjorge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control. 2005;16 : 987–996. doi: 10.1007/s10552-005-3638-3 16132807
22. Otani T, Iwasaki M, Inoue M. Body mass index, body height, and subsequent risk of colorectal cancer in middle-aged and elderly Japanese men and women: Japan public health center-based prospective study. Cancer Causes Control. 2005;16 : 839–850. doi: 10.1007/s10552-005-4573-z 16132794
23. Bowers K, Albanes D, Limburg P, Pietinen P, Taylor PR, Virtamo J, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol. 2006;164 : 652–664. doi: 10.1093/aje/kwj253 16877536
24. MacInnis RJ, English DR, Haydon AM, Hopper JL, Gertig DM, Giles GG. Body size and composition and risk of rectal cancer (Australia). Cancer Causes Control. 2006;17 : 1291–1297. doi: 10.1007/s10552-006-0074-y 17111261
25. MacInnis RJ, English DR, Hopper JL, Gertig DM, Haydon AM, Giles GG. Body size and composition and colon cancer risk in women. Int J Cancer. 2006;118 : 1496–1500. doi: 10.1002/ijc.21508 16187280
26. Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98 : 920–931. doi: 10.1093/jnci/djj246 16818856
27. Oxentenko AS, Bardia A, Vierkant RA, Wang AH, Anderson KE, Campbell PT, et al. Body size and incident colorectal cancer: a prospective study of older women. Cancer Prev Res (Phila). 2010;3 : 1608–1620. doi: 10.1158/1940-6207.CAPR-10-0116
28. Hughes LAE, Simons CCJM, van den Brandt PA, Goldbohm RA, van Engeland M, Weijenberg MP. Body size and colorectal cancer risk after 16.3 years of follow-up: an analysis from the Netherlands Cohort Study. Am J Epidemiol. 2011;174 : 1127–1139. doi: 10.1093/aje/kwr247 21984660
29. Shin A, Joo J, Bak J, Yang H-R, Kim J, Park S, et al. Site-specific risk factors for colorectal cancer in a Korean population. PLoS ONE. 2011;6:e23196. doi: 10.1371/journal.pone.0023196 21853085
30. Wiren S, Haggstrom C, Ulmer H, Manjer J, Bjorge T, Nagel G, et al. Pooled cohort study on height and risk of cancer and cancer death. Cancer Causes Control. 2014;25 : 151–159. doi: 10.1007/s10552-013-0317-7 24173535
31. Boursi B, Haynes K, Mamtani R, Yang Y-X. Height as an independent anthropomorphic risk factor for colorectal cancer. Eur J Gastroenterol Hepatol. 2014;26 : 1422–1427. doi: 10.1097/MEG.0000000000000209 25264984
32. Severson RK, Grove JS, Nomura AM, Stemmermann GN. Body mass and prostatic cancer: a prospective study. BMJ. 1988;297 : 713–715. 3147735
33. Le Marchand L, Kolonel LN, Wilkens LR, Myers BC, Hirohata T. Animal fat consumption and prostate cancer: a prospective study in Hawaii. Epidemiology. 1994;5 : 276–282. 8038241
34. Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6 : 863–873. 9367058
35. Nilsen TI, Vatten LJ. Anthropometry and prostate cancer risk: a prospective study of 22,248 Norwegian men. Cancer Causes Control. 1999;10 : 269–275. 10482485
36. Habel LA, Van Den Eeden SK, Friedman GD. Body size, age at shaving initiation, and prostate cancer in a large, multiracial cohort. Prostate. 2000;43 : 136–143. 10754529
37. Putnam SD, Cerhan JR, Parker AS, Bianchi GD, Wallace RB, Cantor KP, et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol. 2000;10 : 361–369. 10964002
38. Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151 : 541–549. 10733035
39. Engeland A, Tretli S, Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003;89 : 1237–1242. doi: 10.1038/sj.bjc.6601206 14520453
40. Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15 : 1977–1983. 17035408
41. Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S. Association of body mass index and height with risk of prostate cancer among middle-aged Japanese men. Br J Cancer. 2006;94 : 740–742. doi: 10.1038/sj.bjc.6602983 16465189
42. Sequoia JSP, Wright ME, McCarron P, Pietinen P, Taylor PR, Virtamo J, et al. A prospective investigation of height and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15 : 2174–2178. doi: 10.1158/1055-9965.EPI-06-0467 17119043
43. Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121 : 1571–1578. doi: 10.1002/ijc.22788 17450530
44. Littman AJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007;165 : 1271–1279. doi: 10.1093/aje/kwm013 17395597
45. Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17 : 3252–3261. doi: 10.1158/1055-9965.EPI-08-0609 18990768
46. Zuccolo L, Harris R, Gunnell D, Oliver S, Lane JA, Davis M, et al. Height and prostate cancer risk: a large nested case-control study (ProtecT) and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17 : 2325–2336. doi: 10.1158/1055-9965.EPI-08-0342 18768501
47. Ahn J, Moore SC, Albanes D, Huang W-Y, Leitzmann MF, Hayes RB. Height and risk of prostate cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Br J Cancer. 2009;101 : 522–525. doi: 10.1038/sj.bjc.6605159 19568244
48. Hernandez BY, Park S-Y, Wilkens LR, Henderson BE, Kolonel LN. Relationship of body mass, height, and weight gain to prostate cancer risk in the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2009;18 : 2413–2421. doi: 10.1158/1055-9965.EPI-09-0293 19723920
49. Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E. A prospective Swedish study on body size, body composition, diabetes, and prostate cancer risk. Br J Cancer. 2009;100 : 1799–1805. doi: 10.1038/sj.bjc.6605077 19436298
50. Stocks T, Hergens M-P, Englund A, Ye W, Stattin P. Blood pressure, body size and prostate cancer risk in the Swedish Construction Workers cohort. Int J Cancer. 2010;127 : 1660–1668. doi: 10.1002/ijc.25171 20087861
51. Bassett JK, Severi G, Baglietto L, MacInnis RJ, Hoang HN, Hopper JL, et al. Weight change and prostate cancer incidence and mortality. Int J Cancer. 2012;131 : 1711–1719. doi: 10.1002/ijc.27414 22213024
52. Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer. 2012;12 : 25. doi: 10.1186/1471-2407-12-25 22260413
53. Zhang B, Shu X-O, Delahanty RJ, Zeng C, Michailidou K, Bolla MK, et al. Height and breast cancer risk: evidence from prospective studies and Mendelian randomization. J Natl Cancer Inst. 2015;107:djv219. doi: 10.1093/jnci/djv219 26296642
54. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135 : 1301–1309. 1626547
55. Il’yasova D, Hertz-Picciotto I, Peters U, Berlin JA, Poole C. Choice of exposure scores for categorical regression in meta-analysis: a case study of a common problem. Cancer Causes Control. 2005;16 : 383–388. doi: 10.1007/s10552-004-5025-x 15953980
56. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37 : 658–665. doi: 10.1002/gepi.21758 24114802
57. Hung RJ, Ulrich CM, Goode EL, Brhane Y, Muir K, Chan AT, et al. Cross cancer genomic investigation of inflammation pathway for five common cancers: lung, ovary, prostate, breast, and colorectal cancer. J Natl Cancer Inst. 2015;107:djv246. doi: 10.1093/jnci/djv246 26319099
58. Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467 : 832–838. doi: 10.1038/nature09410 20881960
59. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44 : 512–525. doi: 10.1093/ije/dyv080 26050253
60. Humerfelt S, Eide GE, Kvåle G, Gulsvik A. Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) variability in asymptomatic never-smoking men. Clin Physiol. 1998;18 : 387–396. 9715766
61. Zacny JP, Stitzer ML, Brown FJ, Yingling JE, Griffiths RR. Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. J Pharmacol Exp Ther. 1987;240 : 554–564. 3806411
62. Tashkin DP, Clark VA, Coulson AH, Bourque LB, Simmons M, Reems C, et al. Comparison of lung function in young nonsmokers and smokers before and after initiation of the smoking habit. A prospective study. Am Rev Respir Dis. 1983;128 : 12–16. 6870055
63. Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4 : 505–518. doi: 10.1038/nrc1387 15229476
64. Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu J-L, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110 : 771–781. doi: 10.1172/JCI15463 12235108
65. Rosenfeld RG. Insulin-like growth factors and the basis of growth. N Engl J Med. 2003;349 : 2184–2186. doi: 10.1056/NEJMp038156 14657423
66. Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363 : 1346–1353. doi: 10.1016/S0140-6736(04)16044-3 15110491
67. Fürstenberger G, Senn H-J. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3 : 298–302. 12067807
68. Huang XP, Zhou WH, Zhang YF. Genetic variations in the IGF-IGFR-IGFBP axis confer susceptibility to lung and esophageal cancer. Genet Mol Res. 2014;13 : 2107–2119. doi: 10.4238/2014.January.24.17 24615087
69. Smith WJ, Underwood LE, Clemmons DR. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J Clin Endocrinol Metab. 1995;80 : 443–449. doi: 10.1210/jcem.80.2.7531712 7531712
70. Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92 : 1592–1600. 11018095
71. Boyle T, Fritschi L, Heyworth J, Bull F. Long-term sedentary work and the risk of subsite-specific colorectal cancer. Am J Epidemiol. 2011;173 : 1183–1191. doi: 10.1093/aje/kwq513 21421743
72. Zhang H-J, Wang H-Y, Zhang H-T, Su J-M, Zhu J, Wang H-B, et al. Transforming growth factor-β1 promotes lung adenocarcinoma invasion and metastasis by epithelial-to-mesenchymal transition. Mol Cell Biochem. 2011;355 : 309–314. doi: 10.1007/s11010-011-0869-3 21695462
73. Shaw A, Gipp J, Bushman W. The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene. 2009;28 : 4480–4490. doi: 10.1038/onc.2009.294 19784071
74. Yue D, Li H, Che J, Zhang Y, Tseng H-HK, Jin JQ, et al. Hedgehog/Gli promotes epithelial-mesenchymal transition in lung squamous cell carcinomas. J Exp Clin Cancer Res. 2014;33 : 34. doi: 10.1186/1756-9966-33-34 24758269
75. Thrift AP, Gong J, Peters U, Chang-Claude J, Rudolph A, Slattery ML, et al. Mendelian randomization study of height and risk of colorectal cancer. Int J Epidemiol. 2015;44 : 662–672. doi: 10.1093/ije/dyv082 25997436
76. Davies NM, Gaunt TR, Lewis SJ, Holly J, Donovan JL, Hamdy FC, et al. The effects of height and BMI on prostate cancer incidence and mortality: a Mendelian randomization study in 20,848 cases and 20,214 controls from the PRACTICAL consortium. Cancer Causes Control. 2015;26 : 1603–1616. doi: 10.1007/s10552-015-0654-9 26387087
77. Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. 2014;43 : 922–929. doi: 10.1093/ije/dyu005 24608958
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 9- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Reporting of Adverse Events in Published and Unpublished Studies of Health Care Interventions: A Systematic Review
- A Public Health Framework for Legalized Retail Marijuana Based on the US Experience: Avoiding a New Tobacco Industry
- Improving Research into Models of Maternity Care to Inform Decision Making
- Associations between Extending Access to Primary Care and Emergency Department Visits: A Difference-In-Differences Analysis
- Sex Differences in Tuberculosis Burden and Notifications in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis
- Pre-exposure Prophylaxis Use by Breastfeeding HIV-Uninfected Women: A Prospective Short-Term Study of Antiretroviral Excretion in Breast Milk and Infant Absorption
- A Comparison of Midwife-Led and Medical-Led Models of Care and Their Relationship to Adverse Fetal and Neonatal Outcomes: A Retrospective Cohort Study in New Zealand
- Scheduled Intermittent Screening with Rapid Diagnostic Tests and Treatment with Dihydroartemisinin-Piperaquine versus Intermittent Preventive Therapy with Sulfadoxine-Pyrimethamine for Malaria in Pregnancy in Malawi: An Open-Label Randomized Controlled Trial
- Tenofovir Pre-exposure Prophylaxis for Pregnant and Breastfeeding Women at Risk of HIV Infection: The Time is Now
- The Policy Dystopia Model: An Interpretive Analysis of Tobacco Industry Political Activity
- International Criteria for Acute Kidney Injury: Advantages and Remaining Challenges
- Chronic Kidney Disease in Primary Care: Outcomes after Five Years in a Prospective Cohort Study
- Potential for Controlling Cholera Using a Ring Vaccination Strategy: Re-analysis of Data from a Cluster-Randomized Clinical Trial
- Association between Adult Height and Risk of Colorectal, Lung, and Prostate Cancer: Results from Meta-analyses of Prospective Studies and Mendelian Randomization Analyses
- The Incidence Patterns Model to Estimate the Distribution of New HIV Infections in Sub-Saharan Africa: Development and Validation of a Mathematical Model
- Antimicrobial Resistance: Is the World UNprepared?
- A Médecins Sans Frontières Ethics Framework for Humanitarian Innovation
- Reduced Emergency Department Utilization after Increased Access to Primary Care
- "The Policy Dystopia Model": Implications for Health Advocates and Democratic Governance
- Interplay between Diagnostic Criteria and Prognostic Accuracy in Chronic Kidney Disease
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Sex Differences in Tuberculosis Burden and Notifications in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis
- International Criteria for Acute Kidney Injury: Advantages and Remaining Challenges
- Potential for Controlling Cholera Using a Ring Vaccination Strategy: Re-analysis of Data from a Cluster-Randomized Clinical Trial
- The Policy Dystopia Model: An Interpretive Analysis of Tobacco Industry Political Activity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




