-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRisk of Adverse Pregnancy Outcomes among Women Practicing Poor Sanitation in Rural India: A Population-Based Prospective Cohort Study
Pinaki Panigrahi and colleagues examine the association between adverse pregnancy outcomes and sanitation practices in pregnant women in rural India.
Published in the journal: . PLoS Med 12(7): e32767. doi:10.1371/journal.pmed.1001851
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001851Summary
Pinaki Panigrahi and colleagues examine the association between adverse pregnancy outcomes and sanitation practices in pregnant women in rural India.
Introduction
The burden of adverse pregnancy outcomes (APOs), which includes both preterm births and low birth weights [1,2], is substantial in both developed and developing countries [1–3]. More than 60% of preterm births take place in south Asia and sub-Saharan Africa [3]. A recent study estimated that 12.8 million babies were born small for gestational age in India alone in the year 2010, a prevalence of 47% of all births [1]. Preterm birth and low birth weight are critical determinants of child survival, disabilities, stunting, and long-term adverse consequences for the onset of non-communicable diseases in the life course and demand appropriate public health interventions [1,4]. Despite India’s impressive economic growth in the last two decades, access to improved sanitation services in rural and vulnerable communities is extremely limited.
The World Health Organization (WHO) defines a birth weight of <2,500 g as low birth weight and a delivery before 37 completed weeks of gestation as preterm birth [5]. We adopted the WHO guidelines that define an APO as an event of low birth weight, preterm birth, stillbirth, or abortion. APO is a complex, multifactorial, physiological outcome in women, and despite decades of research, a clear causal mechanism for APOs has not been established. Studies have reported numerous risk factors for APOs such as malaria [6], infection [7–12], anaemia [13–16], obesity [17], hypertension [18], hyperglycaemia [19], diabetes [20], periodontal disease [21], endometriosis [22], history of abortion [23], antenatal complications [24], antenatal care (ANC) [24], environmental pollution [25–29], violence [30], and other socio-economic disparities [31–33]. In many low - and middle-income countries, access to improved sanitation facilities is limited, but the link between sanitation and APOs has not been explored.
The WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation (JMP) defines an improved sanitation facility as a facility that hygienically separates human excreta from human contact, such as a flush toilet, piped sewer system, septic tank, flush/pour flush to pit latrine, ventilated improved pit latrine, pit latrine with slab, or composting toilet [34]. Similarly, the JMP defines unimproved sanitation facilities as flush/pour flush to elsewhere, pit latrine without slab, bucket, hanging toilet or hanging latrine, no facilities, or bush or field. Globally, 1.1 billion people still practices open defecation, of which 638 million are in India [34]. Poor sanitation, alongside unsafe drinking water and hygiene, are responsible for a considerable proportion of the global burden of disease [35,36]. Water, sanitation, and hygiene (WASH) interventions have been linked to improvements in a number of important health outcomes, including diarrhoeal diseases [37], helminth infections [38,39], and childhood stunting [40,41]. Recent studies in India have failed to show that programmes to improve sanitation in India lead to health gains in children aged under 5 y, although critical in these findings were the low levels of improved sanitation use among the population [42,43]. Although little work has been done to evaluate the effects of WASH interventions on APOs, a recent review identified over 60 biological and social mechanisms linking poor WASH practices to various maternal and reproductive health outcomes [44].
At least some of these identified plausible mechanisms linking open defecation to APOs are supported by existing evidence. For example, there is good evidence that poor sanitation can promote hookworm infestation [39], which is a risk factor for maternal anaemia [10,45,46], which, in turn, is directly linked to APOs [16,47]. In India, the prevalence of anaemia and chronic energy deficiency (measured as low body mass index [BMI]) in women aged 15–49 y is as high as 55.3% and 35.6%, respectively [48,49].
Exposure to unsafe water, unimproved sanitation, and poor waste management during pregnancy may increase the risk of infection, causing downstream effects such as low birth weight and preterm delivery. A recent systematic review [50] and a conceptual framework [44] concluded that a lack of improved sanitation facilities appears to be associated with maternal mortality, and highlighted the paucity of primary studies assessing the impact of water and sanitation practices on pregnancy outcomes [44,50].
To the best of our knowledge, this is the first rigorous attempt to quantify the risk of APOs with access to improved sanitation and practice using a population-based cohort, with specific aims to quantify the prevalence of open defecation among pregnant women and its association with APOs.
Methods
Ethical Approval
Ethical approval for the study was obtained from the Ethical Review Committee of the Asian Institute of Public Health(Bhubaneswar, India), and the Institutional Review Board at Emory University (Atlanta, Georgia). Written informed consent to participate in the study was obtained from each study participant at the time of recruitment. Participants were informed about the purpose of the study, and that they were free to withdraw from the study at any point. The survey team also received cultural competency and confidentiality training from a qualified trainer.
Study Settings and Participants
The state of Odisha, home to 41.9 million people, has one of the highest infant mortality rates (53 per 1,000 live births) and maternal mortality rates (235 per 100,000 women of reproductive age) in India [51]. Odisha faces a number of serious challenges: frequent natural disasters, high levels of unemployment, and over 40% of the population living below the poverty line [51]. In 2011, only 18.2% of households had access to an improved latrine, and more than 75% of households practiced open defecation [52].
Since improved sanitation access and uses are associated with class, caste, and geography [53,54], we attempted to include a diverse and representative sample by including two geographically predominant and distinct areas of Odisha state (S1 Fig). Lathikata and Kuarmunda, the administrative revenue blocks of Sundargarh, a northwest inland tribal district, and Balianta and Balipatana, the revenue blocks of Khurda, a typical rural district located on the east coast of the country, were chosen for this study. Villages in the inland tribal populations (Sundargarh study setting) are spread over hilly and mining areas where communities follow their traditional lifestyle with minimal outside interaction. In contrast, inhabitants of the coastal rural population (Khurda study setting) depend on irrigated agriculture, farming, and small-scale business. Some individuals work in government offices and other small service industries. Individuals in the Khurda study setting are relatively more affluent and live in densely populated villages that are close to each other.
For the current study, we utilised an existing population-based surveillance cohort with a combined population of 360,000, with approximately 60,000 married women of reproductive age (13–49 y). This cohort was established for the recently completed Aetiology of Neonatal Infection in South Asia (ANISA) study [55], where all pregnancies were being tracked and recorded using the GPS coordinates of the mothers’ homes. A small subset of pregnant women was randomly chosen from this existing cohort for the current study. We trained our community health volunteers (CHVs), women from the same villages as the participants, to recruit eligible pregnant women in our study. Pregnant women (10–12 wk of gestation) who were residents of the locality, who were between 18 and 48 y of age, and who provided informed consent were eligible to participate in the study. We followed a three-tiered monitoring and reporting system. Each study setting (Sundargarh and Khurda) had one CHV responsible for following 4–5 pregnancies, supervised by an area coordinator (one per ten CHVs), who reported to a programme manager. Our CHVs worked very closely with government personnel, including Anganwadi workers and Accredited Social Health Activists, of the study settings.
Study Design
We conducted a population-based prospective cohort study. Assuming a 20.0% prevalence of APOs [24,56–58], we calculated that the sample size needed to be 582 to detect a relative risk (the difference in incidence of APOs between women with and without improved sanitation) of 1.5 with 95% confidence at 80% power. All pregnant women satisfying the eligibility criteria in the study population were recruited into the study. Estimating an anticipated 15% dropout rate, our final target sample size was 670. There were 708 eligible pregnant women in the two study settings (coastal and inland). A random number generator selected the 670 geocoded households/women who were enrolled through a household visit by the CHV. A schematic diagram of the study design is shown in Fig 1. Baseline assessment at recruitment and three home visits (one in the second trimester and two in the third trimester) were designed to ensure retention and learn about study outcomes during the course of pregnancy, followed by a home visit at birth to document pregnancy outcomes. Sanitation exposures measured at baseline were used to estimate the risks of pregnancy outcomes.
Fig. 1. Study design and sampling scheme. 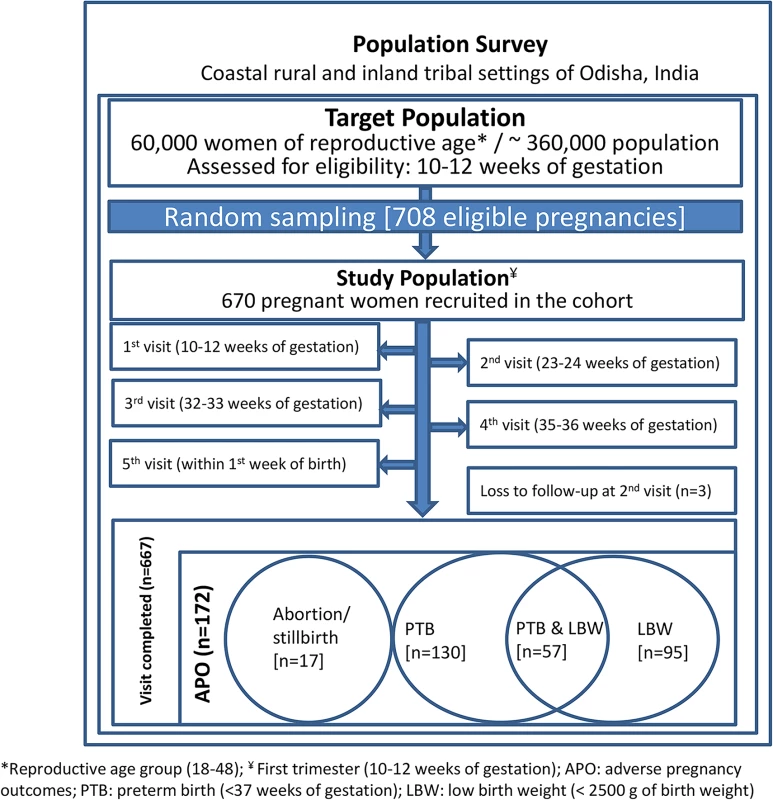
Exposure Measures
The survey instruments (questionnaires and observation checklists) were developed in three stages. First, a preliminary survey questionnaire was developed after literature review and inputs from key stakeholders. Second, a draft sanitation exposure assessment questionnaire was developed through focus group discussions with selected pregnant women and key informants in the community. Third, a pre-test was conducted using the preliminary questionnaire in nearby non-study villages to prevent contamination. After triangulation, the main survey administered by the trained CHVs addressed specific questions on sanitation and hygiene practices and conditions during recruitment. Visual observations of defecation sites were performed to confirm the interview response. Where a latrine was used, observational checklists were also used to inspect for the presence of a functional water source or water storage container at the latrine, type of latrine, visible faecal contamination on latrine floors, and presence of a hand-washing station with soap, detergent, or ash at or near the toilet. Other information, such as hand-washing practice after defecation and source of bathing water, was also collected. We defined “poor sanitation” according the JMP criteria of unimproved sanitation facilities and latrine use behaviour [34].
Outcome Measures
We used the WHO definitions for all outcome measures [5]. The primary outcome of interest was incidence of an APO, defined as an event of preterm birth, low birth weight, spontaneous abortion, or stillbirth [5]. Infant demographics such as gestational age and birth weight were abstracted from medical charts at delivery by the CHV. Gestational age was ascertained by the dating method from the last menstrual period. Our female field personnel were extensively trained with the lunar calendar (used by the women in Odisha state) and how to record the first day of the last menstrual period. In our study settings, all of the deliveries were conducted at health centres by a qualified health care service provider.
Potential Covariates and Confounders
Household socio-demographic information, including maternal age at enrolment, education, religion, caste, and previous pregnancy history, was collected by CHVs using a structured instrument in face-to-face interviews. Since most of the pregnant women had limited knowledge on their family’s monthly income, we derived an indirect measure of household economic information from household characteristics and asset data [59] obtained at enrolment. Household characteristics included type of house, household electrification, drinking water source, cooking fuel, light source for household, number of rooms used for sleeping, ownership of agricultural land, whether agricultural land was irrigated or not, ownership of business establishments, and household assets included radios, televisions, fans, mobile telephones, refrigerators, bicycles, motorcycles/scooters, and cars. During the enrolment visit, the height and weight of the participants were measured using standard protocols and digital weighing machines calibrated in the field study office every morning. Relevant data on haemoglobin (Hb) was obtained from the Mother & Child Tracking System card (issued by the Ministry of Health and Family Welfare to pregnant women) of the participants. For women for whom this information was unavailable, a study supervisor conducted the haemoglobin estimation from finger prick blood samples using a portable haemoglobin analyzer (HemoCue Hb 301). Pregnant women’s ANC coverage at birth was also recorded.
Data Collection and Confidentiality
The survey instruments, including the informed consent document, were translated into the local language (Odiya) and administered by the trained female CHVs. A unique study ID was assigned to each participant and used subsequently on all study forms. Surveys were administered inside the home of the participants, and all study forms, including the informed consent document, were transported by supervisors to a field office and then on to the study hospitals for storage in secure, locked metal cabinets. All personal identifiers were removed from the dataset and were kept along with the informed consent documents securely under the custody of the principal investigator. Only trained personnel had access to the rest of the survey documents for data entry and management.
Data Quality Assurance
The survey instruments were piloted in villages from similar settings that were not included in the study. The quality of the data collected by the CHVs was ensured through direct supervision by respective field supervisors and subsequently by the programme manager. Supervisory visits and standardisation exercise sessions were organised routinely to ensure the quality of the data collected. Every reported outcome of interest was confirmed by a repeat visit to the household by supervisory staff. CHVs submitted data forms to their supervisor, who checked the forms for completeness and consistency. Double entry was done for all study forms into a custom-designed database management interface using the EpiInfo platform. The quality of the primary outcome measures (gestational age and birth weight) was ensured through a quality indicator of high gestational age at birth (i.e., ≥44 wk); all birth weights were abstracted from medical charts at delivery. The quality of key exposure data (latrine access and use) was crosschecked with observational data supporting use of latrine or open defecation. For analysis and reporting purposes, all of the dataset passed the above quality assurance measures.
Statistical Analysis
We conducted cross-tabulation to explore frequencies and bivariate associations between key independent variables and the outcomes (APO, preterm birth, and low birth weight). The main independent variable (sanitation) was categorised into access to a latrine (private or neighbour’s) versus open defecation.
We used principal component analysis with varimax rotation for computing a wealth index [59] from the household characteristics and asset data. Based on the distribution of the wealth index, the households were then divided into four groups (quartiles) of socio-economic status: low, lower medium, upper medium, and high (S1 Table).
We estimated unadjusted odds ratios (ORs) and adjusted odds ratios (AORs) of the relationship between improved sanitation access and APOs using logistic regression models. Our predefined analysis using a generalized linear model was changed to logistic regression in response to reviewer requests and because of the types of data included in the model (S1 Text).
We included a priori covariates—such as materials used to wash hands after defecation, source of bathing water, place of residence, maternal age, maternal parity, BMI, maternal haemoglobin, ANC, poverty, educational level, caste, and religion—for APOs that were thought to be important confounding factors on theoretical grounds [60]. The covariate poverty ascertained from below poverty line status was changed to the wealth index at the peer-review stage. Where applicable, we incorporated a categorical/continuous parameterization into the multivariate model to better control for confounders. Data were analysed using STATA (version 13).
Results
Of the 670 women recruited, 667 completed the study, of which 172 (28.2%) experienced APOs (Fig 1). Among these women, 130 (19.4%) had preterm births, 95 (14.2%) gave birth to babies with low birth weight, 11 (1.6%) had spontaneous abortions, and six (0.9%) had stillbirths. Detailed socio-demographic, anthropometric, and clinical characteristics of study participants stratified by APO are shown in Table 1. In our study population, about 85% of the women were 20–29 y-old, 72% had normal BMI (range 18.5–24.9 kg/m2), 36.1% did not have anaemia (Hb ≥ 110 g/l), 35.4% were primiparous, and 15.1% had no formal education (Table 1).
Tab. 1. Pregnant women’s selected socio-demographic, anthropometrics, and clinical characteristics stratified by adverse pregnancy outcome (n = 667). 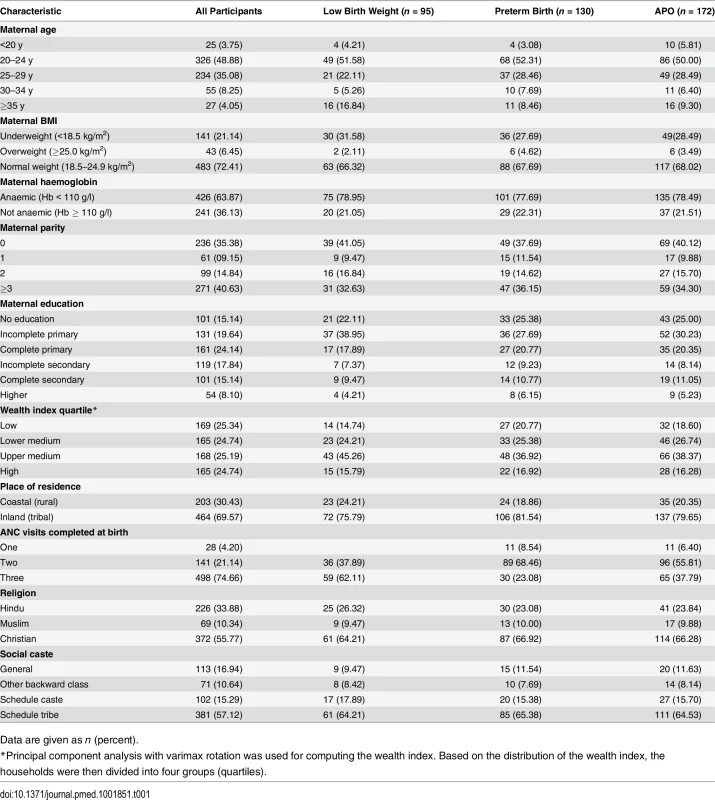
Data are given as n (percent). Table 2 provides the prevalence of sanitation access and practices with unadjusted ORs for APOs. Of the 667 women, 388 (58.2%) had no access to a latrine and reported open defecation at recruitment. About half (45.8%) of the pregnant women living in a household with latrine access used the latrine on a regular basis, and 32% reported rare use of the facility. The majority (72.4%) of the latrine facilities were simple pit latrines. About 60% of the latrines had a water source in or at the latrine, and 21.5% of the latrines had visible faecal contamination on the latrine floor (an indication of poor sanitation practice). About half of the households had a hand-washing station with soap at or near the latrine. In our population, 58% of pregnant women did not wash their hands with soap or detergent after defecation. Only 14.7% of participants in our study used piped water for bathing or body washing.
Tab. 2. Pregnant women’s sanitation access and use with unadjusted odds ratios for adverse pregnancy outcomes. 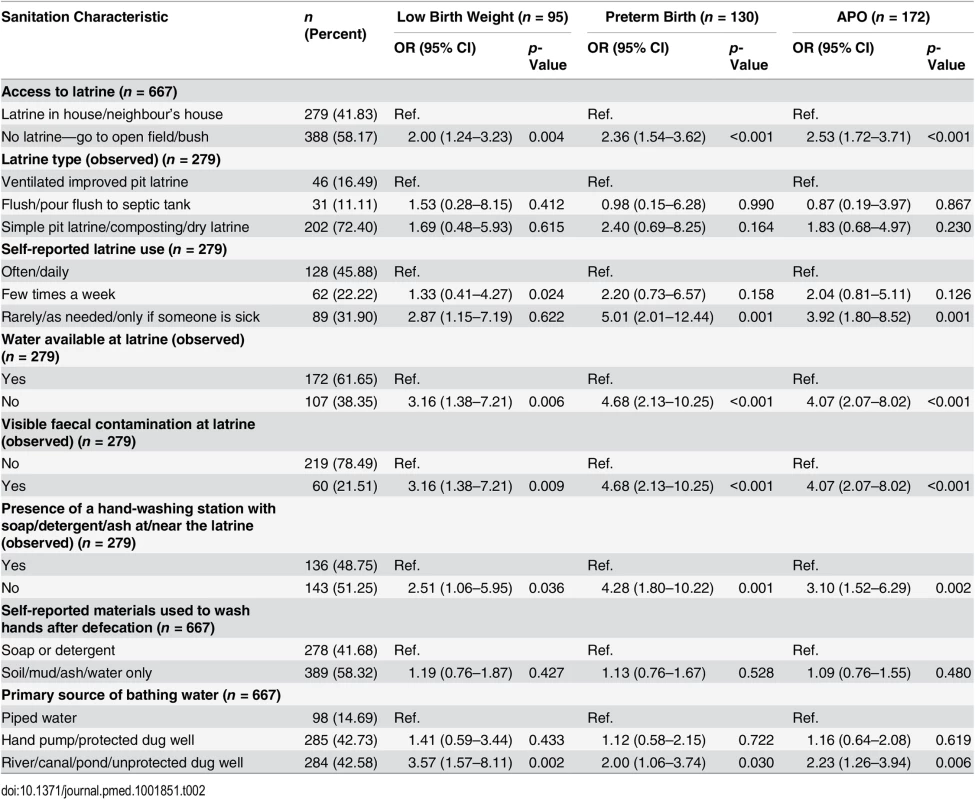
Unadjusted bivariate associations of each of these sanitation factors with APOs are presented in Table 2. Compared to latrine access, open defecation was associated with higher odds of APO (OR: 2.53; 95% CI: 1.72–3.71), preterm birth (OR: 2.36; 95% CI: 1.54–3.62), and low birth weight (OR: 2.00; 95% CI: 1.24–3.23). Risk of APO (OR: 3.92; 95% CI: 1.80–8.52) was also considerably higher for women who used a latrine only occasionally. Water not being available at the latrine was also associated with an increased odds of APO (OR: 4.07; 95% CI: 2.07–8.02). Women who reported bathing or body washing with an open source of water such as a pond, river, or canal also had significantly higher odds of APO (Table 2).
Table 3 shows the results of the multivariable model adjusted for covariates that were selected using a priori criteria, as related to latrine access and APOs. The model estimate in the multivariable analysis for the association of open defecation with APO (AOR: 2.38; 95% CI: 1.49–3.80) was minimally attenuated (Table 3). We also observed that higher wealth index was not associated with a reduction in the odds of APOs (AOR: 0.97; 95% CI: 0.80–1.18); however, higher education was found to be associated with a reduction in the odds of APO (AOR: 0.68; 95% CI: 0.59–0.79). Higher haemoglobin in the first trimester was found to be significantly associated with lower odds of APOs (AOR: 0.50; 95% CI: 0.31–0.81) (Table 3). We further investigated the association between latrine use and APOs among participants with latrine access (Table 4). Our results specifically demonstrate that latrine access alone is not associated with a reduction in the burden of APOs; however, latrine use is. Our model estimated 7-fold higher odds of APOs among pregnant women who had access to a latrine but used it only rarely (AOR: 7.10; 95% CI: 2.18–23.11) compared to women who used a latrine often/daily. The association of poor sanitation practices with APOs was independent of poverty in our study settings.
Tab. 3. Multivariable adjusted models for the association between sanitation characteristics and adverse pregnancy outcomes (n = 667). 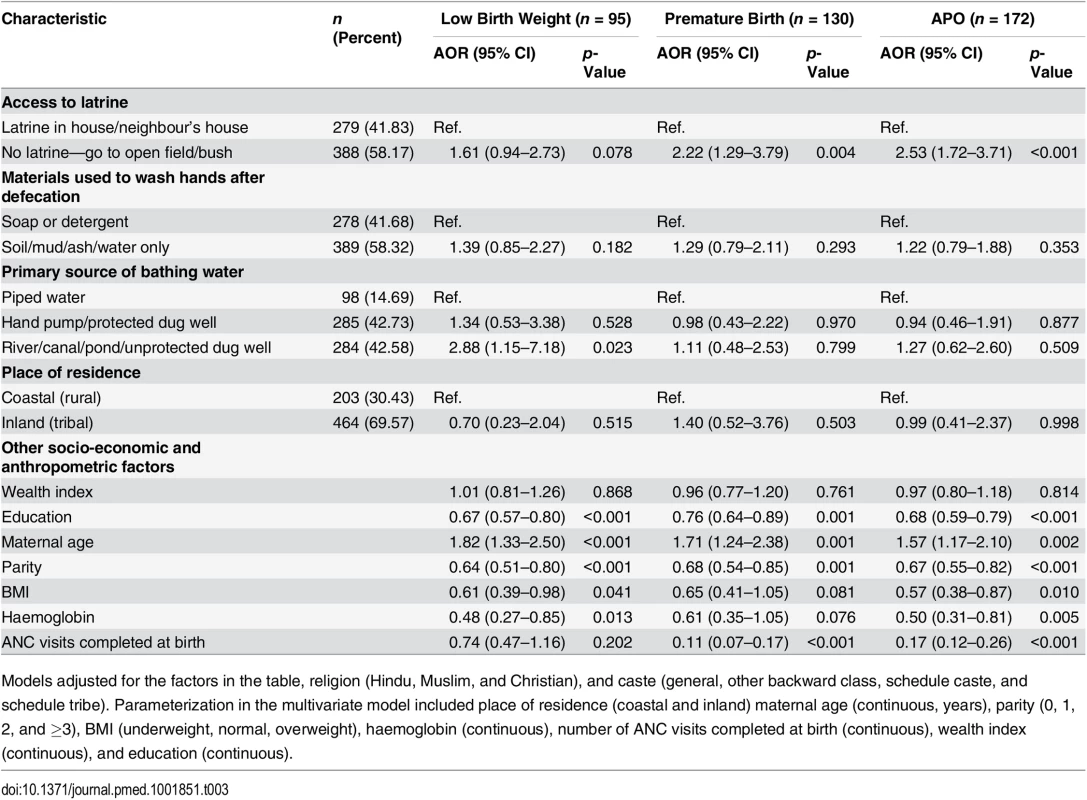
Models adjusted for the factors in the table, religion (Hindu, Muslim, and Christian), and caste (general, other backward class, schedule caste, and schedule tribe). Parameterization in the multivariate model included place of residence (coastal and inland) maternal age (continuous, years), parity (0, 1, 2, and ≥3), BMI (underweight, normal, overweight), haemoglobin (continuous), number of ANC visits completed at birth (continuous), wealth index (continuous), and education (continuous). Tab. 4. Association between sanitation use and adverse pregnancy outcomes among participants with latrine access (n = 279). 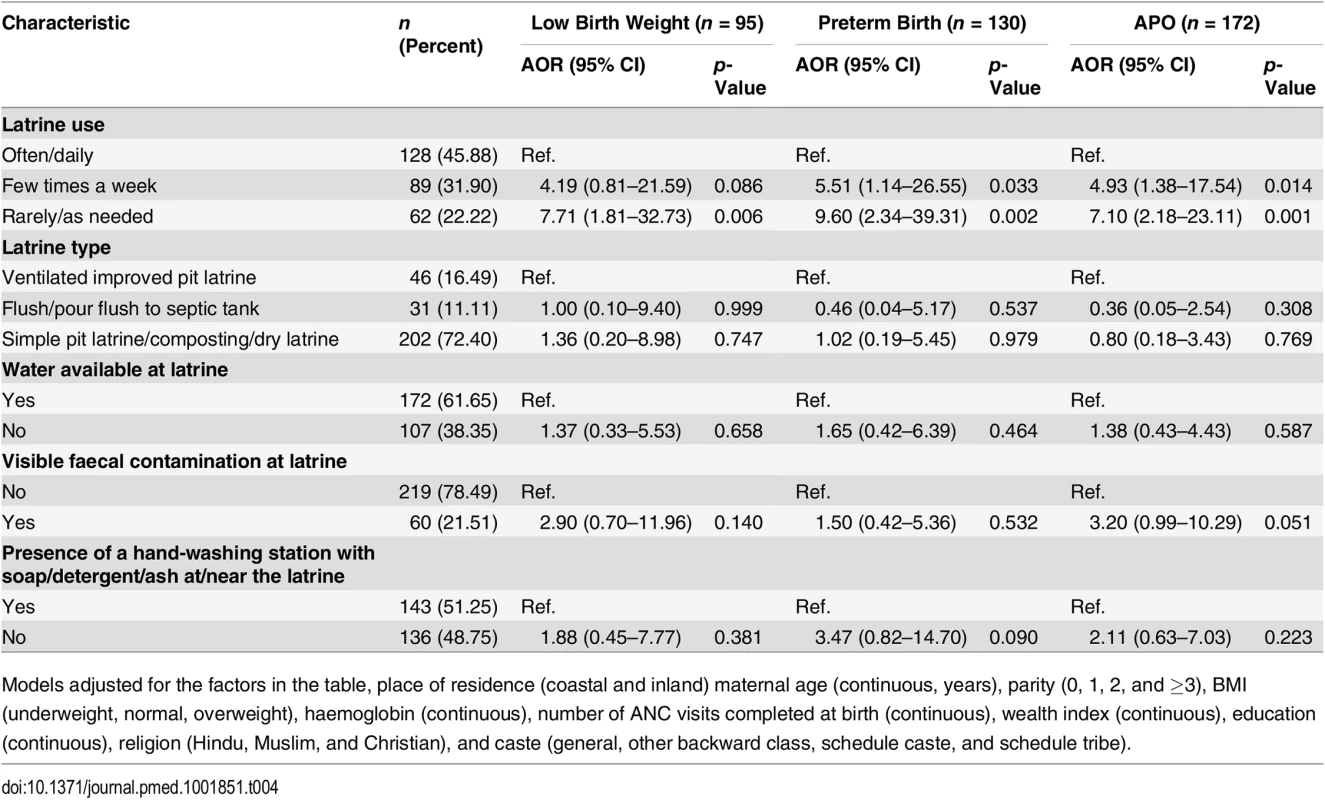
Models adjusted for the factors in the table, place of residence (coastal and inland) maternal age (continuous, years), parity (0, 1, 2, and ≥3), BMI (underweight, normal, overweight), haemoglobin (continuous), number of ANC visits completed at birth (continuous), wealth index (continuous), education (continuous), religion (Hindu, Muslim, and Christian), and caste (general, other backward class, schedule caste, and schedule tribe). Discussion
In this prospective study, we examined the relationship between maternal sanitation behaviour and APOs. After adjusting for socio-demographic, anthropometric, and other sanitation-related behaviours, we observed that women who reported poor sanitation practices in the early phase of pregnancy (10–12 wk of gestation) were more likely to experience an APO, independent of the established confounding factors of poverty and caste. To our knowledge, this is the first community-based prospective study to demonstrate that practicing open defecation is associated with a higher risk of APOs. Using a large existing population-based cohort, all pregnancies could be enrolled and followed longitudinally from the first trimester to pregnancy. The diverse nature of our study sites also allowed us to enrol a representative rural Indian sample including individuals of different castes, class, religion, and socio-economic status, with a range of sanitation-related practices.
Population-based data on the prevalence of APOs as reported here are uncommon in India. Among our cohort, we found that 19.5% of births were preterm and 14.2% of babies had low birth weight during the study period. Studies from south India (Tamil Nadu) have reported a prevalence of low birth weight and preterm birth of 17.0% and 12.3%, respectively, although the prevalence of low birth weight has also been estimated to be as high as 30%–40% in India [57]. The rate of preterm delivery (<37 wk) in neighbouring Bangladesh [24] was 22.3% in a setting similar to India. In sub-Saharan Africa, the prevalence of preterm birth and low birth weight in an urban setting was 19.9% and 10.2%, respectively [56].
Given the paucity of research linking open defecation to APOs, it is difficult to assess our findings in the context of other study findings. A hospital-based study in sub-Saharan Africa found a higher risk of preterm birth, but not low birth weight, among babies born to mothers using shared sanitation facilities (OR: 1.26; 95% CI: 1.07–1.48) [56].
Our study reports a protective role of maternal haemoglobin (Hb ≥ 110 g/l) in regards to the risk of APOs among the study populations which is in line with other findings. A recent systematic review and meta-analysis showed a significantly higher risk of low birth weight (OR: 1.29; 95% CI: 1.09–1.53) and preterm birth (OR: 1.21; 95% CI: 1.13–1.30) with anaemia in the first or second trimester [47]. The same study also estimated that each 1-g/l increase in mean haemoglobin corresponded to birth weight being increased by 14.0 g (95% CI: 6.8–21.8). Another study in India revealed 30% of women to be anaemic (Hb < 110 g/dl), and maternal anaemia predicted a 2.4-fold greater risk of preterm delivery (p < 0.01) and an increased risk of low birth weight (p = 0.05) [16]. We observed that higher haemoglobin was associated with low odds (AOR: 0.50; 95% CI: 0.31–0.81) of APOs among women in the multivariable model.
Another important finding of our study was that education remained a key determinant of APOs in the multivariable model. We observed higher education to be associated with lower odds (AOR: 0.68; 95% CI: 0.59–0.79) of APOs among women in the multivariable model. This finding is consistent with numerous research findings of higher education being predictive of lower likelihood of APOs [31,61]. A Canadian study showed that not having a high school diploma was associated with low birth weight (OR: 3.20; 95% CI: 2.61–3.91). The inverse association of education with APOs could be attributed to many socio-behavioural factors related to the overall impact of higher levels of education. Education can improve knowledge about safe hygiene practices and assimilation of health-related information and good hygiene behaviour [62,63]. Similarly, an educated person may be more likely to appreciate the positive effect of sanitation and hygiene practices, resulting in sustained healthy behaviour [64].
We adjusted our estimates to account for socio-economic factors by constructing a household wealth index. Poverty is highly correlated with a lack of sanitation access, and both factors have been linked to increased risks regarding maternal health [31,32], stunting [41], and stress. While it is intuitive to expect that individuals with low economic status are more likely to experience APOs because of many concomitant negative determinants of pregnancy, our results demonstrate that open defecation poses significant health risks that are not explained by poverty.
In our study, although we adjusted our estimates for a priori covariates and considered several biological plausibilities, we did not adjust for many potential risk factors for APOs such as maternal smoking, alcohol use, history of sexually transmitted diseases, history of antenatal complications, history of abortions, etc. Whereas each of these factors has been associated with an increased risk of APOs, we are unaware of any data suggesting that these variables are associated with defecation practice. Hence, we considered these variables unlikely to be confounders in our analysis [60]. Several questions remained unanswered in this study, and we have not been able to address the biological or behavioural basis of these findings. One mechanism may be related to the adverse outcomes of restricting food and water intake to cope with sanitation challenges. It has been shown that women, when confronted with poor sanitation choices, may choose to limit their intake of food and water to avoid the need to use the toilet. Another potential mechanism may be related to community-level or household-level coverage and use of sanitation and the resulting increase in disease prevalence associated with environmental contamination in drinking water or in food. The pathogenesis of each of the adverse birth outcomes is unique and potentially independent, and we have not identified the specific exposure pathways (such as incidence of bacterial vaginosis) that may play a role in our observed outcomes. A third potential mechanism may be related to the lack of sanitation and psycho-social stress. Women throughout their life course may experience tangible threats to physical health as a result of sanitation insecurity, a mechanism currently being explored as part of this study.
This study indicates that, in the context of maternal and child health promotion research, sanitation is an important dimension of women’s health and distinct from poverty and caste. Additional research is warranted that addresses the underlying mechanisms of sanitation-related APOs.
Supporting Information
Zdroje
1. Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Heal. 2013;1:e26–36.
2. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88 : 31–38. doi: 10.2471/BLT.08.062554 20428351
3. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A - B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379 : 2162–2172. doi: 10.1016/S0140-6736(12)60820-4 22682464
4. Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35 : 706–718. 16556647
5. World Health Organization. International classification of diseases and related health problems. 10th revision. Geneva: World Health Organization; 2004.
6. Walker PGT, ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Heal. 2014;2:e460–7.
7. Odibo AO, Patel KR, Spitalnik A, Odibo L, Huettner P. Placental pathology, first-trimester biomarkers and adverse pregnancy outcomes. J Perinatol. 2014;34 : 186–191. doi: 10.1038/jp.2013.176 24434779
8. Gilbert NM, O’Brien VP, Hultgren S, Macones G, Lewis WG, Lewis AL. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob Adv Health Med. 2013;2 : 59–69. doi: 10.7453/gahmj.2013.061 24416696
9. Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333 : 1737–1742. 7491137
10. Getachew M, Tafess K, Zeynudin A, Yewhalaw D. Prevalence soil transmitted helminthiasis and malaria co-infection among pregnant women and risk factors in Gilgel Gibe Dam area, southwest Ethiopia. BMC Res Notes. 2013;6 : 263. doi: 10.1186/1756-0500-6-263 23837685
11. Nielsen SY, Henriksen TB, Hjøllund NH, Mølbak K, Andersen AMN. Risk of adverse pregnancy outcome in women exposed to livestock: a study within the Danish National Birth Cohort. Epidemiol Infect. 2014;142 : 1545–1553. doi: 10.1017/S0950268813002203 24054461
12. Kumar A, Begum N. Hepatitis E in pregnancy: an insight into etiopathogenesis. 2010;23 : 281–283.
13. Sukrat B, Wilasrusmee C, Siribumrungwong B, McEvoy M, Okascharoen C, Attia J, et al. Hemoglobin concentration and pregnancy outcomes: a systematic review and meta-analysis. Biomed Res Int. 2013;2013 : 769057. doi: 10.1155/2013/769057 23984406
14. Goswami TM, Patel VN, Pandya NH, Mevada AK, Desai KS, Solanki KB. Maternal anaemia during pregnancy and its impact on perinatal outcome. Int J Biomed Adv Res. 2014;5 : 99–102.
15. Koura GK, Ouedraogo S, Le Port A, Watier L, Cottrell G, Guerra J, et al. Anaemia during pregnancy: impact on birth outcome and infant haemoglobin level during the first 18 months of life. Trop Med Int Health. 2012;17 : 283–291. doi: 10.1111/j.1365-3156.2011.02932.x 22146105
16. Finkelstein J, Duggan C, Thomas T, Bose B, Samuel T, Srinivasan K, et al. Maternal anemia, iron deficiency, and pregnancy outcomes in India (804.10). FASEB J. 2014;28 (Suppl 1):804.10. http://www.fasebj.org/content/28/1_Supplement/804.10.short. Accessed 29 May 2015.
17. Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368 : 1164–1170. 17011943
18. Ankumah N - A, Cantu J, Jauk V, Biggio J, Hauth J, Andrews W, et al. Risk of adverse pregnancy outcomes in women with mild chronic hypertension before 20 weeks of gestation. Obstet Gynecol. 2014;123 : 966–972. doi: 10.1097/AOG.0000000000000205 24785847
19. Darling AM, Liu E, Aboud S, Urassa W, Spiegelman D, Fawzi W. Maternal hyperglycemia and adverse pregnancy outcomes in Dar es Salaam, Tanzania. Int J Gynaecol Obstet. 2014;125 : 22–27. doi: 10.1016/j.ijgo.2013.10.007 24508349
20. Hughes RCE, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37 : 2953–2959. doi: 10.2337/dc14-1312 25190675
21. Madianos PN, Bobetsis YA, Offenbacher S. Adverse pregnancy outcomes (APOs) and periodontal disease: pathogenic mechanisms. J Clin Periodontol. 2013;40 (Suppl 1):S170–S180.
22. Aris A. A 12-year cohort study on adverse pregnancy outcomes in Eastern Townships of Canada: impact of endometriosis. Gynecol Endocrinol. 2014;30 : 34–37. doi: 10.3109/09513590.2013.848425 24134807
23. Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357 : 648–653. 17699814
24. Shah R, Mullany LC, Darmstadt GL, Mannan I, Rahman SM, Talukder RR, et al. Incidence and risk factors of preterm birth in a rural Bangladeshi cohort. BMC Pediatr. 2014;14 : 112. doi: 10.1186/1471-2431-14-112 24758701
25. Ahmad SA, Sayed MHSU, Barua S, Khan MH, Faruquee MH, Jalil A, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109 : 629–631. 11445518
26. Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci. 2008;15 : 631–650. doi: 10.1177/1933719108322436 18836129
27. Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health. 2013;12 : 6. doi: 10.1186/1476-069X-12-6 23320899
28. Kile ML, Rodrigues EG, Mazumdar M, Dobson CB, Diao N, Golam M, et al. A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh. Environ Health. 2014;13 : 29. doi: 10.1186/1476-069X-13-29 24735908
29. Yucra S, Tapia V, Steenland K, Naeher LP, Gonzales GF. Maternal exposure to biomass smoke and carbon monoxide in relation to adverse pregnancy outcome in two high altitude cities of Peru. Environ Res. 2014;130 : 29–33. doi: 10.1016/j.envres.2014.01.008 24561394
30. Petersen R, Gazmararian JA, Spitz AM, Rowley DL, Goodwin MM, Saltzman LE, et al. Violence and adverse pregnancy outcomes: a review of the literature and directions for future research. Am J Prev Med. 1997;13 : 366–373. 9315269
31. Luo Z - C, Wilkins R, Kramer MS. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. CMAJ. 2006;174 : 1415–1420. 16682708
32. Andersen A - MN, Mortensen LH. Socioeconomic inequality in birth outcomes: what do the indicators tell us, and where do we find the data? CMAJ. 2006;174 : 1429–1430. 16682710
33. Ververs M-T, Antierens A, Sackl A, Staderini N, Captier V. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr. 2013 Jun 7.
34. World Health Organization, United Nations Children’s Fund. Progress on sanitation and drinking-water: 2013 update. Geneva: World Health Organization; 2013.
35. Schmidt W-P. The elusive effect of water and sanitation on the global burden of disease. Trop Med Int Health. 2014;19 : 522–527. doi: 10.1111/tmi.12286 24576060
36. Freeman MC, Stocks ME, Cumming O, Jeandron A, Higgins JPT, Wolf J, et al. Hygiene and health: systematic review of handwashing practices worldwide and update of health effects. Trop Med Int Health. 2014;19 : 906–916. doi: 10.1111/tmi.12339 24889816
37. Cairncross S, Hunt C, Boisson S, Bostoen K, Curtis V, Fung ICH, et al. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol. 2010;39 (Suppl 1):i193–i205. doi: 10.1093/ije/dyq035 20348121
38. Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9:e1001162. doi: 10.1371/journal.pmed.1001162 22291577
39. Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001620. doi: 10.1371/journal.pmed.1001620 24667810
40. Dangour AD, Watson L, Cumming O, Boisson S, Che Y, Velleman Y, et al. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev. 2013;8:CD009382.
41. Spears D, Ghosh A, Cumming O. Open defecation and childhood stunting in India: an ecological analysis of new data from 112 districts. PLoS ONE. 2013;8:e73784. doi: 10.1371/journal.pone.0073784 24066070
42. Patil SR, Arnold BF, Salvatore AL, Briceno B, Ganguly S, Colford JM, et al. The effect of India’s Total Sanitation Campaign on defecation behaviors and child health in rural Madhya Pradesh: a cluster randomized controlled trial. PLoS Med. 2014;11:e1001709. doi: 10.1371/journal.pmed.1001709 25157929
43. Clasen T, Boisson S, Routray P, Torondel B, Bell M, Cumming O, et al. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. Lancet Glob Health. 2014;2:e645–53. doi: 10.1016/S2214-109X(14)70307-9 25442689
44. Campbell OMR. Getting the basics right—the role of water, sanitation and hygiene in maternal and reproductive health; a conceptual framework. Trop Med Int Health. 2015;20 : 252–267. doi: 10.1111/tmi.12439 25430609
45. Bora R, Sable C, Wolfson J, Boro K, Rao R. Prevalence of anemia in pregnant women and its effect on neonatal outcomes in Northeast India. J Matern Fetal Neonatal Med. 2014;27 : 887–891. doi: 10.3109/14767058.2013.845161 24041147
46. Clasen T, Boisson S, Routray P, Cumming O, Jenkins M, Ensink JHJ, et al. The effect of improved rural sanitation on diarrhoea and helminth infection: design of a cluster-randomized trial in Orissa, India. Emerg Themes Epidemiol. 2012;9 : 7. doi: 10.1186/1742-7622-9-7 23148587
47. Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2013;346:f3443. doi: 10.1136/bmj.f3443 23794316
48. Jose S. Adult undernutrition in India: is there a huge gender gap? Econ Polit Wkly. 2011;46 : 95–102.
49. Kulasekaran R. Influence of mothers’ chronic energy deficiency on the nutritional status of preschool children in Empowered Action Group states in India. Int J Nutr Pharmacol Neurol Dis. 2012;2 : 198.
50. Benova L, Cumming O, Campbell OMR. Systematic review and meta-analysis: association between water and sanitation environment and maternal mortality. Trop Med Int Health. 2014;19 : 368–387. doi: 10.1111/tmi.12275 24506558
51. Government of India Ministry of Health and Family Welfare National Health Mission. State wise information: Orissa. http://nrhm.gov.in/nrhm-in-state/state-wise-information/odisha.html#state_profile. Accessed 30 November 2014.
52. Kumar A, Das KC. Drinking water and sanitation facility in India and its linkages with diarrhoea among children under five : evidences from recent data. Int J Humanit Soc Sci Invent. 2014;3 : 50–60.
53. Heijnen M, Rosa G, Fuller J, Eisenberg JNS, Clasen T. The geographic and demographic scope of shared sanitation: an analysis of national survey data from low - and middle-income countries. Trop Med Int Heal. 2014;19 : 1334–1345.
54. Pullan RL, Freeman MC, Gething PW, Brooker SJ. Geographical inequalities in use of improved drinking water supply and sanitation across sub-Saharan Africa: mapping and spatial analysis of cross-sectional survey data. PLoS Med. 2014;11:e1001626. doi: 10.1371/journal.pmed.1001626 24714528
55. Satpathy R, Nanda P, Nanda NC, Bal HB, Mohanty R, Misra A, et al. Challenges in implementation of the ANISA protocol at the Rourkela-Bhubaneswar site in India. Pediatr Infect Dis J. In press.
56. Olusanya BO, Ofovwe GE. Predictors of preterm births and low birthweight in an inner-city hospital in sub-Saharan Africa. Matern Child Health J. 2010;14 : 978–986. doi: 10.1007/s10995-009-0528-4 19795198
57. George K, Prasad J, Singh D, Minz S, Albert DS, Muliyil J, et al. Perinatal outcomes in a South Asian setting with high rates of low birth weight. BMC Pregnancy Childbirth. 2009;9 : 5. doi: 10.1186/1471-2393-9-5 19203384
58. Padhi B, Panigrahi P, Elliott L. Prevalence and determinants of low birth weight and prematurity among rural, urban, and indigenous tribal communities in India [abstract]. Abstract number 5378. 27th Conference of the International Society for Environmental Epidemiology; 30 Aug–3 Sept 2015; São Paulo, Brazil. http://ehp.niehs.nih.gov/isee/p-1-33-04/. Accessed 2 June 2015.
59. Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21 : 459–468. 17030551
60. Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia: Lippincott - Raven; 1998.
61. Auger N, Luo Z-C, Platt RW, Daniel M. Do mother’s education and foreign born status interact to influence birth outcomes? Clarifying the epidemiological paradox and the healthy migrant effect. J Epidemiol Community Health. 2008;62 : 402–409. doi: 10.1136/jech.2007.064535 18413452
62. Dreibelbis R, Winch PJ, Leontsini E, Hulland KRS, Ram PK, Unicomb L, et al. The integrated behavioural model for water, sanitation, and hygiene: a systematic review of behavioural models and a framework for designing and evaluating behaviour change interventions in infrastructure-restricted settings. BMC Public Health. 2013;13 : 1015. doi: 10.1186/1471-2458-13-1015 24160869
63. Golden SD, Earp JAL. Social ecological approaches to individuals and their contexts: twenty years of health education & behavior health promotion interventions. Health Educ Behav. 2012;39 : 364–372. doi: 10.1177/1090198111418634 22267868
64. Briceno B, Coville A, Martinez S. Promoting handwashing and sanitation evidence from a large-scale randomized trial in rural Tanzania. World Bank Policy Research Working Paper No. 7164. Washington (District of Columbia): World Bank; 2015. http://ssrn.com/abstract=2550526. Accessed 2 June 2015.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Flexofytol® – přírodní revoluce v boji proti osteoartróze kloubů
-
Všechny články tohoto čísla
- Noncommunicable Diseases: A Globalization of Disparity?
- The Individualised versus the Public Health Approach to Treating Ebola
- Ebola Virus Disease: Experience and Decision Making for the First Patients outside of Africa
- Risk of Adverse Pregnancy Outcomes among Women Practicing Poor Sanitation in Rural India: A Population-Based Prospective Cohort Study
- Health, Health Inequality, and Cost Impacts of Annual Increases in Tobacco Tax: Multistate Life Table Modeling in New Zealand
- Connecting the Dots: Health Information Technology Expansion and Health Disparities
- Searching for Public Health Law’s Sweet Spot: The Regulation of Sugar-Sweetened Beverages
- Glitazone Treatment and Incidence of Parkinson’s Disease among People with Diabetes: A Retrospective Cohort Study
- Individual Participant Data (IPD) Meta-analyses of Randomised Controlled Trials: Guidance on Their Use
- AA Genotype Exacerbates Effect of Diabetes on Dementia and Alzheimer Disease: A Population-Based Longitudinal Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Ebola Virus Disease: Experience and Decision Making for the First Patients outside of Africa
- Searching for Public Health Law’s Sweet Spot: The Regulation of Sugar-Sweetened Beverages
- Glitazone Treatment and Incidence of Parkinson’s Disease among People with Diabetes: A Retrospective Cohort Study
- Individual Participant Data (IPD) Meta-analyses of Randomised Controlled Trials: Guidance on Their Use
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



