-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPrices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis
Suzanne Hill and colleagues provide an economic analysis of the prices, costs, and affordability of new hepatitis C medicines in 30 countries.
Published in the journal: . PLoS Med 13(5): e32767. doi:10.1371/journal.pmed.1002032
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002032Summary
Suzanne Hill and colleagues provide an economic analysis of the prices, costs, and affordability of new hepatitis C medicines in 30 countries.
Introduction
Hepatitis C virus (HCV) infection is a significant global public health problem. Although the precise prevalence is uncertain, a recent analysis estimated that 80 million people globally were living with HCV viraemia (also known as chronic HCV infection), with a range of 64–103 million people [1]. If left untreated, chronic HCV infection can cause liver cirrhosis and cancer, leading to an estimated 700,000 deaths per year worldwide [2].
Until recently, standard guidelines for treating HCV infection recommended combination therapy with pegylated interferon and ribavirin. While resulting in sustained virological response (SVR) in 54% to 63% of clinical trial participants [3], this combination requires 24 to 48 wk of therapy and has severe side effects such as haemolytic anaemia and flu-like symptoms [4]. Recently developed direct-acting antivirals (DAAs) have markedly improved treatment efficacy and shortened and simplified the treatment regimen. In late 2013, sofosbuvir was approved in the United States (US) as the first once-daily orally administered therapy for HCV without interferon. Clinical trials showed SVR in up to 93% of trial participants following 12 wk of treatment with sofosbuvir [5]. In 2014, a combination product of ledipasvir/sofosbuvir was approved in the US based on evidence of a SVR rate of up to 99% after 12 wk of treatment [6]. Since then, other DAA regimens have gained regulatory approval, but sofosbuvir and ledipasvir/sofosbuvir dominate the market [7].
The initial published list prices in the US for 12-wk courses of treatment with sofosbuvir and ledipasvir/sofosbuvir were US$84,000 and US$94,500, respectively. The manufacturer claimed the new regimen was equal to or less expensive than prior standard of care regimens because the new regimen had much higher cure rates and would reduce the total treatment costs of HCV, including the costs of medications, side effects, complications, and additional health services required [8,9]. A number of published economic studies from high-income countries supported the cost-effectiveness of sofosbuvir at the proposed price, although the budget impact was substantial [10,11]. A recent study estimated the cost of production of sofosbuvir to be US$68–US$136 for a 12-wk course of treatment based on the same manufacturing methods used in the large-scale generic production of HIV/AIDS medicines [12], and its findings have not been challenged. The difference between the estimated cost of production and the marketed prices raises questions about the fairness of pricing medicines of public health importance like sofosbuvir and ledipasvir/sofosbuvir [13], echoing similar discussion about new cancer medicines [14–16].
As a consequence of the high prices of the new HCV medicines, payers in high-income countries have been restricting coverage (e.g., United States) [17], negotiating public deals and private discounts and rebates with the manufacturer (e.g., France and Germany [18]), or delaying reimbursement until a reasonable price has been negotiated (e.g., Australia [19]).
A recent report suggested that 73% of people with chronic HCV live in middle-income countries [20]. For some low - and medium-income countries (LMICs), tiered pricing agreements have been negotiated. As a result, countries such as Mongolia, Egypt, and Pakistan have published prices of about US$900 per 12-wk course of sofosbuvir. Voluntary licensing agreements have been established with 11 India-based generic pharmaceutical manufacturers for production and distribution of the medicines in 101 countries [21]. The Indian licensees are selling generic sofosbuvir at prices between US$161 and US$312 per 28-tablet pack [22]. However, the license agreement does not include 39 middle-income countries, including Brazil, China, Mexico, and Turkey [21,23].
This study seeks to systematically compare the prices of sofosbuvir and ledipasvir/sofosbuvir across countries, including generic versions where prices are available. We assessed the affordability and budgetary impact of these treatments, both to health systems and to individual patients paying for the treatment fully out of pocket in the absence of reimbursement from public or private health insurance. We did not include other DAAs (e.g., daclatasvir, simeprevir, and combination products by other manufacturers) in this analysis because they are still only available in a small number of countries.
Methods
Study Design
We conducted a comparative study of the published prices of sofosbuvir and ledipasvir/sofosbuvir in countries where published price information was available. Ex-factory prices were used because prices paid by the consumer include different amounts of taxes, mark-ups, and distribution costs that make them difficult to compare. We included Organisation for Economic Co-operation and Development (OECD) member countries and LMICs for which we had access to reliable, publicly available price information. If price information was unavailable for a country, the country was not included in the analysis. In the case of some European countries, this was usually because the medicines had not yet been marketed, or prices were not publicly disclosed. In India, the ex-factory price was “commercial in confidence”, and we used the publicly listed retail price without further adjustments.
In order to compare prices between countries, we converted prices in national currencies to US dollars using OECD 2014 period average exchange rates [24], without and with adjustment for purchasing power parity (PPP) [24]. We report these as the “nominal price” (US dollars) and the “PPP-adjusted price” (PPP dollars), respectively. PPP adjustment is important when comparing the prices of goods across countries to account for the differences in national income levels and purchasing power in buying goods and services [25,26]. The theory of PPP, based on the “law of one price”, states that, after accounting for transaction costs and trade barriers, identical goods will be sold for the same price in trading countries when their prices are expressed in a common currency [27,28]. Accordingly, the price of a medicine in different countries should in theory be the same when expressed in a common currency (e.g., US dollars) using the PPP exchange rates. S1 Text lists the prices and rates used in the analysis where prices in national currency were divided by the corresponding period average or PPP exchange rate of the national currency per US dollar published by the OECD.
We ranked the nominal and PPP-adjusted prices for all countries. We compared the PPP price for each country according to gross domestic product (GDP) per capita published by OECD [29] and, for Egypt and Mongolia, published by the World Bank [30].
We then estimated the total budget impact of sofosbuvir and ledipasvir/sofosbuvir, allowing for different levels of treatment coverage. To estimate the population requiring HCV treatment, we used the prevalence estimates of viraemic HCV infection by Gower and colleagues [1] rather than the number of persons with anti-HCV antibodies [31] because approximately 25% of persons who acquire HCV clear the infection spontaneously [32]. Based on the WHO treatment guidelines [33], we assumed in our base case analysis that all adults with viraemic HCV infection were eligible for treatment, and patients would receive a 12-wk treatment regimen of sofosbuvir or ledipasvir/sofosbuvir because a majority of patients have non–genotype 3 HCV. Patients with genotype 3 infections were assumed to receive 24 wk of treatment with sofosbuvir in sensitivity analysis.
We compared the total estimated budget impact of sofosbuvir and ledipasvir/sofosbuvir treatment with the country’s total expenditure on pharmaceuticals, based on the reported PPP-adjusted total pharmaceutical expenditure (TPE) per capita [34] multiplied by the population size [35]. For consistency in adjustments, we used the PPP-adjusted prices instead of the nominal prices of medicines when estimating budget impact. For the countries with negotiated tiered prices, we assumed no further rebate.
To assess the affordability of sofosbuvir and ledipasvir/sofosbuvir for individual patients without insurance coverage, we estimated the duration of time that an individual would need to work—earning the average wage of the general population—to obtain sufficient income to pay for a full course of treatment fully out of pocket. We performed a sensitivity analysis using minimum wage because people with HCV infection may have incomes below population average wages due to illness and socioeconomic status [36,37]. Previous studies [38–40] have measured the affordability of medicines using minimum wage as an indicator of patient income.
Data Sources
For the European Union (EU) member states, Norway, and Switzerland, published ex-factory prices for a 12-wk course of treatment were provided by the Pharma Price Information (PPI) service of the Austrian public health institute Gesundheit Österreich GmbH. The PPI service offers medicine price data for all price types (ex-factory price, pharmacy purchasing price, and net and gross pharmacy retail price) for all 28 EU member states, Norway, and Switzerland based on data collection from official national databases. The PPI service provided data for sofosbuvir as of July 2015, and for ledipasvir/sofosbuvir as of September 2015. To validate the collected data and to complete the dataset in other countries, we sought additional information from national government or drug reimbursement authorities and recent press releases (S1 Text).
Insurance agencies and reimbursement organisations often negotiate purchase prices lower than drugs’ published prices (list prices). These arrangements may take the form of confidential discounts, rebates, or refunds after purchase [41]. To account for the impact of these undisclosed agreements on prices, in our base case analysis, we assumed a 23% price reduction from the published price in all countries, except for the countries with a negotiated tiered price as noted above. This price reduction estimate was based on the legislated rebate obtained by the US Centers for Medicare & Medicaid Services [17]. To ensure the appropriateness of the estimated budget impact of sofosbuvir and ledipasvir/sofosbuvir, we conducted two sensitivity analyses assuming (1) no price reduction and (2) a 50% price reduction based on the rebate received by the US Department of Veterans Affairs [42].
Relevant demographic and economic statistics for each country were extracted from the OECD database: population [35], pharmaceutical expenditure per capita [34], GDP per capita [29], currency exchange rates [24], and average annual wages in 2014 PPP dollars (constant price) per full-time - and full-year-equivalent employee [43]. The OECD dataset contained wage information for all countries of interest except for Brazil, Egypt, Iceland, and Turkey. For these countries, we used the median nominal monthly earnings (converted to annual earnings) of employees reported in the United Nations International Labour Organization Global Wage Database [44], with linear extrapolation of historical data to 2014 values and with PPP adjustments.
Statistical Analysis
All descriptive statistical analyses and data visualisation were performed in Microsoft Excel 2010 (version 14.0).
Assumptions and Uncertainty
Table 1 lists the main assumptions used in the analysis and their rationale, data sources, estimates of uncertainty, and likely impact on the outcomes of the analysis.
Tab. 1. Assumptions, data sources, uncertainty estimates, impacts on outcomes, and sensitivity analyses. 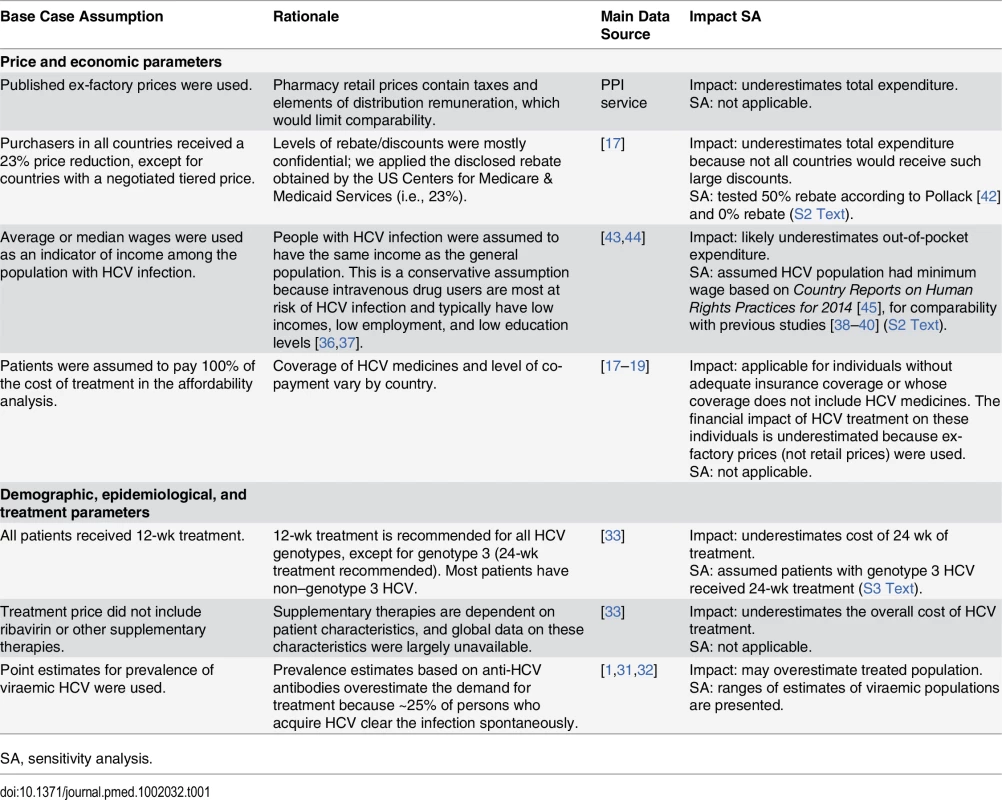
SA, sensitivity analysis. As noted above, there are confidential agreements between manufacturers and insurance agencies/reimbursement organisations, leading to the published prices being higher than the actual prices. We applied a 23% price reduction to estimate the impact of this uncertainty and undertook a sensitivity analysis using a 50% price reduction (S2 Text). The levels of supply chain remuneration and taxes also differ between countries, affecting the pharmacy retail prices. To ensure a conservative approach and to exclude possible bias due to different levels of mark-up and remuneration, we used ex-factory prices for all analyses. This underestimates the total expenditure and costs to the patient.
We estimated TPE based on the mean and range of values of prevalence of hepatitis C viraemia presented in Gower et al. [1].
We also carried out an analysis to adjust the total expenditure on sofosbuvir and ledipasvir/sofosbuvir for the proportion of patients in each country infected with genotype 3 HCV, based on the genotype distribution estimated by Gower et al. [1] and assuming that this group of patients would receive the recommended 24-wk treatment with sofosbuvir (S3 Text). Other factors influencing the duration of treatment are primarily the presence or absence of cirrhosis [33], but reliable data for this factor are not yet available from all countries in our analysis. However, adjusting for this factor would increase the estimates for total expenditure on sofosbuvir and ledipasvir/sofosbuvir because the recommended duration of treatment for patients with cirrhosis is longer (24 wk).
There are two sources of uncertainty when estimating the duration of full-time paid employment required for a patient to pay for a full course of treatment: the income level of HCV patients and the level of out-of-pocket payment. We used the published average or median wage in different countries as the indicator of patient income, assuming that people with HCV infection have the same income as the general population. This is a conservative assumption because some HCV patients are intravenous drug users who typically have low incomes, low employment, and low levels of education [36,37]. To test this assumption, and also for comparability with previous studies on medicine affordability [38–40], we performed a sensitivity analysis using the minimum wage data reported in Country Reports on Human Rights Practices for 2014 [45].
For this analysis of the patient affordability sofosbuvir and ledipasvir/sofosbuvir, we assumed that patients would pay for the treatment fully out of pocket because the level of coverage and co-payments varies considerably across health systems, types of insurance and benefits packages, and characteristics of HCV patients. It could be expected that in high-income countries with universal health coverage, such as European OECD countries, prices of HCV medicines would be fully covered by public payers if the eligibility criteria for reimbursement were met. However, while information from the PPI service showed that some European high-income countries provided full coverage, national price data sources indicated no coverage in other countries. This suggests either full out-of-pocket payments or some specific coverage arrangements beyond regular reimbursement.
We did not include in this analysis the prices of other medicines used in various HCV treatment regimens, the cost of diagnostic tests, and other health service costs. This, again, is a conservative assumption.
Results
Prices of sofosbuvir were obtained for 26 OECD countries and 4 LMICs: Brazil, India, Egypt, and Mongolia (Fig 1A). Ledipasvir/sofosbuvir prices were available in 21 OECD countries, India, Egypt, and Mongolia (Fig 1B). The prices in India were the retail prices of generic products.
Fig. 1. Comparison of nominal and PPP-adjusted prices of sofosbuvir and ledipasvir/sofosbuvir. 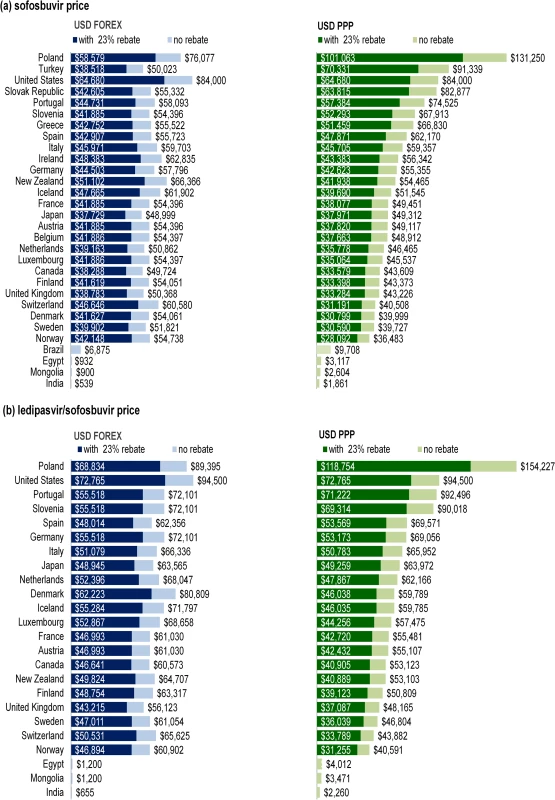
Fig 1 shows the nominal (USD FOREX) and PPP-adjusted (USD PPP) prices of (A) sofosbuvir and (B) ledipasvir/sofosbuvir, with and without a 23% rebate (or price reduction). Dark blue bars show the nominal prices of the medicines assuming a 23% rebate. Light blue bars show the nominal prices of the medicines without rebate. Dark green bars show the PPP-adjusted prices of the medicines assuming a 23% rebate. Light green bars show the PPP-adjusted prices of the medicines without rebate. Price Comparison
Assuming a 23% price reduction on the list ex-factory price, the median nominal price of sofosbuvir for a 12-wk course across all OECD countries was US$42,017, with the price ranging from US$37,729 in Japan to US$64,680 in the US. The 23% reduction was not applied in Brazil, Egypt, India, and Mongolia, where there were special pricing arrangements or generic licensing agreements. The nominal prices of sofosbuvir in Brazil (US$6,875), Egypt (US$932), Mongolia (US$900), and India (US$539) were up to 120 times lower than the nominal price in the US (US$64,680) due to negotiated pricing arrangements and licensing agreements.
In countries with stronger purchasing power, PPP adjustment resulted in lower prices. For example, as illustrated in Fig 1A, the PPP-adjusted price in Norway was 0.67 times less than its nominal price (US$42,148, PPP$28,092). In contrast, countries with weaker purchasing power had a significant increase in price with PPP adjustment. For example, the PPP-adjusted price of sofosbuvir in India (PPP$1,861) was 3.45 times more than the nominal price (US$539). In the OECD, the PPP-adjusted prices were higher in Poland (PPP$101,063), Turkey (PPP$70,331), Slovakia (PPP$63,815), Portugal (PPP$57,384) than in higher-income European economies, particularly the Nordic countries (i.e., Norway, Sweden, Denmark, and Finland). The PPP-adjusted prices for sofosbuvir in Brazil, Egypt, Mongolia, and India were PPP$9,708, PPP$3,117, PPP$2,604, and PPP$1,861, compared to the nominal prices of US$6,875, US$932, US$900, and US$539, respectively.
The nominal price of ledipasvir/sofosbuvir was the highest in the US (US$72,765 with 23% price reduction) and lowest in the UK (US$43,215) among the OECD countries. Assuming no price reduction, the nominal price of ledipasvir/sofosbuvir in Egypt and Mongolia (US$1,200) was 47 times lower than in the United Kingdom (US$56,123) due to negotiated tiered pricing. The nominal price of ledipasvir/sofosbuvir in India (US$655) was 1.2% of the price in the United Kingdom. The price in Norway was the lowest after adjustment for PPP (PPP$31,255), reflecting the stronger purchasing power of its currency. The PPP-adjusted price of a 12-wk course of ledipasvir/sofosbuvir was again higher in Poland (PPP$118,754) than in other countries, with the price being 3.8 times higher than the price in Norway (PPP$31,255).
Relationship of Price with the Standard of Living
Fig 2 shows the relationship between PPP-adjusted price, GDP per capita, and estimated market size. Prices do not increase, and in some cases decrease, with increased standard of living. This pattern is not seen in Brazil, Egypt, India, and Mongolia, however, where prices of sofosbuvir were low because of existing pricing arrangements. In the US, prices of both products were much higher than in countries with comparable GDP per capita.
Fig. 2. Relationship between PPP-adjusted price, GDP per capita, and estimated market size for sofosbuvir and ledipasvir/sofosbuvir. 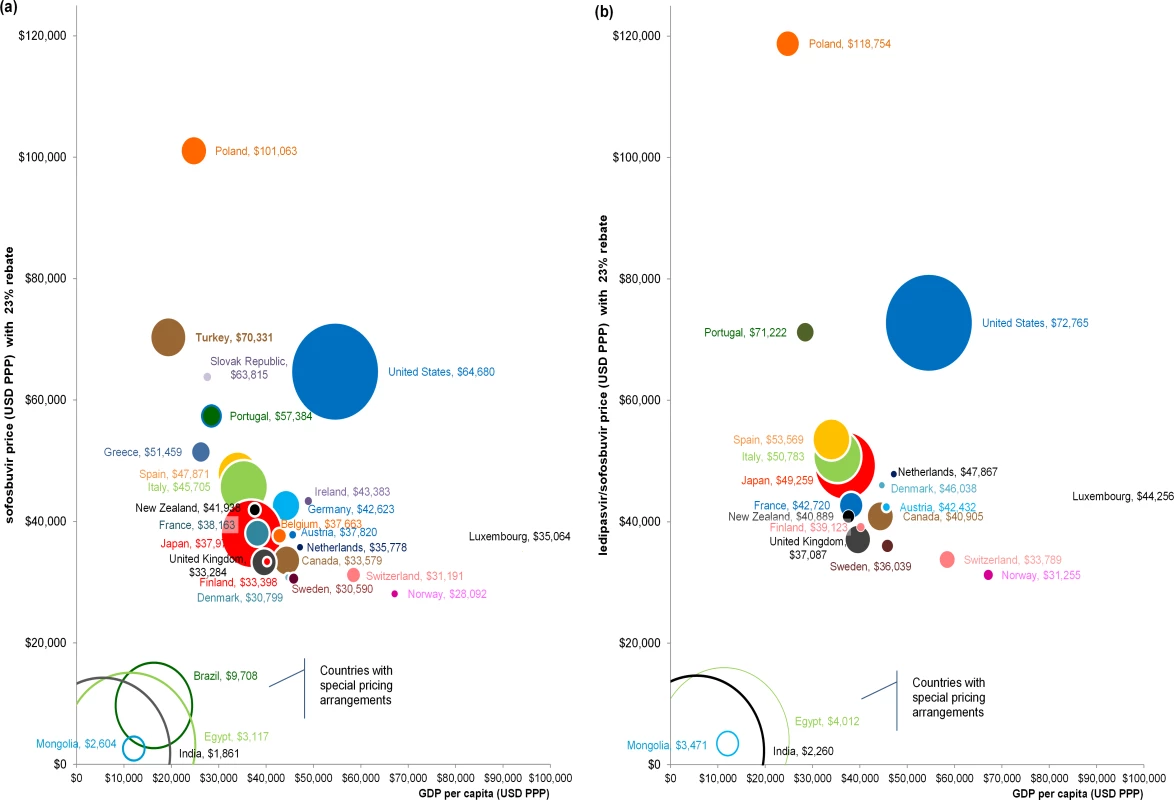
Fig 2 shows the relationship between PPP-adjusted price (y-axis), GDP per capita (USD PPP) (x-axis), and estimated market size (circle size) for (A) sofosbuvir and (B) ledipasvir/sofosbuvir. Solid circles indicate countries where insurance agencies/reimbursement organisations are likely to obtain confidential rebates/price reductions for the medicines, and thus have a 23% rebate in the analysis. Unfilled circles indicate countries that have special pricing arrangements and are unlikely to obtain additional price reductions, and therefore have no further discounts in the analysis. The estimated market size for each country is based on the point estimate of the viraemic population reported by Gower et al. [1]. There was no observable relationship between the PPP-adjusted price and potential market size (Fig 2). For example, Nordic countries had fewer people requiring HCV treatment (based on point estimates reported by Gower et al. [1]) and had higher GDP per capita than countries such as Japan, Italy, and Spain, but the PPP-adjusted prices of sofosbuvir and ledipasvir/sofosbuvir were much lower. Although the GDP per capita in Turkey was only about $3,000 higher than in Brazil, the PPP-adjusted price of sofosbuvir in Turkey (PPP$70,331) was 7.2-fold higher than in Brazil (PPP$9,708). Luxembourg had the smallest estimated HCV population and the highest GDP per capita, but its price for sofosbuvir was less than the price in half of the other OECD countries.
Financial Impact for National Budgets
Fig 3 and Table 2 show the budget impact of treating all infected patients with sofosbuvir or ledipasvir/sofosbuvir for a 12-wk course of treatment, at the PPP-adjusted prices with a 23% reduction, based on the point estimates and range estimates for HCV prevalence from Gower et al. [1]. The budget impact estimates vary from PPP$100.9 million (UI: PPP$56.7 million, PPP$174.1 million) in Luxembourg to PPP$166.6 billion (UI: PPP$153.7 billion, PPP$307.5 billion) in the US. As noted, these estimates do not include the costs of diagnostic testing, ribavirin or other medicines, or other associated health service costs. Fifteen of the 30 countries analysed would require more than PPP$5 billion to provide treatment coverage for the entire infected patient population of their country (Fig 3A). For Poland, Turkey, Spain, and Italy, the point estimates of total budget impact vary from PPP$20 billion to PPP$35 billion. For Japan, the point estimate of the total budget impact of providing treatment for 1.25 million people with HCV viraemia is close to PPP$50 billion. The PPP-adjusted expenditure for treating 2.58 million people with HCV viraemia (point estimate) in the US would be PPP$166.6 billion. These estimates do not include the cost of retreatment for patients who fail treatment or become reinfected. A similar level of financial impact is observed across countries for ledipasvir/sofosbuvir (Fig 3B).
Fig. 3. Financial impact of treatment coverage for the entire estimated population of people with HCV who require treatment with sofosbuvir or ledipasvir/sofosbuvir. 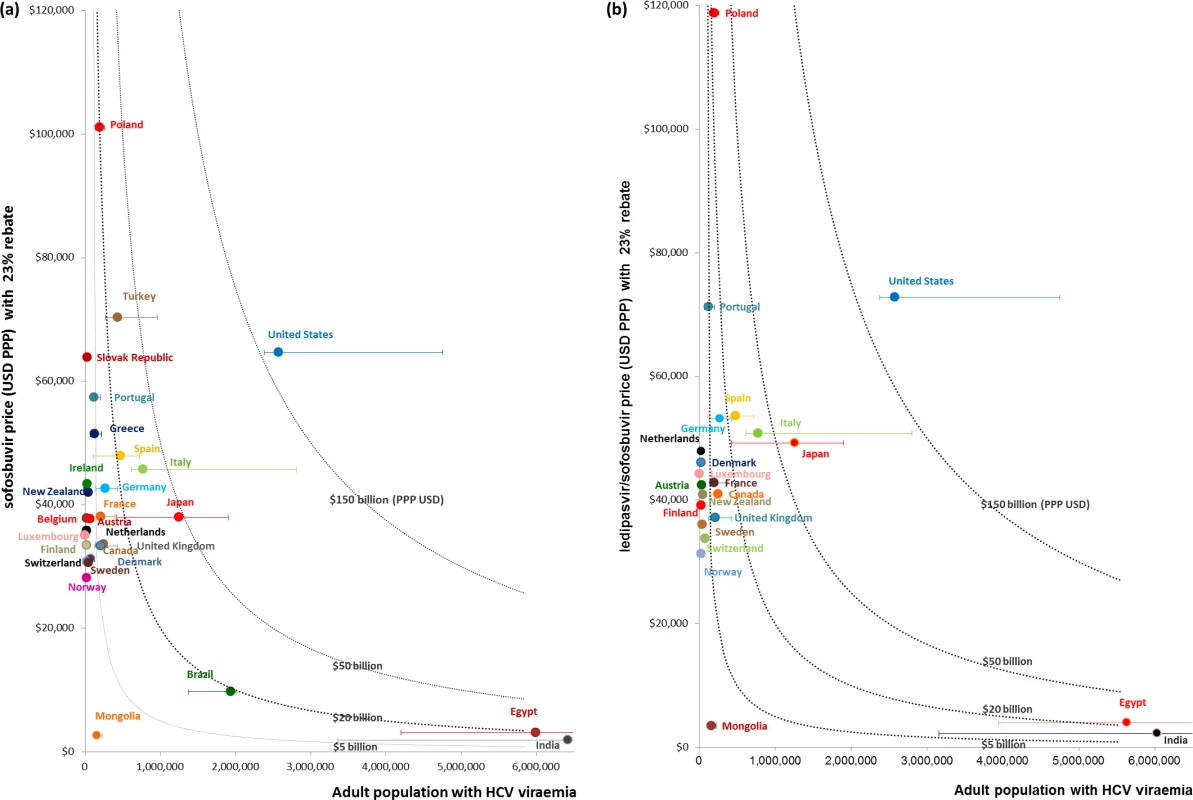
Fig 3 shows the financial impact of covering the entire estimated population of people with HCV who require treatment with (A) sofosbuvir or (B) ledipasvir/sofosbuvir. Financial impact on national budgets is measured by multiplying the PPP-adjusted cost of the medicines (USD PPP) and the point estimates of adult population with HCV viraemia, as reported by Gower et al. [1]. Error bars indicate the financial impact in each country based on the upper and lower estimates of the total adult viraemic population, as reported by Gower et al. The dotted curves indicate countries that may require more than PPP billion, PPP billion, PPP billion, and PPP0 billion to treat 100% of their total adult viraemic population. Tab. 2. PPP-adjusted financial impact of treatment coverage for all patients with viraemic HCV infection (for point estimates and uncertainty intervals) with sofosbuvir or ledipasvir/sofosbuvir. 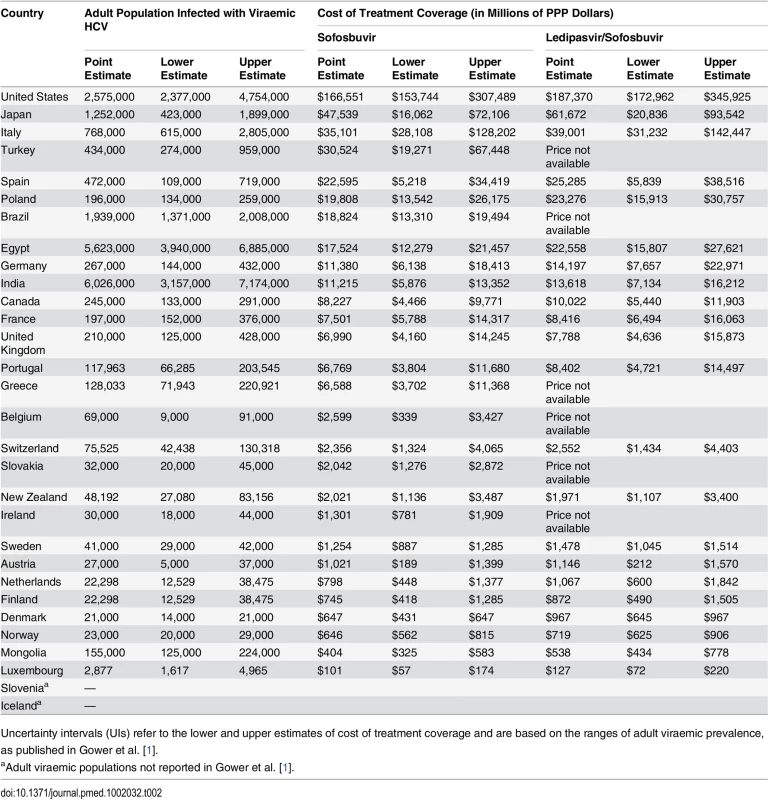
Uncertainty intervals (UIs) refer to the lower and upper estimates of cost of treatment coverage and are based on the ranges of adult viraemic prevalence, as published in Gower et al. [1]. Compared to the PPP-adjusted TPE in each country, treating the entire HCV viraemic population (based on point estimates reported by Gower et al. [1]) with sofosbuvir at the PPP-adjusted price with a 23% price reduction would amount to at least one-tenth of the current TPE in all countries (Table 3). In Poland, treating the whole HCV viraemic population would amount to as much as 1.6 times the current TPE. If only 10% of the HCV viraemic population were treated, the expenditure on sofosbuvir would still be high in proportion to the TPE in Poland (16.2%; UI: 11.1%, 40.6%), New Zealand (15.5%; UI: 8.9%, 26.8%), Portugal (13.3%; UI: 8.7%, 23.0%), Italy (11.1%; UI: 7.5%, 21.4%), and Spain (10.0%; UI: 5.4%, 16.7%) because these countries have relatively high sofosbuvir prices and HCV prevalence. In contrast, in the Netherlands, it would cost an amount equal to 1% (UI: 0.4%, 1.8%) of its current TPE to provide sofosbuvir to 10% of its infected population—even though the price of sofosbuvir is similar to in other OECD countries—because the estimated number of people with hepatitis C is relatively low. The analyses of prices of ledipasvir/sofosbuvir produced similar results, where the expenditure on ledipasvir/sofosbuvir would cost an amount equal to a considerable proportion of the country’s current TPE, particularly in Poland (19.1%; UI: 13.0%, 45.1%), Portugal (16.5%; UI: 9.9%, 28.5%), New Zealand (15.1%; UI: 9.3%, 26.1%), Italy (12.3%; UI: 8.5%, 25.2%), and Spain (11.2%; UI: 5.4%, 17.0%) (Table 3).
Tab. 3. The cost of treatment coverage with sofosbuvir or ledipasvir/sofosbuvir of different proportions of patients with viraemic HCV infection (for point estimates and uncertainty intervals) as a proportion of PPP-adjusted current total pharmaceutical expenditure. 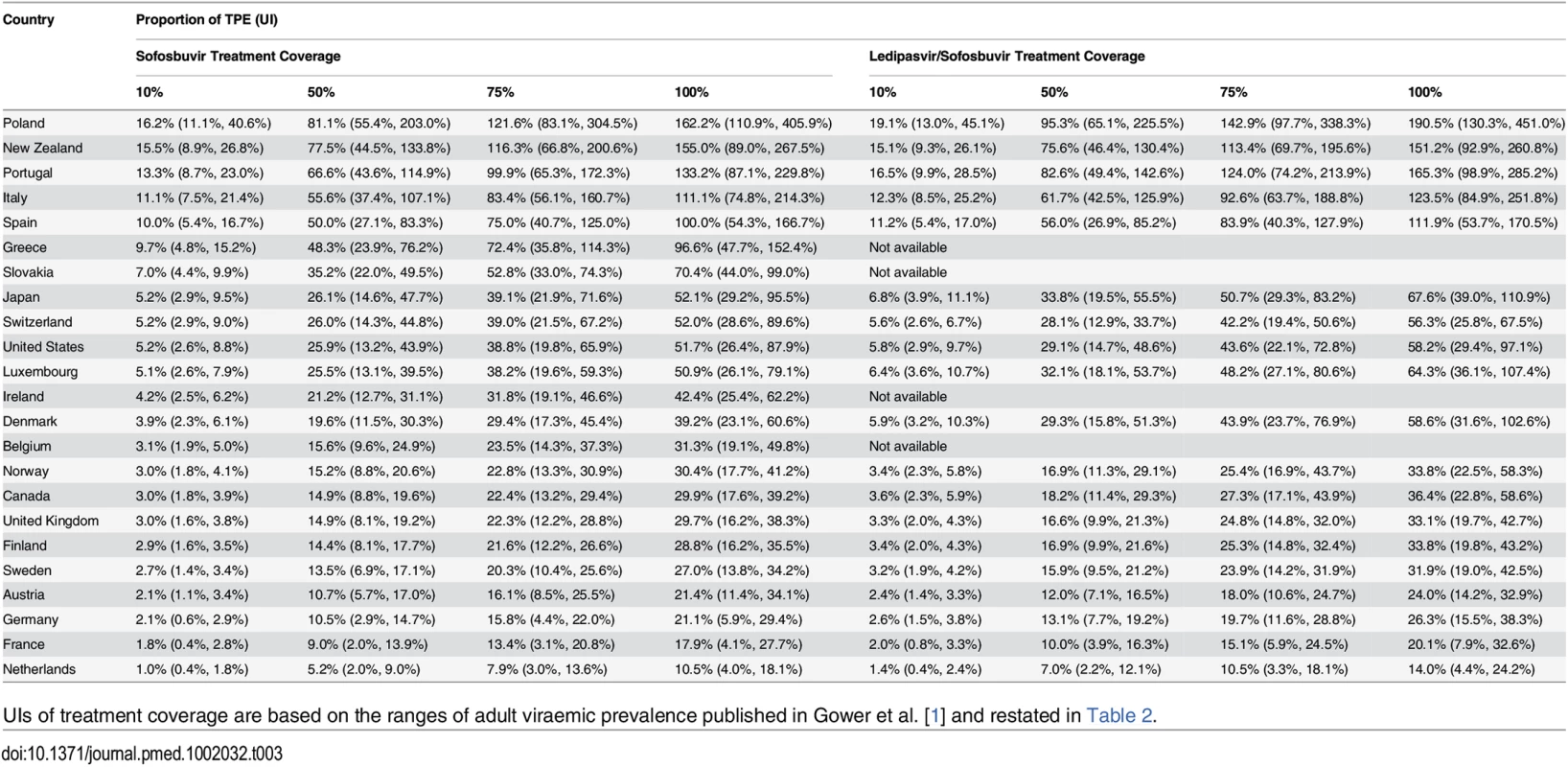
UIs of treatment coverage are based on the ranges of adult viraemic prevalence published in Gower et al. [1] and restated in Table 2. Financial Impact for Individuals
The PPP-adjusted price of a full course of sofosbuvir alone would be equivalent to at least 1 y (365 d) of the PPP-adjusted average earnings for individuals in 12 of the 30 countries analysed (Fig 4A). In Poland, Slovakia, Portugal, and Turkey, a course of sofosbuvir alone would cost at least 2 y of average annual wages. This analysis is conservative because prices were ex-factory prices with an assumed 23% price reduction, and did not include supply chain mark-ups and other costs such as the cost of diagnosis, daclatasvir, ribavirin, and health service costs. We also assumed that all wages were disposable for purchasing these medicines. Assuming no price reduction, a HCV patient in Poland would have to spend 5.55 y and 6.52 y of earnings on a 12-wk course of treatment with sofosbuvir and ledipasvir/sofosbuvir, respectively. Similarly, in ten of the 21 countries where annual wage data and ledipasvir/sofosbuvir prices were available, a person who earned an average wage would need at least 1 y of income to afford a course of ledipasvir/sofosbuvir if no subsidy were offered (Fig 4B). Due to the availability of tiered prices for ledipasvir/sofosbuvir, less than 1 y’s median wage earnings are required in Egypt to pay for treatment. The duration of time that an individual would need to work to pay for a course of treatment out of pocket would be higher with less conservative assumptions.
Fig. 4. Duration of time an individual would need to work to pay for 12 wk of treatment with the hepatitis C medicines sofosbuvir and ledipasvir/sofosbuvir. 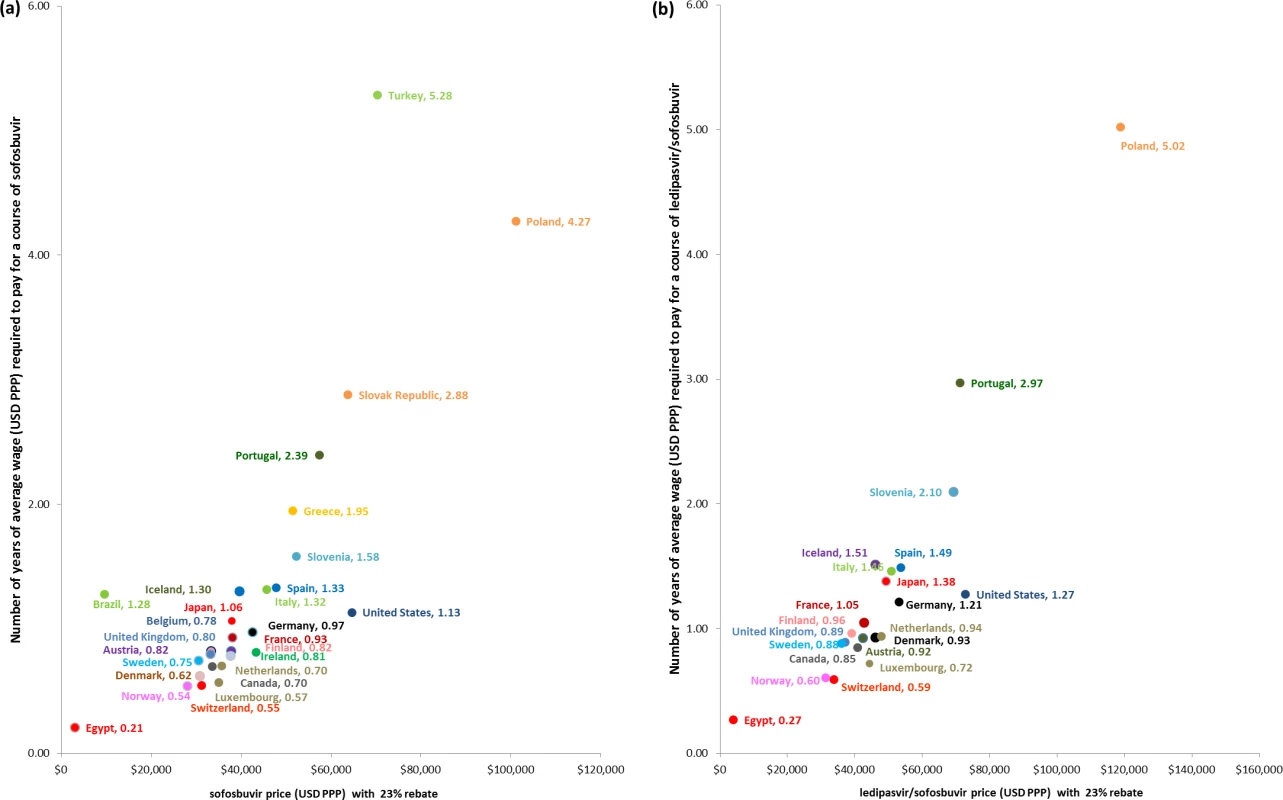
Fig 4 shows duration of time that an individual would need to work to obtain sufficient income to pay for 12 wk of treatment with (A) sofosbuvir and (B) ledipasvir/sofosbuvir. Average annual wage—from OECD average annual wage or, in the case of Brazil, Egypt, Iceland, and Turkey, International Labour Organization Global Wage Database median nominal monthly earnings—is adjusted for PPP (USD PPP). The duration of working time, expressed in years, required by patients to pay for a 12-wk course of treatment for each country is calculated from the PPP dollar price of the treatment and the average wage. The price of treatment is discounted by 23% in all countries except Mongolia, Brazil, Egypt, and India, as they have special pricing arrangements and are not expected to receive additional discounts. Sensitivity Analyses
S2 Text lists the findings of univariate sensitivity analyses assuming 0% and 50% rebates on the prices of sofosbuvir and ledipasvir/sofosbuvir. Based on these assumptions, the estimated percentages of current TPE that would be required for countries to provide treatment for different percentages of the HCV-infected population were within the corresponding range of the values presented in Table 3. The estimated number of years individuals would need to work to pay for a full course of treatment using the minimum wage is an average of 1.55-fold (SA range: 0.8; 2.7) longer compared to the base case findings using average/median wage.
S3 Text shows the findings assuming that patients with genotype 3 HCV would receive the recommended 24 wk of treatment with sofosbuvir. Under this scenario, the estimated percentages of current TPE at different levels of treatment coverage and the number of equivalent income years required to pay for full treatment increased by an average of 1.26-fold (SA range: 1.00; 1.54) compared to the base case estimates.
Discussion
Our analyses show significant price variation for sofosbuvir and ledipasvir/sofosbuvir across countries, especially when accounting for local purchasing power. The lowest and highest nominal prices of sofosbuvir and ledipasvir/sofosbuvir in OECD countries varied, respectively, by 1.71 times between Japan and the US and 1.68 times between the United Kingdom and the US. If the prices in LMICs under tiered pricing arrangements or licensing agreements are included, the prices vary by more than 100-fold. Our analysis also shows that the PPP-adjusted prices of these medicines in Central and Eastern European countries are considerably and consistently higher than in other OECD countries, particularly compared to Nordic countries. Countries that benefit from tiered pricing arrangements and are included in licensing agreements (such as Egypt, Mongolia, and India) have lower prices, which are more affordable compared to their average/median wages. Assuming minimal transaction costs and trade barriers, these are substantial price disparities for goods that are identical in all markets.
We are aware of four recent publications that have also analysed the budget impact and price variation of new HCV medicines [22,46–48]. The studies of budget impact [46–48] were specific to the US and Ireland only, whereas our analysis provides budget impact estimates for a significantly larger sample of countries. Our estimates of budget impact for the US are comparable to those in these studies, given the difference in populations used in each study. Médecins Sans Frontières (MSF) undertook a survey on the prices of six DAAs, including sofosbuvir and ledipasvir/sofosbuvir [22], although our study differs from the MSF study in a number of ways. Our analysis includes a larger range of high-income countries, while the MSF study included a larger range of LMICs. The MSF study obtained price data from key informants, while we obtained our price information from publicly accessible sources. We also obtained data in local currency and then adjusted for exchange rate, purchasing power, and potential price reductions using consistent methods. However, the MSF study found similar variability in prices among high-income countries, “with little correlation between drug prices and gross national income” [22]. Additionally, price disparities in relation to national wealth indicators also occurred among LMICs. The prices of sofosbuvir and ledipasvir/sofosbuvir were higher in Malaysia than in some high-income countries, and the originator version of sofosbuvir in India was less expensive than generic sofosbuvir in Côte d’Ivoire. The MSF study also found that the availability of most DAAs in low-income countries was low at the time of the survey, with the exception of sofosbuvir. For countries included in both studies, foreign exchange rates, time of data collection, and differences in sources are the primary reasons for the differences in prices. Notwithstanding these differences, we concur with the MSF authors in noting that having access to updated and reliable information on prices is essential to allow decision-makers to negotiate prices.
The price disparities in OECD countries may be explained partly by the pharmaceutical price-setting policies used in different countries. For example, some countries set prices according to explicit cost-effectiveness thresholds based on GDP per capita [49], average monthly wage [50], or comparative assessment [51] against interferon-based therapy. These thresholds signal the purchasers’ willingness to pay and may result in the highest possible price that satisfies the threshold, without consideration of budget impact. Another policy for price setting used by many OECD countries is external reference pricing. This is the practice of using the prices of a medicine in one or several countries in order to derive a benchmark, or reference, price for the purpose of setting or negotiating the price. However, the methods used may not explicitly incorporate local purchasing power and status of economic development. For example, the reference countries used by Turkey are France, Greece, Portugal, and Spain, which are selected on the basis of similar “product variety, communicable and common diseases, population and age distribution, and health/disease status” [52,53]. The nominal price of sofosbuvir in Turkey, assuming a 23% price reduction, was lower (US$38,518) than in its reference countries—France, Greece, Spain, and Portugal (ranging from US$41,885 to US$44,731). However, the PPP-adjusted price of sofosbuvir in Turkey (PPP$70,331) was 1.8 times higher than the price in France (PPP$38,077). Turkey’s GDP per capita (US$19,363) is more comparable to Brazil (US$16,320) than its current reference countries, but the nominal and PPP-adjusted prices of sofosbuvir in Turkey were 5.6 and 7.2 times higher, respectively, than the prices in Brazil. Similarly, Slovenia, Poland, and Slovakia use reference prices from countries with mostly richer economies. In the case of sofosbuvir, the nominal price in Norway is comparable to that in most of the other countries it references (i.e., Austria, Belgium, Germany, Denmark, Finland, Ireland, Netherlands, Sweden, and the UK). However, because Norway has greater purchasing power, PPP-adjusted prices indicate that the medicine may be more affordable for Norwegians in comparison to other countries in the OECD. This highlights the challenges of external reference pricing, which include the need to select comparable countries and to consider local purchasing power during price negotiation. There may also be differences between list prices and actual prices that may be hidden due to confidentiality agreements.
Our analysis suggests that sofosbuvir and ledipasvir/sofosbuvir are not “affordable” for most OECD countries at the nominal and PPP-adjusted prices, with Central and Eastern European countries being the most affected. While determining what is affordable or not is a value judgement, funding these treatments in these national health systems would consume large proportions of their TPE and increase pressure on existing budgets. We calculated that even funding treatment for only 10% of the potential population requiring sofosbuvir treatment would amount to at least 1% (UI: 1%, 16.2%) of current TPE in all countries analysed. However, treating only 10% of the infected population is unlikely to be ethically defensible or acceptable to the patient community. The cost of treatment increases substantially if treatment uptake is higher than 10%: if half of the eligible patient population is treated, five countries would spend an amount equivalent to more than half of their current TPE on sofosbuvir. Thus, the potential total cost of treatment presents a dilemma for payers and physicians, with some systems currently restricting access to these medicines to small groups of patients, despite the fact that almost all patients with chronic HCV infection are likely to benefit [17].
Where patients do not have access to subsidised treatment, individuals are unlikely to be able to pay for the medicines out of pocket. Based on ex-factory prices, the price of a 12-wk course of sofosbuvir would be equivalent to at least 1 y of income for the average income earner in 12 of the 30 countries analysed. It is not surprising that, given the price differences, HCV patients in high-income countries have been reported to import sofosbuvir at lower prices or even devise plans to receive treatment in India [54,55]. Assuming a 23% price reduction of nominal prices in both countries, consumers in the US pay US$64,680 for 12 wk of treatment, but if they instead obtained 12 wk of treatment of sofosbuvir in India, inclusive of airfare, hotels, and travel insurance (based on searches in common travel sites), they would pay only approximately US$6,000–US$7,000–10% of the price paid in the US.
Our analysis is limited by the accuracy of the estimates of the numbers of people infected and of the price information that was accessible. We have also not included all likely costs, such as the costs of combination treatment with ribavirin, other health care services, and increases in the duration of treatment in patients with cirrhosis; thus, our budget impact estimates are underestimates of the cost of treatment. We are also aware that in some countries, the prices are probably lower than the publicly accessible prices because of confidential discounts or rebates negotiated with the manufacturer. To minimise overestimation of price and budgetary impact, we made a conservative assumption that all countries, except for countries with special pricing arrangements, had the same price reduction as two of the largest payers—the US Centers for Medicare & Medicaid Services and the US Department of Veterans Affairs. However, neither this price reduction nor the sensitivity analysis using a 50% price reduction changes our overall conclusions about total expenditure on these HCV drugs in relation to TPE. There are also different types of discounts being offered that we have not included, such as rebate schemes that provide the medicines for free after 12-wk of treatment. We also did not attempt to adjust the estimates for pricing agreements based on treating only subgroups of patients. For example, in Portugal, the government has agreed to pay US$28,287 per patient treated irrespective of the duration of treatment. However, this arrangement is limited to less than 10% of the total eligible patient population over 3 y [56,57].
We have analysed the current situation for medicines for hepatitis C, but they are not the only group of medicines where high prices have affected or are affecting patient access. When the antiretrovirals were first launched for HIV, there were similar problems with price and affordability, due to the high price of medicines and the burden of disease. A combination of strategies and interventions—including global purchasing mechanisms, use of voluntary licensing agreements, compulsory licenses, price reduction through tiered pricing, and the rapid development of many high-quality generic products—contributed to improving the situation [58,59]. However, an analysis by Wirtz et al. [60] found persisting and substantial variability in antiretroviral prices in Latin American and Caribbean countries. They suggested that those middle-income countries with comparatively higher prices could afford to treat more patients if prices were lower. They also suggested the need to ensure effective procurement and price negotiation by procurement agencies. Currently, however, there is no global procurement or funding mechanism for hepatitis C medicines. This has limited the potential economies of scale that could be achieved by large-scale generic production. Existing licensing agreements, similar to those that are in place for antiretrovirals, exclude the upper-middle income and OECD countries that are currently paying the highest prices. Perhaps in contrast to the HIV epidemic, the burden of disease due to hepatitis C is more evenly distributed across countries, and the expectations about the outcome of treatment—a cure—are much higher, leading to much greater demand and expectations for access. The argument raised by Wirtz et al. [60] about the need for effective negotiation of prices therefore applies particularly to the medicines for hepatitis C, as there are still not enough alternative treatment options to allow for effective competition.
Setting prices and effective price negotiation is complex. Ramani and Urias [61] used economic game theory to evaluate when compulsory licenses can be effective in price negotiation in developing countries and emphasised the impact that having complete information (or not) about the product can have on negotiation outcomes. Purchasers usually do not have complete information on pharmaceutical products when they set prices. In particular, they usually do not have information about the costs of production or costs of research and development that have been claimed to contribute to the price set by manufacturers [61]. In the case of hepatitis C medicines, the confidential agreements on prices also make it difficult to compare prices accurately across countries, should a purchaser wish to define a “fair price”, for example, by adjusting prices by PPP. Differential or tiered pricing based on the national wealth of countries has been suggested as an approach to setting fair prices, but, to date, there has been no agreement on how to set the tiers [62].
The World Health Organization currently recommends that all patients with chronic HCV should be assessed for treatment [33], but the challenge is clearly how to provide treatment at a total cost that health systems and patients can afford. We had to exclude several countries from the analysis due to the unavailability of price data because, in most cases, the medicines were not publicly funded or not marketed at all. Moreover, as illustrated in our analysis, affordable prices could not be achieved in many OECD countries, even if they have price control systems, which suggests a need for an updated pricing system. While generic competition is likely to reduce prices in countries that are included in voluntary licensing agreements or that will issue compulsory licences, the impact of these strategies is unlikely to impact prices in OECD countries. Tiered pricing agreements are in use for these medicines, but are unlikely to be sufficient to increase access to the medicines for all countries. In order for countries to increase investment and minimise the burden of hepatitis C, governments and industry stakeholders will need to jointly develop and implement fairer pricing frameworks that deliver lower and more affordable prices.
Supporting Information
Zdroje
1. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–S57. doi: 10.1016/j.jhep.2014.07.027 25086286
2. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385 : 117–171. doi: 10.1016/S0140-6736(14)61682-2 25530442
3. Feuerstadt P, Bunim AL, Garcia H, Karlitz JJ, Massoumi H, Thosani AJ, et al. Effectiveness of hepatitis C treatment with pegylated interferon and ribavirin in urban minority patients. Hepatology. 2010;51 : 1137–1143. doi: 10.1002/hep.23429 20049907
4. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 Suppl 1):S237–S244. doi: 10.1053/jhep.2002.36810 12407599
5. Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370 : 1993–2001. doi: 10.1056/NEJMoa1316145 24795201
6. Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370 : 1483–1493. doi: 10.1056/NEJMoa1316366 24725238
7. UNITAID. Hepatitis C medicines technology and market landscape. 2015 Feb [cited 20 Oct 2015]. Available: http://www.unitaid.eu/images/marketdynamics/publications/HCV_Meds_Landscape_Feb2015.pdf.
8. Berkrot B, Humor C, Wills K. U.S. health insurers say Gilead hepatitis C drug too costly. Reuters. 2014 May 20 [cited 13 Aug 2015]. Available: http://www.reuters.com/article/2014/05/20/us-insurance-gilead-sciences-drugcosts-idUSKBN0E029U20140520.
9. Bichoupan K, Martel-Laferriere V, Sachs D, Ng M, Schonfeld EA, Pappas A, et al. Costs of telaprevir-based triple therapy for hepatitis C: $189,000 per sustained virological response. Hepatology. 2014;60 : 1187–1195. doi: 10.1002/hep.27340 25065814
10. Ollendorf DA, Tice JA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir for chronic hepatitis C virus infection. JAMA Intern Med. 2014;174 : 1170–1171. doi: 10.1001/jamainternmed.2014.2151 24798321
11. Tice JA, Ollendorf DA, Chahal HS, Kahn JG, Marseille E, Weissberg J, et al. The comparative clinical effectiveness and value of simeprevir and sofosbuvir in the treatment of chronic hepatitis C infection: a technology assessment. 2014 Apr 15 [cited 13 Aug 2015]. Boston: Institute for Clinical and Economic Review. Available: http://icer-review.org/sites/default/files/assessments/CTAF_Hep_C_Apr14_final.pdf.
12. Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58 : 928–936. doi: 10.1093/cid/ciu012 24399087
13. Sachs J. The cure for Gilead. 2015 Aug 3 [cited 13 Aug 2015]. Huffpost Business. Available: http://www.huffingtonpost.com/jeffrey-sachs/the-cure-for-gilead_b_7924300.html.
14. Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12 : 933–980. doi: 10.1016/S1470-2045(11)70141-3 21958503
15. Tefferi A, Kantarjian H, Rajkumar SV, Baker LH, Abkowitz JL, Adamson JW, et al. In support of a patient-driven initiative and petition to lower the high price of cancer drugs. Mayo Clin Proc. 2015;90 : 996–1000. doi: 10.1016/j.mayocp.2015.06.001 26211600
16. Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121 : 4439–4442. doi: 10.1182/blood-2013-03-490003 23620577
17. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163 : 215–223. doi: 10.7326/M15-0406 26120969
18. Burger L. Gilead agrees 41,000 eur hep-C drug price for 12-week treatment. Reuters. 2015 Feb 12 [cited 13 Aug 2015]. Available: http://www.reuters.com/article/2015/02/12/gilead-sciences-hepatitis-germany-idUSL5N0VM4NC20150212.
19. Tyrrell H. Time for new cures—time to list new hepatitis C medicines. Hepatitis Australia. 2015 Sep 24 [cited 21 Oct 2015]. Available: http://static1.squarespace.com/static/50ff0804e4b007d5a9abe0a5/t/560a2f6ee4b0a09442e297ce/1443508078862/Dear_Minister_HCV_meds_2015-09-24_final.pdf.
20. Londeix P. New treatments for hepatitis C virus: strategies for achieving universal access. Médecins du Monde. 2014 Mar [cited 20 Oct 2015]. Available: http://www.hepcoalition.org/IMG/pdf/web_daas_strategies_for_achieving_universal_access_en.pdf.
21. Gilead. Chronic hepatitis C treatment expansion: generic manufacturing for developing countries. 2015 Aug [cited 13 Aug 2015]. Available: http://www.gilead.com/~/media/files/pdfs/other/hcvgenericagreementfactsheet.pdf?la=en.
22. Andrieux-Meyer I, Cohn J, de Araujo ES, Hamid SS. Disparity in market prices for hepatitis C virus direct-acting drugs. Lancet Glob Health. 2015;3:e676–e677. doi: 10.1016/S2214-109X(15)00156-4 26475012
23. Médecins Sans Frontières. Strategies to secure access to generic hepatitis C medicines: overcoming patent and regulatory barriers to secure access to generic hepatitis C medicines. 2015 May [cited 30 Aug 2015]. Available: http://www.msfaccess.org/sites/default/files/MSF_assets/HepC/Docs/HepC_brief_OvercomingbarriersToAccess_ENG_2015.pdf.
24. Organisation for Economic Co-operation and Development. PPPs and exchange rates. OECD National Accounts Statistics. Paris: Organisation for Economic Co-operation and Development; 2015.
25. Callen T. PPP versus the market: which weight matters? Finance Dev. 2007 Mar [cited 1 Aug 2015]. Available: http://www.imf.org/external/pubs/ft/fandd/2007/03/basics.htm.
26. Organisation for Economic Co-operation and Development. Purchasing power parities—frequently asked questions (FAQs). 2015 [cited 1 Aug 2015]. Available: https://www.oecd.org/std/ppp/faq.
27. Vogel F. What is purchasing power parity? World Bank. [cited 12 Jan 2016]. Available: http://siteresources.worldbank.org/ICPINT/Resources/270056-1255977254560/6483625-1338834270350/FVogel_WhatisPurchasingPowerParity.pdf.
28. Lafrance R, Schembri L. Purchasing-power parity: definition, measurement, and interpretation. Bank of Canada Review. 2002 [cited 14 Jan 2016]. Available: http://www.bankofcanada.ca/wp-content/uploads/2010/06/lafrance_e.pdf.
29. Organisation for Economic Co-operation and Development. Level of GDP per capita and productivity. OECD Productivity Database. Paris: Organisation for Economic Co-operation and Development; 2015.
30. Bank World. GDP per capita, PPP (current international $). World Development Indicators. Washington (District of Columbia): World Bank; 2015.
31. Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17 : 107–115. doi: 10.1111/j.1469-0691.2010.03432.x 21091831
32. Grebely J, Prins M, Hellard M, Cox AL, Osburn WO, Lauer G, et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect Dis. 2012;12 : 408–414. doi: 10.1016/S1473-3099(12)70010-5 22541630
33. World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. 2014 Apr [cited 28 Apr 2016]. Available: http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/
34. Organisation for Economic Co-operation and Development. Total expenditure on pharmaceuticals and other medical non-durables, /capita, US$ purchasing power parity. OECD Health Statistics. Paris: Organisation for Economic Co-operation and Development; 2014.
35. Organisation for Economic Co-operation and Development. Historical population data and projections (1950–2050). OECD Demography and Population. Paris: Organisation for Economic Co-operation and Development; 2015.
36. Cunningham EB, Applegate TL, Lloyd AR, Dore GJ, Grebely J. Mixed HCV infection and reinfection in people who inject drugs-impact on therapy. Nat Rev Gastroenterol Hepatol. 2015;12 : 218–230. doi: 10.1038/nrgastro.2015.36 25782091
37. Long C, DeBeck K, Feng C, Montaner J, Wood E, Kerr T. Income level and drug related harm among people who use injection drugs in a Canadian setting. Int J Drug Policy. 2014;25 : 458–464. doi: 10.1016/j.drugpo.2013.11.011 24380808
38. World Health Organization, Health Action International. Measuring medicine prices, availability, affordability and price components. 2nd ed. Geneva: World Health Organization; 2008.
39. Cameron A, Bansal A, Dua T, Hill SR, Moshe SL, Mantel-Teeuwisse AK, et al. Mapping the availability, price, and affordability of antiepileptic drugs in 46 countries. Epilepsia. 2012;53 : 962–969. doi: 10.1111/j.1528-1167.2012.03446.x 22432967
40. Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373 : 240–249. doi: 10.1016/S0140-6736(08)61762-6 19042012
41. Vogler S, Zimmermann N, Habl C, Piessnegger J, Bucsics A. Discounts and rebates granted to public payers for medicines in European countries. South Med Rev. 2012;5 : 38–46. 23093898
42. Pollack A. Sales of Sovaldi, new Gilead hepatitis C drug, soar to $10.3 billion. New York Times. 3 Feb 2015 [cited 13 Aug 2015]. Available: http://www.nytimes.com/2015/02/04/business/sales-of-sovaldi-new-gilead-hepatitis-c-drug-soar-to-10-3-billion.html?_r=0.
43. Organisation for Economic Co-operation and Development. Average annual wages. OECD.Stat. 2016 [cited 18 Jan 2016]. Available: http://stats.oecd.org/Index.aspx?DataSetCode=AV_AN_WAGE.
44. International Labour Organization. Median nominal monthly earnings of employees (local currency). 2016 [cited 18 Jan 2016]. Available: http://www.ilo.org/ilostat/faces/help_home/data_by_subject/subject-details/indicator-details-by-subject;jsessionid=mgRp8W1J3WgwjZj8CEZOYbt2U384qdp_XwWOSIEvaQCMqZeFvAXE!1575150460?subject=&indicator=EAR_MNEE_NOC_NB&datasetCode=&collectionCode=GWR&_afrLoop=886404755919478#%40%3Findicator%3DEAR_MNEE_NOC_NB%26subject%3D%26_afrLoop%3D886404755919478%26datasetCode%3D%26collectionCode%3DGWR%26_adf.ctrl-state%3Dzr7cw9rmh_4.
45. US Department of State. Country reports on human rights practices for 2014. 2014 [cited 13 Aug 2015]. Available:http://www.state.gov/j/drl/rls/hrrpt/2014humanrightsreport/index.htm#wrapper.
46. Nguyen JT, Rich JD, Brockmann BW, Vohr F, Spaulding A, Montague BT. a budget impact analysis of newly available hepatitis C therapeutics and the financial burden on a state correctional system. J Urban Health. 2015;92 : 635–649. doi: 10.1007/s11524-015-9953-4 25828149
47. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162 : 397–406. doi: 10.7326/M14-1336 25775312
48. Kieran J, Bennett K, Coghlan M, Bergin C, Barry M. The budget impact of hepatitis C treatment in Ireland 2001–2012. Ir Med J. 2015;108 : 166–169. 26182797
49. Jakubiak-Lasocka J, Jakubczyk M. Cost-effectiveness versus cost-utility analyses: what are the motives behind using each and how do their results differ?—A Polish example. Value Health Reg Issues. 2014;4 : 66–74. doi: 10.1016/j.vhri.2014.06.008
50. Skoupá J, Annemans L, Hájek P. Health economic data requirements and availability in the European Union: results of a survey among 10 European countries. Value Health Reg Issues. 2014;4 : 53–57. doi: 10.1016/j.vhri.2014.06.003
51. Leopold C, Vogler S, Mantel-Teeuwisse AK, de Joncheere K, Leufkens HG, Laing R. Differences in external price referencing in Europe: a descriptive overview. Health Policy. 2012;104 : 50–60. doi: 10.1016/j.healthpol.2011.09.008 22014843
52. Ministry of Health of Turkey. Beşeri İlaçların Fiyatlandırılmasına Dair Kararda Değişiklik Yapılması Hakkında Karar. Ankara: Ministry of Health of Turkey; 2010.
53. Koçkaya G, Kiliç P. Pharmaceutical policies and market access in Turkey. ISPOR Connections. 2012;18 : 8–10.
54. Pettypiece S, Gokhale K. US hepatitis C patients travel to India for cheaper Sovaldi versions. Live Mint. 2015 Jun 2 [cited 28 Aug 2015]. Available: http://www.livemint.com/Politics/B1zuQ1onYrhT9IXz0U9HzK/US-patients-get-extreme-to-obtain-Hepatitis-drug-thats-1-t.html.
55. Atkin M, Keep J. Hepatitis C sufferer imports life-saving drugs from India, takes on global pharmaceutical company. Australian Broadcasting Company. 2015 Aug 20 [cited 28 Aug 2015]. Available: http://www.abc.net.au/news/2015-08-20/hepatitis-c-sufferer-imports-life-saving-drugs-from-india/6712990.
56. Borja-Santos R. Hepatite C: acordo prevê que farmacêutica só será paga se doentes ficarem curados. Publico. 2015 Feb 6 [cited 13 Aug 2015]. Available: http://www.publico.pt/sociedade/noticia/hepatite-c-acordo-sobre-medicamento-preve-pagar-por-doente-tratado-1685250?page=-1.
57. Sedge M. Governo só pagará à Gilead por doentes curados. Observador. 2015 Feb 6 [cited 13 Aug 2015]. Available: http://observador.pt/2015/02/06/paulo-macedo-fizemos-o-melhor-acordo-de-todos/.
58. Hoen E, Berger J, Calmy A, Moon S. Driving a decade of change: HIV/AIDS, patents and access to medicines for all. J Int AIDS Soc. 2011;14 : 15. doi: 10.1186/1758-2652-14-15 21439089
59. Waning B, Diedrichsen E, Moon S. A lifeline to treatment: the role of Indian generic manufacturers in supplying antiretroviral medicines to developing countries. J Int AIDS Soc. 2010;13 : 35. doi: 10.1186/1758-2652-13-35 20840741
60. Wirtz V, Santa-Ana-Tellez Y, Trout C, Kaplan W. Allocating scarce financial resources for HIV treatment: benchmarking prices of antiretroviral medicines in Latin America. Health Policy Plan. 2012;27 : 638–648. doi: 10.1093/heapol/czs011 22367770
61. Ramani S, Urias E. Access to critical medicines: when are compulsory licenses effective in price negotiations? Soc Sci Med. 2015;135 : 75–83. doi: 10.1016/j.socscimed.2015.04.023 25957163
62. Towse A, Pistollato M, Mestre-Ferrandiz J, Khan Z, Kaura S, Garrison L. European Union pharmaceutical markets: a case for differential pricing? Int J Econ Business. 2015;22 : 263–275.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 5- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study
- Steroid-Based Therapy and Risk of Infectious Complications
- How Much Can the USA Reduce Health Care Costs by Reducing Smoking?
- Interpreting the Global Enteric Multicenter Study (GEMS) Findings on Sanitation, Hygiene, and Diarrhea
- Health Research and the World Humanitarian Summit—Not a Thousand Miles Apart
- A Public Health Paradox: The Women Most Vulnerable to Malaria Are the Least Protected
- Toward a Common Secure Future: Four Global Commissions in the Wake of Ebola
- The Clinical Challenge of Sepsis Identification and Monitoring
- All-Cause Mortality of Low Birthweight Infants in Infancy, Childhood, and Adolescence: Population Study of England and Wales
- Smoking Behavior and Healthcare Expenditure in the United States, 1992–2009: Panel Data Estimates
- Estimating the Risk of Chronic Pain: Development and Validation of a Prognostic Model (PICKUP) for Patients with Acute Low Back Pain
- Initiating Antiretroviral Therapy for HIV at a Patient’s First Clinic Visit: The RapIT Randomized Controlled Trial
- Prioritizing Surgical Care on National Health Agendas: A Qualitative Case Study of Papua New Guinea, Uganda, and Sierra Leone
- Effectiveness of and Financial Returns to Voluntary Medical Male Circumcision for HIV Prevention in South Africa: An Incremental Cost-Effectiveness Analysis
- Risk of Advanced Neoplasia in First-Degree Relatives with Colorectal Cancer: A Large Multicenter Cross-Sectional Study
- Common Infections in Patients Prescribed Systemic Glucocorticoids in Primary Care: A Population-Based Cohort Study
- Sanitation and Hygiene-Specific Risk Factors for Moderate-to-Severe Diarrhea in Young Children in the Global Enteric Multicenter Study, 2007–2011: Case-Control Study
- A Revolution in Treatment for Hepatitis C Infection: Mitigating the Budgetary Impact
- Nondisclosure of Financial Interest in Clinical Practice Guideline Development: An Intractable Problem?
- Financial Relationships between Organizations That Produce Clinical Practice Guidelines and the Biomedical Industry: A Cross-Sectional Study
- Prices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Estimating the Risk of Chronic Pain: Development and Validation of a Prognostic Model (PICKUP) for Patients with Acute Low Back Pain
- Prioritizing Surgical Care on National Health Agendas: A Qualitative Case Study of Papua New Guinea, Uganda, and Sierra Leone
- A Revolution in Treatment for Hepatitis C Infection: Mitigating the Budgetary Impact
- Toward a Common Secure Future: Four Global Commissions in the Wake of Ebola
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



