-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCervical Screening at Age 50–64 Years and the Risk of Cervical Cancer at Age 65 Years and Older: Population-Based Case Control Study
Background:
There is little consensus, and minimal evidence, regarding the age at which to stop cervical screening. We studied the association between screening at age 50–64 y and cervical cancer at age 65–83 y.Methods and Findings:
Cases were women (n = 1,341) diagnosed with cervical cancer at age 65–83 y between 1 April 2007 and 31 March 2012 in England and Wales; age-matched controls (n = 2,646) were randomly selected from population registers. Screening details from 1988 onwards were extracted from national databases. We calculated the odds ratios (OR) for different screening histories and subsequent cervical cancer. Women with adequate negative screening at age 65 y (288 cases, 1,395 controls) were at lowest risk of cervical cancer (20-y risk: 8 cancers per 10,000 women) compared with those (532 cases, 429 controls) not screened at age 50–64 y (20-y risk: 49 cancers per 10,000 women, with OR = 0.16, 95% CI 0.13–0.19). ORs depended on the age mix of women because of the weakening association with time since last screen: OR = 0.11, 95% CI 0.08–0.14 at 2.5 to 7.5 y since last screen; OR = 0.27, 95% CI 0.20–0.36 at 12.5 to 17.5 y since last screen. Screening at least every 5.5 y between the ages 50 and 64 y was associated with a 75% lower risk of cervical cancer between the ages 65 and 79 y (OR = 0.25, 95% CI 0.21–0.30), and the attributable risk was such that in the absence of screening, cervical cancer rates in women aged 65+ would have been 2.4 (95% CI 2.1–2.7) times higher. In women aged 80–83 y the association was weaker (OR = 0.49, 95% CI 0.28–0.83) than in those aged 65–69 y (OR = 0.12, 95% CI 0.09–0.17). This study was limited by an absence of data on confounding factors; additionally, findings based on cytology may not generalise to human papillomavirus testing.Conclusions:
Women with adequate negative screening at age 50–64 y had one-sixth of the risk of cervical cancer at age 65–83 y compared with women who were not screened. Stopping screening between ages 60 and 69 y in women with adequate negative screening seems sensible, but further screening may be justifiable as life expectancy increases.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(1): e32767. doi:10.1371/journal.pmed.1001585
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001585Summary
Background:
There is little consensus, and minimal evidence, regarding the age at which to stop cervical screening. We studied the association between screening at age 50–64 y and cervical cancer at age 65–83 y.Methods and Findings:
Cases were women (n = 1,341) diagnosed with cervical cancer at age 65–83 y between 1 April 2007 and 31 March 2012 in England and Wales; age-matched controls (n = 2,646) were randomly selected from population registers. Screening details from 1988 onwards were extracted from national databases. We calculated the odds ratios (OR) for different screening histories and subsequent cervical cancer. Women with adequate negative screening at age 65 y (288 cases, 1,395 controls) were at lowest risk of cervical cancer (20-y risk: 8 cancers per 10,000 women) compared with those (532 cases, 429 controls) not screened at age 50–64 y (20-y risk: 49 cancers per 10,000 women, with OR = 0.16, 95% CI 0.13–0.19). ORs depended on the age mix of women because of the weakening association with time since last screen: OR = 0.11, 95% CI 0.08–0.14 at 2.5 to 7.5 y since last screen; OR = 0.27, 95% CI 0.20–0.36 at 12.5 to 17.5 y since last screen. Screening at least every 5.5 y between the ages 50 and 64 y was associated with a 75% lower risk of cervical cancer between the ages 65 and 79 y (OR = 0.25, 95% CI 0.21–0.30), and the attributable risk was such that in the absence of screening, cervical cancer rates in women aged 65+ would have been 2.4 (95% CI 2.1–2.7) times higher. In women aged 80–83 y the association was weaker (OR = 0.49, 95% CI 0.28–0.83) than in those aged 65–69 y (OR = 0.12, 95% CI 0.09–0.17). This study was limited by an absence of data on confounding factors; additionally, findings based on cytology may not generalise to human papillomavirus testing.Conclusions:
Women with adequate negative screening at age 50–64 y had one-sixth of the risk of cervical cancer at age 65–83 y compared with women who were not screened. Stopping screening between ages 60 and 69 y in women with adequate negative screening seems sensible, but further screening may be justifiable as life expectancy increases.
Please see later in the article for the Editors' SummaryIntroduction
There is a lack of consensus regarding the appropriate upper age for cervical screening and little direct evidence on which to base policy [1],[2]. Until recently recommendations from the US have been for women to be screened into their 80 s, and cervical screening over age 65 y is common [3],[4]. The current recommendation from both the American Congress of Obstetricians and Gynecologists and the American Cancer Society is that screening should be stopped at age 65 y for women with evidence of adequate prior negative screening and no history of cervical intraepithelial neoplasia grade 2+ [5],[6], but this recommendation was based only on expert opinion and modelling because of the lack of empirical data. More radically, in 1993, Van Wijngaarden and Duncan [7] proposed that screening over the age of 50 y was of little value in those previously well screened. However Rebolj et al. [8] disagreed with these findings and reported a similar 10-y cumulative incidence of cervical cancer in women whose third consecutive negative test was taken at age 45–54 y and in those whose third test was at age 30–44 y. Internationally, the upper age for cervical screening varies from 59 or 60 y in Denmark, Finland, and Scotland, to 70 y and over in Japan, Korea, and Uruguay [9]. In England and Wales women receive their last test between the ages of 60 and 64 y. The justification for stopping screening in older women is based on the natural history of cervical cancer. Incident human papillomavirus (HPV) infections in women aged 55 y or over are rare and are unlikely to have sufficient time to progress to invasive cancer in the woman's lifetime. However, we know of no direct evidence of the impact of cervical screening in older women in the period 7 to 15 y after their last screening test.
We studied the association between screening women at age 50–64 y and cervical cancer diagnosed at age 65–83 y. Our aim was to provide policy-makers with evidence to help address the following questions. (1) Are well-screened women with a history of negative tests and no high-grade results at sufficiently low risk of cervical cancer that screening can cease at age 65? If so, how low is their risk, and how does it change as they age? (2) Are women who regularly participate in screening at age 50–64 y at reduced risk of cervical cancer at age 65–83 y?
Methods
Ethics Statement
The collection of data as part of this audit has been approved since 2003 (PIAG 1-08(a)/2003) under Section 251 of the National Health Service Act 2006, which re-enacted Section 60 of the Health and Social Care Act 2001. The analysis of anonymised data in this context is considered service evaluation and therefore according to UK guidelines is considered ethical without further consideration by a research ethics committee [10].
Participants
There is free universal health care in England and Wales provided by the National Health Service (NHS), and all adult females registered with the NHS have a record in the national Cervical Screening Call/Recall System. Cases were women aged 65 y or older who were diagnosed with cervical cancer (ICD-10 C53) in England (between 1 April 2007 and 31 March 2012) and Wales (between 1 January 2007 and 31 December 2009) (October 2012 dataset) and who were registered with an NHS general practitioner (GP). Any other woman registered with an NHS GP at the time of a case diagnosis was eligible as a control for that case. Controls were randomly selected (using a computer program) from women satisfying the matching criteria. Two controls were individually matched to each case based on age and place of residence: one control had the same GP as the case, and a second control had a different GP but was within the same administrative area. Occasionally, only one control could be identified. The study design did not allow for collection of data on potential confounders such as sexual behaviour, parity, and smoking. Matching on the same GP was to provide a crude surrogate for socio-economic status and ethnicity. The reason for selecting a control from a different GP was to avoid overmatching if screening uptake was dependent on the GP's enthusiasm for cervical screening. Data were collected on all selected controls, so there was no study selection or participation bias.
Data on screening histories were abstracted from routinely recorded cervical cytology records held on the Cervical Screening Call/Recall System (and as such were not subject to recall bias). These records include all NHS (and many private provider) smears taken in the UK since 1988.
After local NHS staff linked screening data to cases and controls, the data were anonymised locally before being transferred for cleaning and analysis. Guidelines on the collection of data for this audit and details of the design have been published previously [11]–[13].
Delays in the inclusion of newly diagnosed cancers in our audit resulted in advanced stage cancers and cases diagnosed in the most recent period being underrepresented. It was estimated that the database contained 78% of all cancers in women aged 65–83 y diagnosed during the study period in England [14].
Women aged 60 y or over on 1 January 1988 were excluded because they may not have been invited for screening as part of the Cervical Screening Call/Recall System; therefore, relatively few women in the study were diagnosed with cancer over the age of 80.
Cytology results were classified according to the British Society for Clinical Cytology system [15]. The British Society for Clinical Cytology terminology can be broadly compared to the Bethesda System, as follows: “borderline changes” include atypical squamous cells, atypical glandular cells, and “borderline, high-grade dyskaryosis not excluded” (equivalent to the Bethesda System's ASC-H); “mild dyskaryosis” corresponds to low-grade squamous intraepithelial lesion (LSIL); “moderate and severe dyskaryosis” corresponds to high-grade squamous intraepithelial lesion (HSIL); and there are separate categories, “query invasive” and “query glandular neoplasia”, for squamous cell carcinoma and adenocarcinoma in situ/cervical glandular intraepithelial neoplasia/adenocarcinoma, respectively. Tests classified as “inadequate” should have resulted in immediate repeat testing and were therefore ignored in this analysis. The exception was when they resulted in a referral to colposcopy (guidelines recommended referral after a third consecutive inadequate test).
Statistical Analysis
We used conditional logistic regression to estimate the odds ratio (OR) of cervical cancer (at age 65 y or older) for women with various screening patterns compared to those with no cervical cytology (except possibly inadequate test[s] not resulting in referral) between the ages 50 and 64 y. To exclude screen-detected cancers (diagnosed following screening at age 64 y), we excluded women (including control women) diagnosed at age 65.0–65.5 y with a cytology test within 6 mo of case diagnosis. Age of diagnosis was defined for controls using the date of diagnosis for their matched case.
We calculated absolute risks using a weighted logistic regression model, with weights calculated by case status, age category (65–69, 70–74, 75–79, and 80–84 y), and year of diagnosis. Weights for cases were calculated as the total number of cases diagnosed (according to the official cancer registration statistics for England [MB1 series] [16] [age-specific data for 1975–2011 were prepared by Cancer Research UK] and for Wales [17]) divided by the number recorded in the audit (see Table S1). As official figures have not yet been published for 2012, the 2011 weights were used. For controls, for each year of diagnosis and age group, the weights were calculated using the following formula:
Cervical cancer incidence rates were calculated as the number of cases diagnosed according to the MB1 series divided by the mid-year population estimate. We used the ORs and the percentage of cases in each category to calculate the population attributable risk (under the assumption of the association being causal) in two ways. We calculated how much greater cervical cancer rates (in women aged 65–79 y) would have been in the absence of screening beyond age 50. Additionally, we calculated the proportion of cancers (that did occur) that might have been prevented had all women been screened at least every 5.5 y between the ages 50 and 64 y. Confidence intervals for the population attributable risks were calculated by bootstrapping (percentile method based on 2,000 replications).We studied the association between cervical screening at age 50–64 y and the risk of cervical cancer at age 65–83 y by answering the following questions. (1) What is the risk of cervical cancer at age 65 y and older in women with a history of negative tests and no high-grade results at age 50–64 y? Does the risk relative to women not screened at age 50–64 y change with time since last test (i.e., as women age)? (2) What is the risk of cervical cancer at age 65–83 y in women who regularly participate (defined as having a test at least every 5.5 y) in screening at age 50–64 y compared with the risk in women not screened at all at age 50–64 y?
To address the first question, screening histories for women between the ages of 50 and 64 y were divided into four categories: (1) “adequate negative screening”, defined as women with at least three tests at age 50–64 y (with at least one at age 60–64 y), the last three of which were negative, and no HSIL or worse cytology since age 50; (2) “sub-optimal but negative screening”, defined as women not satisfying “adequate negative screening” but with either at least one negative test and no abnormal tests, or with three consecutive negative tests and no HSIL, but the last test was under age 60; (3) “abnormal screening”, defined as women with HSIL cytology at age 50–64 y, regardless of whether or not it was followed by three consecutive negatives, or with a low-grade result (atypical squamous cells of undetermined significance [ASC-US] or LSIL) that was not followed by three consecutive negatives; (4) “no screening”, defined as women with no test at age 50–64 y (excluding inadequate tests not resulting in referral to colposcopy). The first category approximates the group that the American Congress of Obstetricians and Gynecologists would discharge from screening. As we have no access to histology records of cervical intraepithelial neoplasia, we cannot identify those with cervical intraepithelial neoplasia grade 2+ and instead exclude women with HSIL cytology at any time since age 50.
Attenuation of the OR with increasing age was investigated non-parametrically (ORs were calculated separately for individuals diagnosed at different ages using overlapping 4-y intervals every 6 mo from age 65.0–69.0 y to age 79.0–83.0 y) and parametrically (a trend line and 95% confidence interval were calculated by including an interaction term between negative screening and age at diagnosis in the regression model). We also studied the risk of cervical cancer in women with at least three consecutive negative tests and no HSIL cytology at age 50–64 y by time since last test (at age 50–64 y).
To address the second question (if screening per se is associated with a lower risk of cervical cancer), a woman's (maximum) screening interval was defined as the longest period during the 15 y from age 50 to age 64 y in which she did not have an adequate cytology test (for women who were over 50 y in 1988, we considered only the interval from 1 January 1988 until their 65th birthday). In England and Wales, women in this age group were invited either every 3 y or every 5 y (depending on local policy at the time); therefore, we defined women whose maximum interval between tests during the 15 y period under study was at most 5.5 y to have attended screening regularly. Women with only inadequate tests were considered not to have been screened at age 50–64 y unless referred to colposcopy as a result of the inadequate cytology, in which case they were considered to be in category 3, “abnormal screening”.
The main analyses were repeated in a number of subgroups (e.g., by histological type) and with a number of exclusions (e.g., considering only cancers known to be stage 1B or worse). Additionally, we performed a sensitivity analysis of the possible impact of unknown confounders (such as smoking or number of sexual partners) on our results, and estimated the potential impact of changing the age at last screen on cervical cancer rates. We considered a risk score of the sort obtained from questionnaire data (not including screening attendance), divided into five risk levels, with 10% of screened women in each of the extreme groups, 20% in each of the high - and low-risk groups, and 40% in the middle group. We allowed for a 5-fold difference in risk between the highest and lowest risk groups, a difference in risk we believe to be plausible but extreme [18]. We assumed that the distribution of risk levels in unscreened women is a logistic shift of the distribution in screened women (shifted by −ln[2.25]) corresponding to an OR of 4.25 between the extreme groups.
Analyses were done in STATA 12 (StataCorp).
Results
A total of 1,341 women with invasive cervical cancer diagnosed at age 65–83 y and 2,646 matched controls were included in the study. Thirty-six cases (2.7%) had only one control—either because, for example, the other potential control was born before 1928 and the case was not, or because only one control that fulfilled the matching criteria was found. The distribution of cases by age, year of diagnosis, International Federation of Gynecology and Obstetrics (FIGO) stage, and histology is shown in Table 1. Similar numbers of women (404–435) were diagnosed in each 5-y age group for 65–79 y, but only 97 women were aged 80–83 y. Most of the cancers in this study were diagnosed as FIGO stage 2 or worse, and over 70% were squamous cell carcinoma.
Tab. 1. Number and percent of invasive cervical cancer cases by age, year of diagnosis, FIGO stage, and histology. 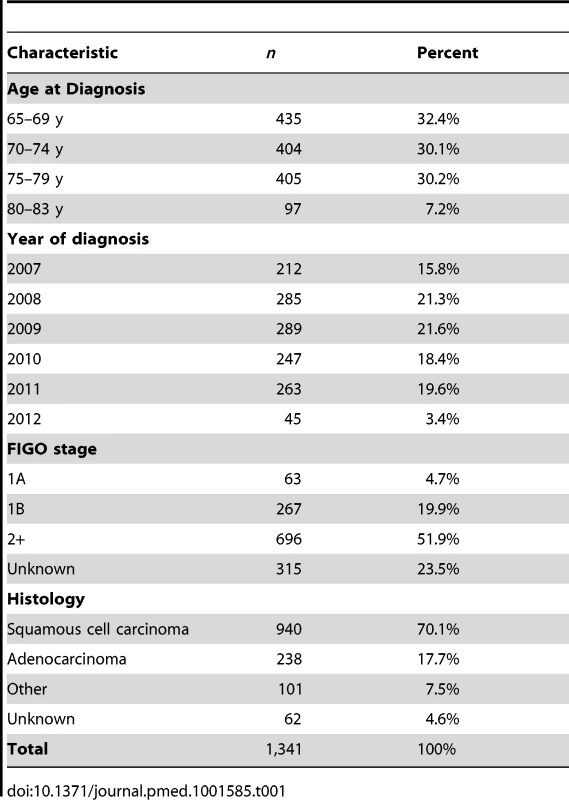
Table 2 shows the risk of cervical cancer diagnosed at age 65–83 y by screening history in the age interval 50–64 y. The highest risk was observed in those with a history of abnormal cytology (OR = 1.83, 95% CI 1.37–2.43, when compared with women without any tests at age 50–64 y). Women with cervical cancer were more likely to have had no screening at age 50–64 y than the general population (40% versus 16%) and less likely to have had adequate negative screening (21% versus 53%). Women with adequate negative screening were approximately six times less likely to be diagnosed with cervical cancer at age 65 y or older (OR 0.16, 95% CI 0.13–0.19) compared with women with no (adequate) tests since age 50.
Tab. 2. Risk of cervical cancer at age 65–83 y by screening history at age 50–64 y. 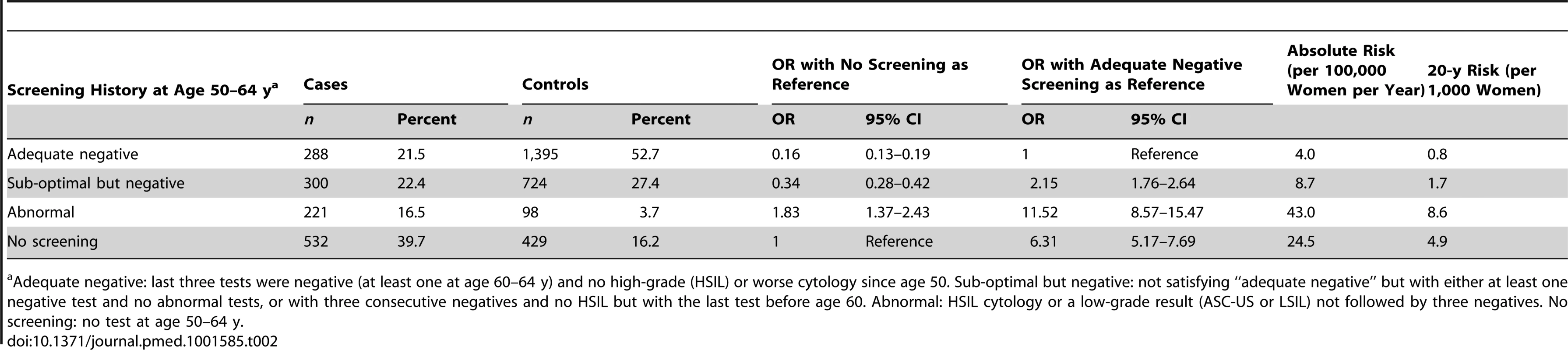
a Adequate negative: last three tests were negative (at least one at age 60–64 y) and no high-grade (HSIL) or worse cytology since age 50. Sub-optimal but negative: not satisfying “adequate negative” but with either at least one negative test and no abnormal tests, or with three consecutive negatives and no HSIL but with the last test before age 60. Abnormal: HSIL cytology or a low-grade result (ASC-US or LSIL) not followed by three negatives. No screening: no test at age 50–64 y. The OR for cervical cancer in the adequate negative screening group increased with age (Figure 1), from 0.07 (95% CI 0.05–0.11) for ages 65–69 y to 0.28 (95% CI 0.20–0.41) for ages 75–79 y and to 0.37 (95% CI 0.16–0.82) for ages 80–83 y (Figure 2; Table 3). Similarly, in women whose last three tests were negative and who had no HSIL results at age 50–64 y, the low risk associated with a negative screen weakened with time since last screen (Figure 3). The OR was 0.11 (95% CI 0.08–0.14) for women diagnosed within 2.5 to 7.5 y of their last screen, but the OR was 0.27 (95% CI 0.20–0.36) for those diagnosed 12.5 to 17.5 y after the last screen. There was no evidence that this tapering effect depended on the age at last screen, but we had little power to address this issue, as 80% of women with three negatives had their last test between 60.9 and 64.6 y of age.
Fig. 1. Odds ratio of cervical cancer at age 65–83 y in those with adequate negative screening compared with no screening at age 50–64 y by age at diagnosis. 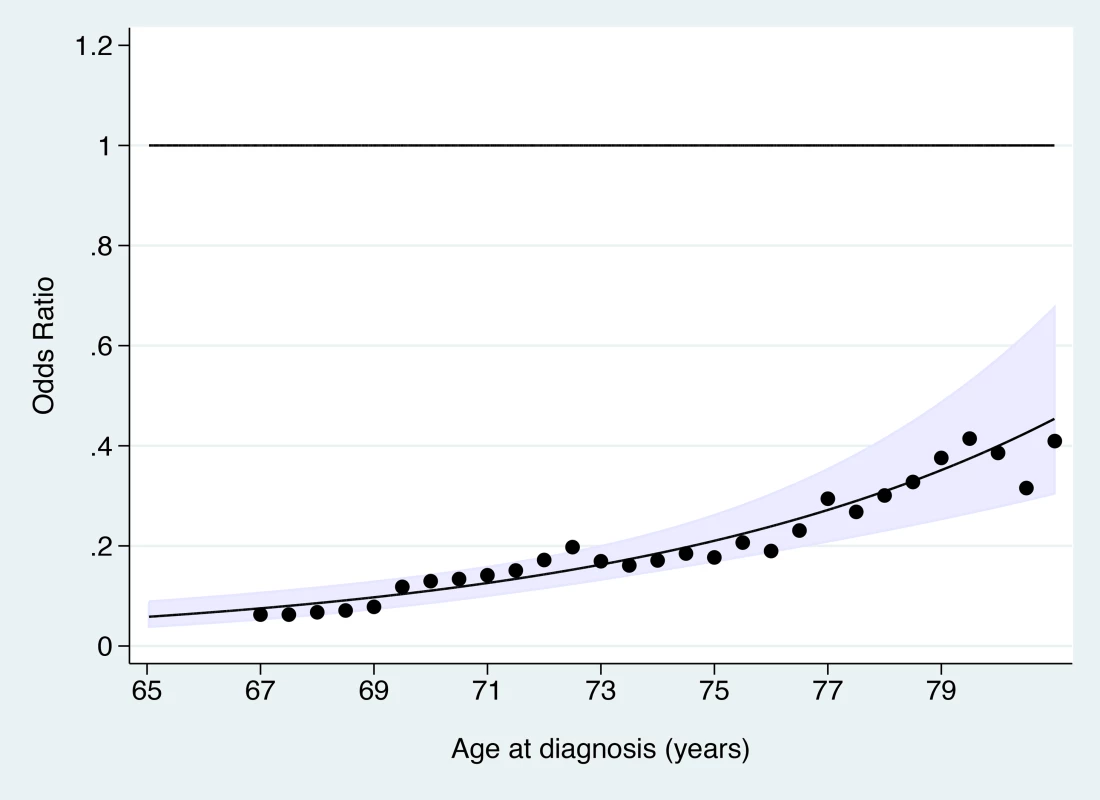
The line shows the log-linear trend, the shaded area shows the 95% confidence interval for the trend line, and the dots provide estimates based on data within 2 y of the x-axis values. Fig. 2. Subgroup analyses—odds ratios of cervical cancer at age 65–83 y for women with adequate negative screening relative to no screening at age 50–64 y. 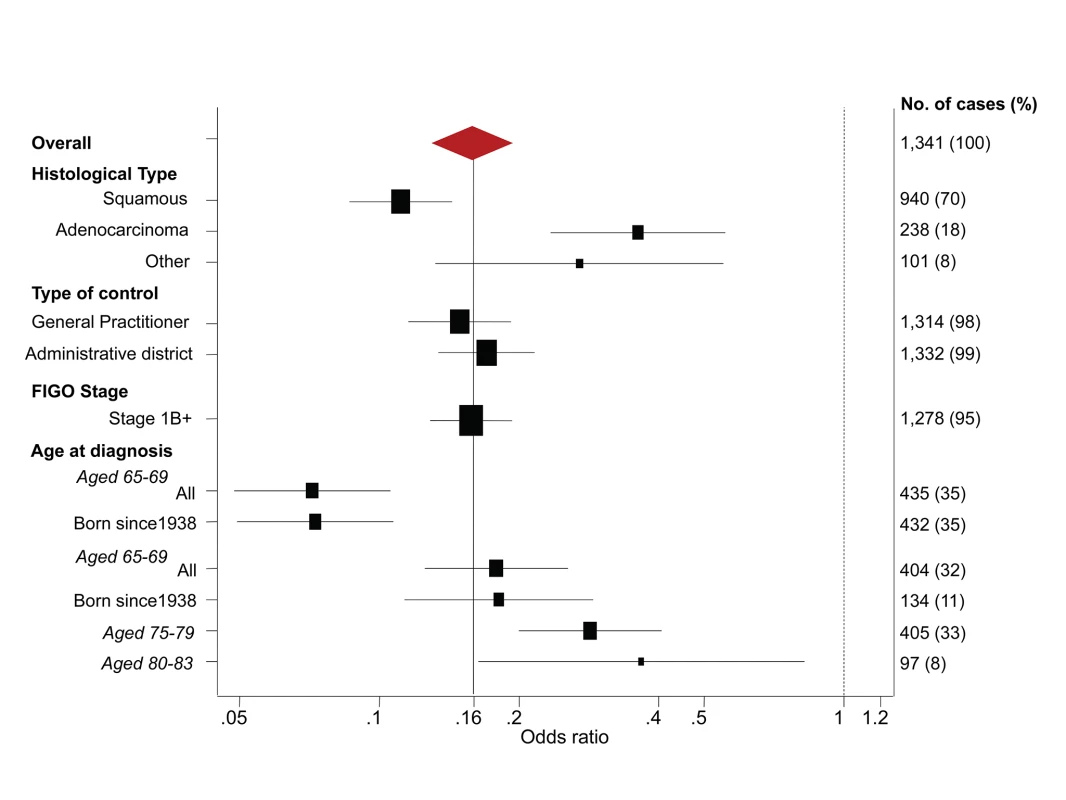
Fig. 3. Odds ratio of cervical cancer in those with adequate negative screening compared with no screening at age 50–64 y by time since last screen. 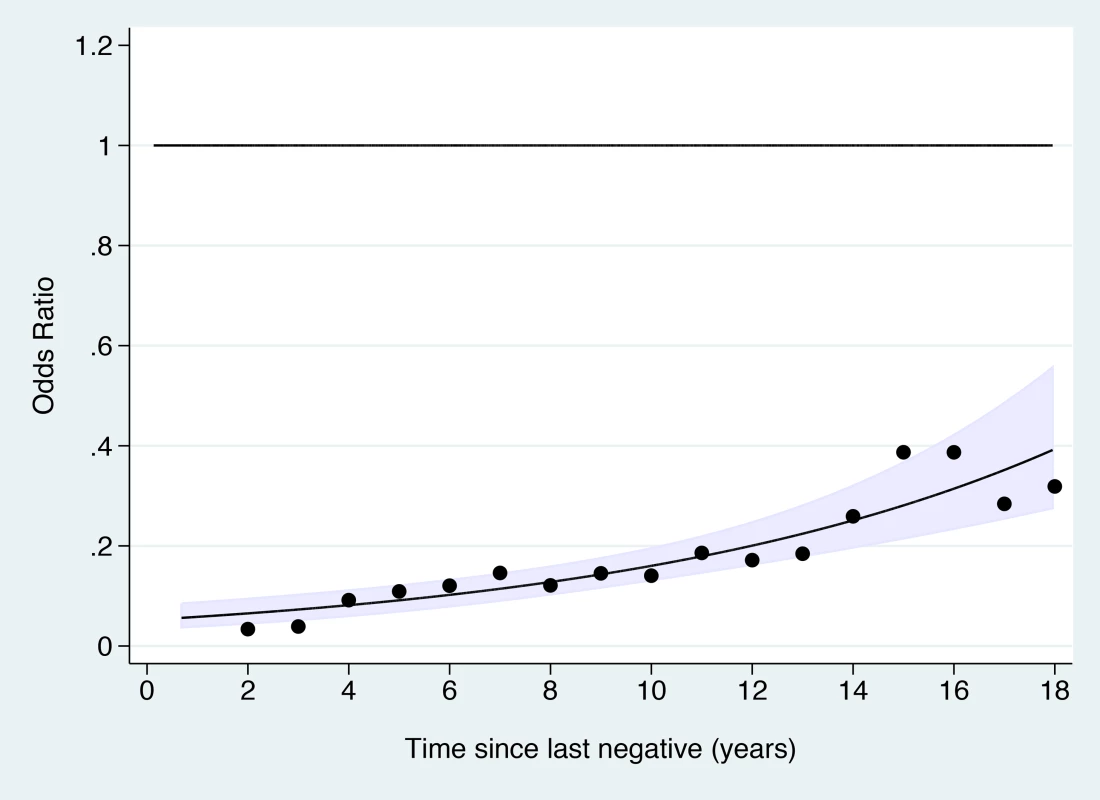
The line shows the log-linear trend, the shaded area shows the 95% confidence interval for the trend line, and the dots provide estimates based on data within 2 y of the x-axis values. Tab. 3. Risk of cervical cancer according to screening history at age 50–64 y by age at diagnosis. 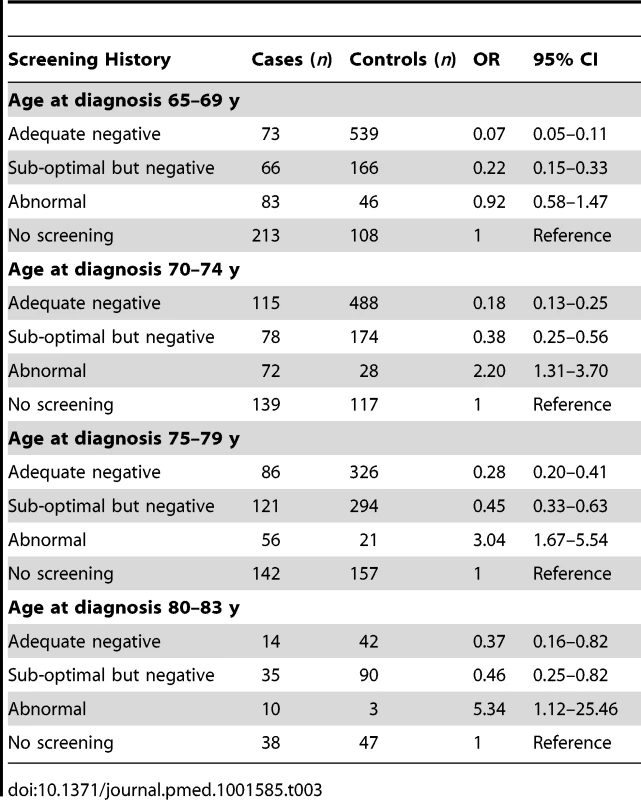
Based on cervical cancer incidence in women aged 65–84 y in 2007–2011, we estimated the absolute rate per 100,000 woman-years to be 4.0 in adequately negatively screened women, 24.5 in unscreened women, and 43.0 in women with an abnormal screening history. These absolute risks translate to a 20-y risk per 10,000 women of eight for adequately negatively screened women and 86 for those with abnormal screening.
The OR of squamous cell carcinoma (Table 4; Figure 2) associated with adequate negative screening was lower than for all cervical cancer (OR = 0.11, 95% CI 0.09–0.14), whereas the OR for adenocarcinoma of the cervix was greater (OR = 0.36, 95% CI 0.23–0.56).
Tab. 4. Risk of cervical cancer at age 65–83 y by screening history at age 50–64 y and histological type. 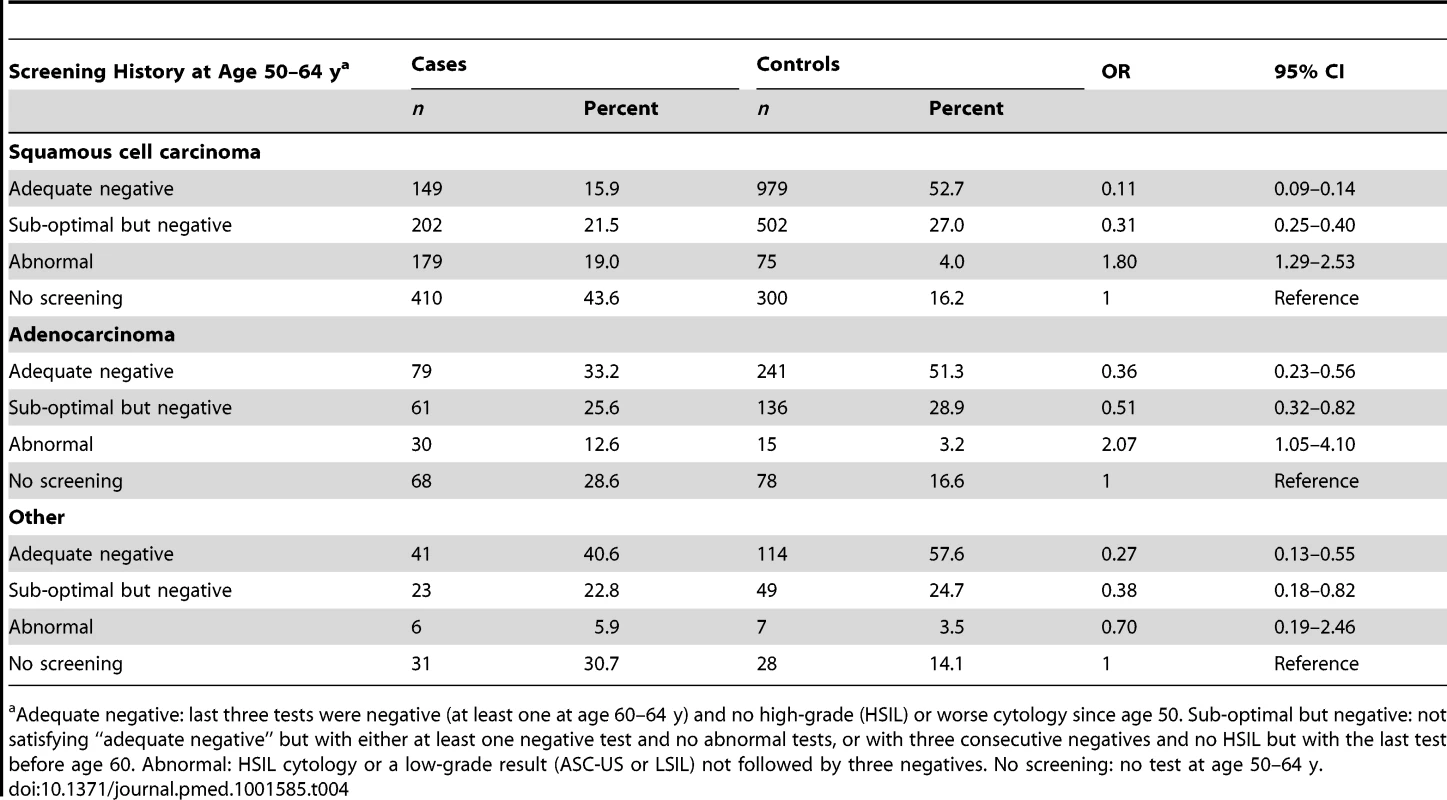
a Adequate negative: last three tests were negative (at least one at age 60–64 y) and no high-grade (HSIL) or worse cytology since age 50. Sub-optimal but negative: not satisfying “adequate negative” but with either at least one negative test and no abnormal tests, or with three consecutive negatives and no HSIL but with the last test before age 60. Abnormal: HSIL cytology or a low-grade result (ASC-US or LSIL) not followed by three negatives. No screening: no test at age 50–64 y. To explore the effect of screening per se (as opposed to the association with negative screening), we estimated the association (OR) between a diagnosis of cervical cancer at age 65–83 y and the maximum screening interval at ages 50–64 y (Table 5). The lowest risk was seen for those with a screening interval of ≤5.5 y (OR = 0.25, 95% CI 0.21–0.30, compared with those with no screen recorded at age 50–64 y): screening intervals of ≤3.5 y were no more “protective” than those of 3.5–5.5 y. Even women with a screening interval of 9–15 y had significantly lower risk than those not screened at all at age 50–64 y (OR = 0.54, 95% CI 0.40–0.71). The lower risk associated with the currently recommended screening every 5 y (interval ≤5.5 y) at ages 50–64 y diminished with increasing age at diagnosis: 65–69 y, OR = 0.12 (95% CI 0.09–0.17); 70–74 y, OR = 0.27 (95% CI 0.19–0.36); 75–79 y, OR = 0.46 (95% CI 0.35–0.61); and 80–83 y, OR = 0.49 (95% CI 0.28–0.83) (Figure 4; Table 6).
Fig. 4. Subgroup analyses—odds ratios of cervical cancer at age 65–79 y for women screened at least every 5.5 y at age 50–64 y relative to no screening at age 50–64 y. 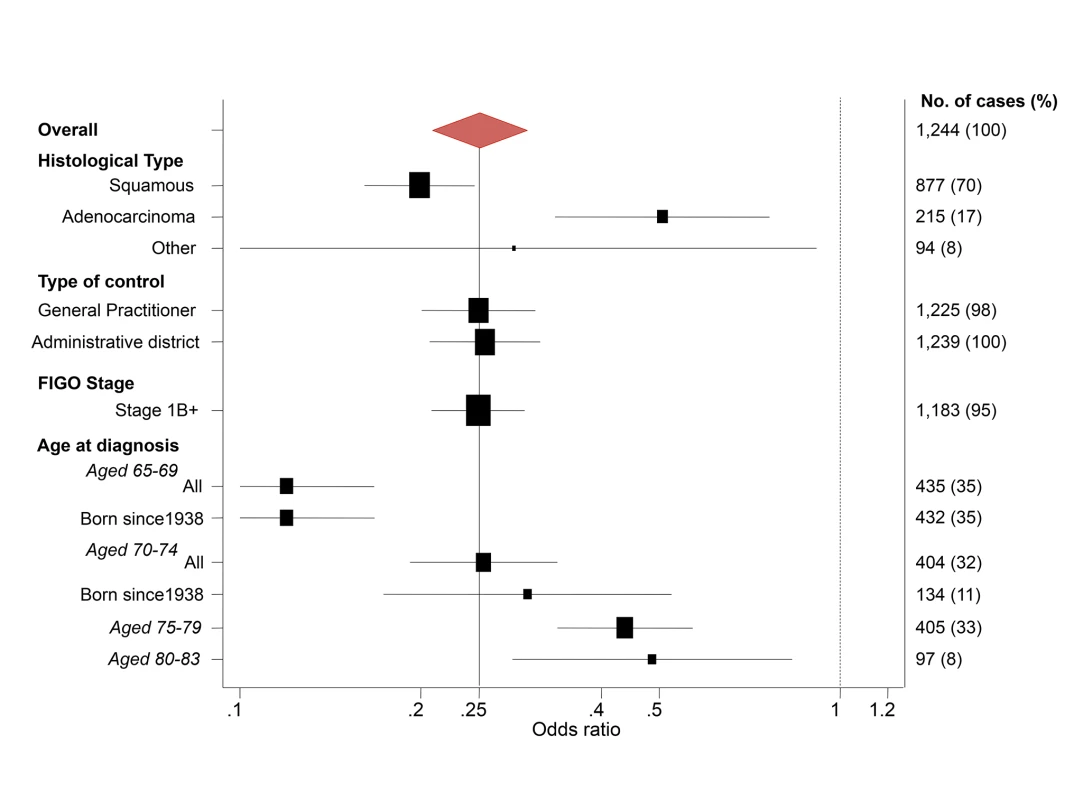
Tab. 5. Risk of cervical cancer at age 65–79 y by maximum screening interval between the ages 50 and 64 y. 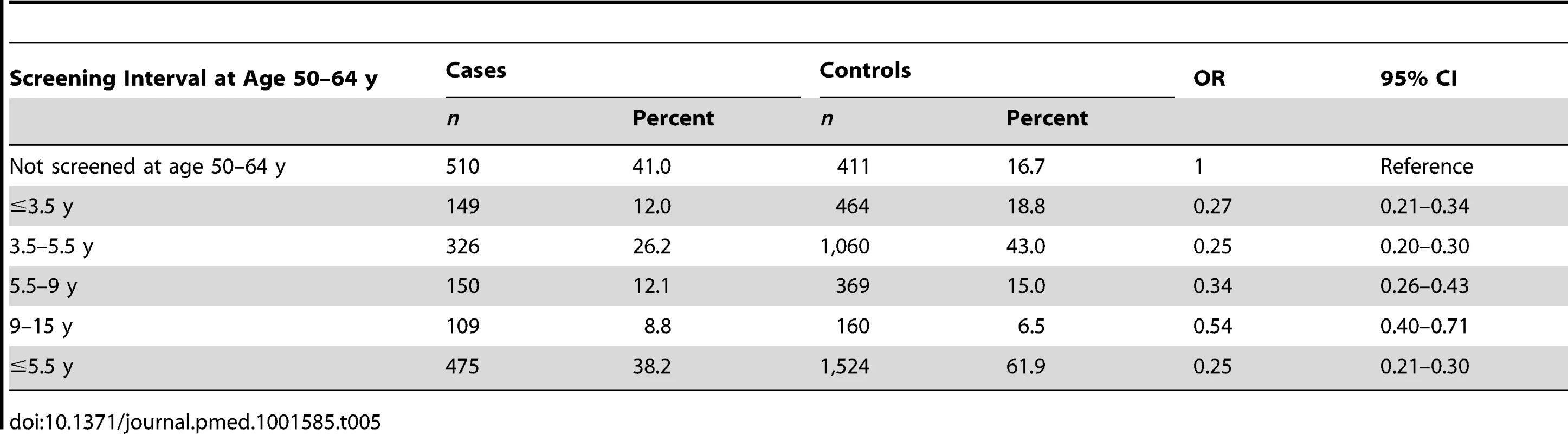
Tab. 6. Odds ratios of cervical cancer by maximum screening interval at age 50–64 y relative to no screening, by age at diagnosis. 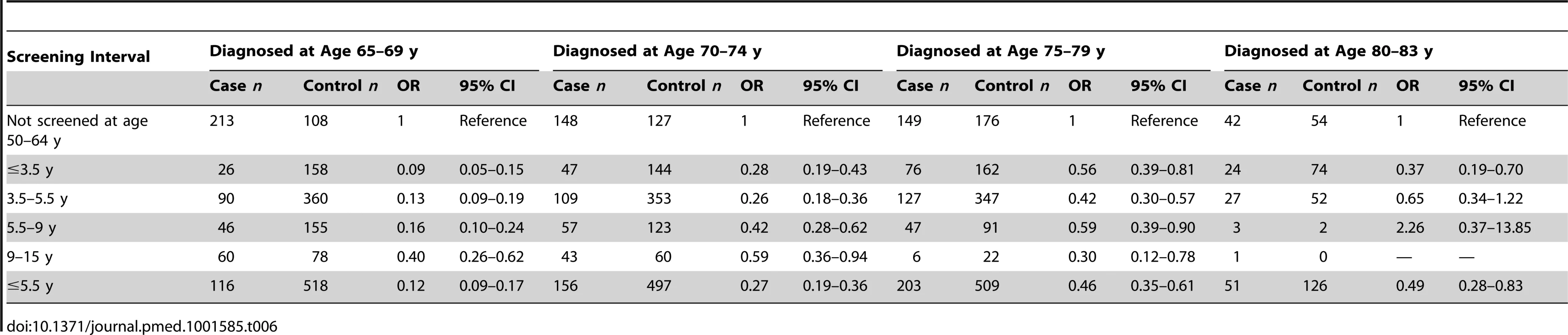
The estimated ORs depended on the age at diagnosis and histological type, but were otherwise very similar in different subgroups (Figures 2 and 4). Restricting analysis to cases known to have stage 1B or worse cancer or to cases born since 1938 gave (age-specific) ORs that were extremely similar to those without such restrictions. Similarly, the results were extremely similar using either only the GP control or only the district control.
Treating the associations in Table 5 as causal, we estimated that in the absence of screening at ages 50–64 y, cervical cancer rates in women aged 65–79 y (currently 9.6 per 100,000 woman-years) [19] would have been 2.42 (95% CI 2.11–2.71) times higher (i.e., 23 per 100,000 woman-years). Conversely, had all women been screened at intervals of ≤5.5 y between the ages 50 and 64 y, population rates of cervical cancer in women aged 65–79 would have been 38% (95% CI 32%–42%) lower than those observed (corresponding to a rate of 5.9 per 100,000 woman-years).
It is possible that the distribution of risk factors (other than cervical screening) for cervical cancer differed between screened and unscreened women. Taking what we consider to be a plausible but extreme scenario (Table 7), resulted in an 18% ( = 1/0.85−1) increase in the estimated ORs, i.e., the ORs of cervical cancer in regularly screened women compared with never screened women would be 0.14 for women aged 65–69 y, 0.32 for women aged 70–74 y, and 0.54 for women aged 75–79 y.
Tab. 7. Estimated relative risks of cervical cancer associated with questionnaire-type risk factor data (e.g., economic deprivation, number of sexual partners, and smoking). 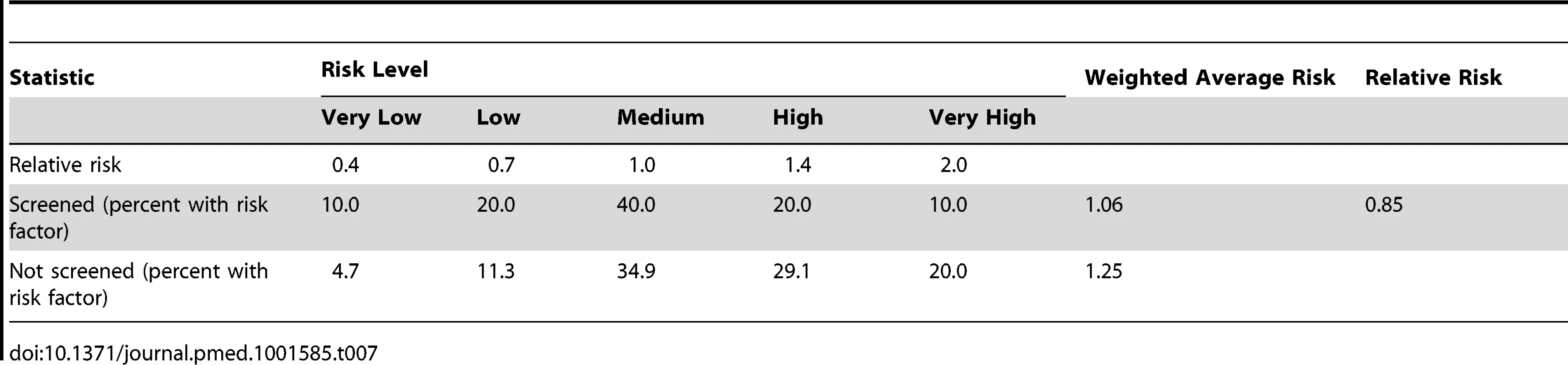
For illustration of what might be the effect of changing the age at last screen, we have estimated the potential impact of stopping screening at age 65 y (as per our data), age 55, or age 75 y (Table 8). In 1975 the cumulative incidence was 892 cervical cancers per 100,000 women. With screening until age 65, this value would be reduced to 211 using the ORs from this study as relative risks, and 250 with the adjustment for unobserved confounding. Using the observed relative risks, there would be an additional 182 cancers if cervical cancer screening ceased at age 55, and 103 fewer if it continued until age 75. Interestingly, the added benefit of prolonged screening is greater with the adjusted relative risks: 216 additional cervical cancers when stopping screening at age 55, and 122 fewer when continuing screening to age 75.
Tab. 8. Modelling of the effect of ceasing screening at age 55 or age 75(for unknown confounders) relative risks for women screened at least every 5.5 y up to age 65 y. 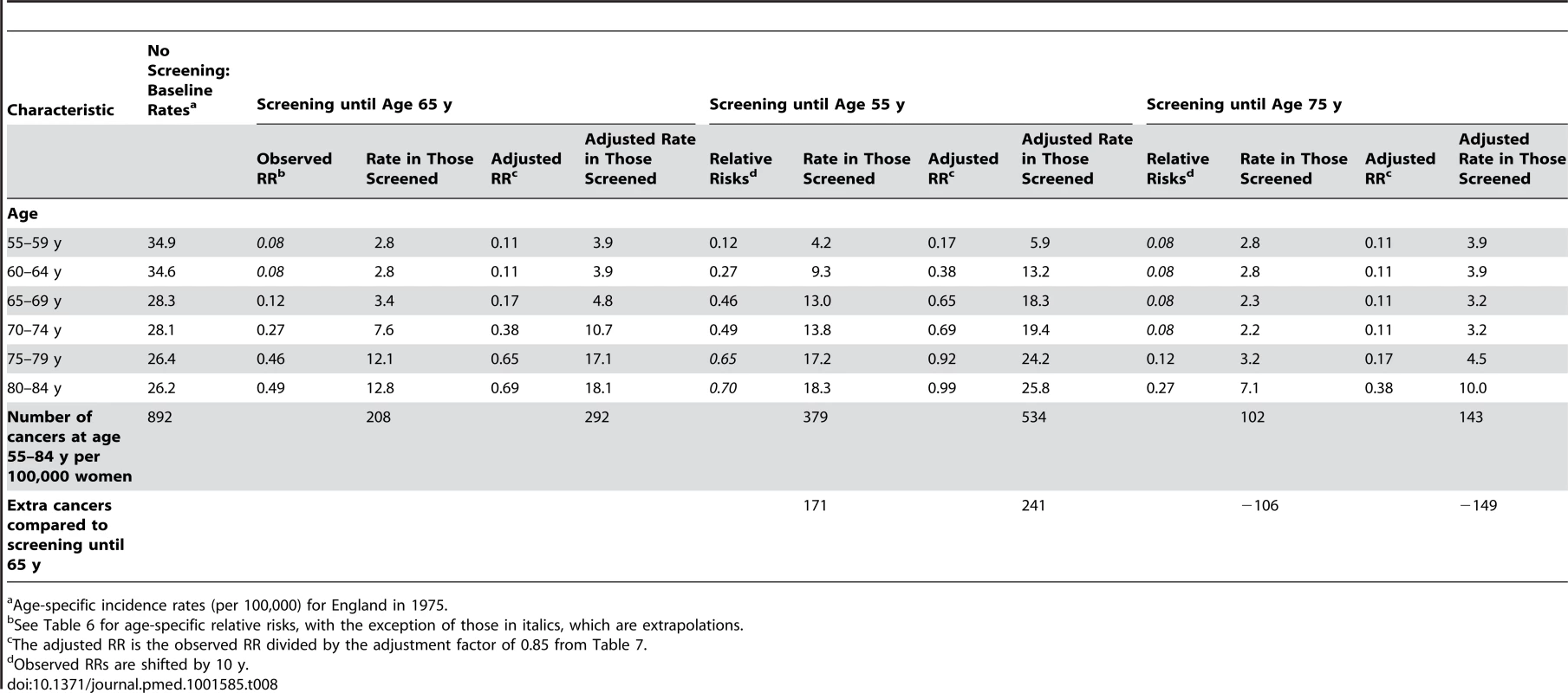
a Age-specific incidence rates (per 100,000) for England in 1975. Discussion
This study showed that women with adequate negative screening at age 50–64 y (women whose last three tests were negative [with at least one at age 60–64 y] and who had no high-grade cytology between the ages of 50 and 64 y) were at particularly low risk of being diagnosed with cervical cancer at age 65 y or older: the risk is 84% less than in unscreened women. The 20-y absolute risk of cervical cancer was eight per 10,000 women in those so screened compared to 49 per 10,000 women in those not screened between the ages of 50 and 64 y. Similarly, low risk of cervical cancer was observed among women whose interval between tests at ages 50–64 y was no greater than 5.5 y. The “protection” of adequate negative screening at age 50–64 y was greater for women aged 65–69 y and decreased steadily with time since last negative screen. It was considerably less 15 y after screening than 10 y after screening. Similarly, regular screening (interval ≤5.5 y) at ages 50–64 y was associated with a low risk of cervical cancer until age 75; thereafter, the “effect” of screening weakened, and by age 80 y the risk in well-screened women was about half the risk of unscreened women. These results were robust to a number of sensitivity analyses.
This is the largest study looking at cervical screening and the risk of cervical cancer at age 65 y and older. Controls were automatically selected from the database of all women invited for cervical screening in England and Wales, minimising the selection bias, and linkage was used to obtain all screening histories, ensuring completeness of the data and no recall bias. The design (a population-based case control study) allowed estimation of absolute risks.
The main limitation of this observational study is that the database containing screening histories (from which the controls were identified) did not record information on risk factors. The association with screening in this study is greater for squamous cell carcinoma than for adenocarcinoma. The risk factors for both histological types are similar [18], and the vast majority of in situ lesions detected by screening are squamous cell carcinomas [20]. Together these three facts suggest that the greater association with squamous cell carcinoma is due to screening and not confounding. Additionally, the association was greater within 5 y (compared with 12.5–17.5 y) of screening, and we see no reason why the impact of unrecorded risk factors would change with time from last screen. Note that screening was not offered and was extremely rare after the age of 64 y, and coverage in women aged 50–64 y has been stable at about 80% over the last 16 y [20],[21]. Nevertheless, the underlying risk of cervical cancer in a woman who has never been screened may be greater than in a woman who is screened regularly. However, the only study that we could find of screening and cervical cancer that adjusts for risk factors (smoking) found that the adjusted and unadjusted ORs were similar [22]. Further, a study comparing never-attenders at cervical screening to attenders in Denmark found that never-attenders had no overrepresentation of cancer risk factors [23]. A small number of women in our cohort may have been screened before 1988 but not since, and we would not know about such early screening. However, analyses restricted to women who were under age 50 y in 1988 did not substantially change the age-specific effects of screening. Another limitation of our study is that we had only 97 cases over age 80 y and none over age 83 y, so estimates beyond age 80 y have wider confidence intervals, and we are unable to say what happens 20 y after the last screen taken at age 60–64 y (i.e., in women adequately negatively screened).
There has been only limited evidence and a lack of consensus regarding the optimal upper limit for screening. Even at younger ages there has been little study of the risk of cervical cancer more than 6 y after the last screening test. A smaller study by Kamineni et al. [22] including 69 cases in women aged 55–79 y (with a maximum time since last negative test of 7 y) also found a reduction in risk of developing cervical cancer within 5 y of the last negative test.
Our results do not give cause for concern regarding the current US recommendations to cease cervical screening in previously well-screened women at age 65 y. This recommendation has been supported by recently published data from a study in northern California [24]. However, while screening at age 50–64 y offers some protection long term, the magnitude of the protection decreases with time.
Screening in older women can be uncomfortable, and a lack of oestrogen can make obtaining an adequate cytology sample and interpreting it difficult. Although HPV testing on a sample collected without a speculum would overcome these problems for women testing HPV negative, those testing positive would still (currently) require cytology triage, and some would require colposcopy. Further, those women who test positive for HPV but who have normal cytology (approximately 4% [25]) pose a challenge for clinical management. Indeed, in the context of primary HPV screening, there will be the need for ongoing reassessment about the optimal age of stopping screening in relation to screening history.
The absolute risks in this paper are based on current rates of cervical cancer in older women in England. The absolute impact in the UK may be greater over the next 15 y because of the increasing underlying risk in cohorts born since 1950 [26]–[28]. Applying the same relative risks to a population with an underlying age-specific annual rate of cervical cancer of about 80 per 100,000 women between the ages 65 and 79 y, for every 1,000 women screened regularly between the ages 50 and 64 y, there would be approximately nine fewer cancers between the ages of 65 and 79 y.
Taken with the considerable evidence that cervical screening causally reduces cervical cancer incidence [29], the results here suggest that screening women aged 50–64 y substantially reduces their risk of cervical cancer at age 65 y and older, but that the magnitude of that protection decreases with time since last screen. The low risk of cervical cancer in those with a test at least every 5.5 y from age 50 to age 64 y (OR = 0.25) can to a large extent be attributed, in the authors' view, to the protection offered by screening. Based on this result, we estimated the annual rate of cervical cancer in the absence of screening to be 25 per 100,000 women. Interestingly this rate is slightly lower than the rate in women aged 65–79 y in England (27.6 per 100,000) before the screening programme was introduced (i.e., 1975–1988) [16].
The lower OR associated with adequate negative screening (0.16) than with regular screening (0.25) represents the ability of screening to identify those at particularly low risk of developing cancer in the following few years. This finding suggests that the guidelines recommending that women should exit screening only if their last three tests were all normal (and they satisfy the criteria for being adequately negatively screened) are sensible. Similarly, the high risk of cervical cancer (20-y absolute risk of 0.86%) in women with an unresolved abnormal test result in their history when they reach age 65 y emphasises the need for continued surveillance for such women (at least until three consecutive negatives are obtained). Among those with adequate negative screening, ceasing screening at age 50 y is unlikely to provide lifelong protection, but continuing screening till age 80 y is not necessary. As life expectancy increases (at age 65 y it is about 20 additional years for women in many industrialised countries [30]), consideration should be given to increasing the upper age of screening, possibly by extending the final screening interval from 5 to 10 y. This study provides evidence that should help produce cost-effectiveness analyses that would enable policy-makers to reach a rational decision.
Cervical screening is changing; most countries with organised screening have moved from conventional cytology to liquid-based cytology, and many are now considering primary HPV testing. This study was done within the UK with a mix of conventional cytology and liquid-based cytology, and as such, these results may not be generalisable to HPV-based screening programmes. Since the long-term negative predictive value of HPV testing is even better than that of cytology, one would expect the period of low risk to be longer following an HPV test, but there are currently no studies looking at the risk 15–20 y after a negative HPV test.
Cervical screening in women aged 50–64 y has a substantial impact on cervical cancer rates not only at age 50–64 y, but for many years thereafter. Screening up to age 65 y greatly reduces the risk of cervical cancer in the following decade, but the protection weakens with time and is substantially less 15 y after the last screen. In the light of increasing life expectancy, it would seem inappropriate for countries that currently stop screening between the ages 60 and 69 y to consider reducing the age at which screening ceases. To the contrary, consideration should be given to cost-effective ways of increasing the age of the last screening.
Supporting Information
Zdroje
1. IsideanSD, FrancoEL (2013) Counterpoint: cervical cancer screening guidelines—approaching the golden age. Am J Epidemiol 178 : 1023–1026.
2. RustagiAS, KamineniA, WeissNS (2013) Point: cervical cancer screening guidelines should consider observational data on screening efficacy in older women. Am J Epidemiol 178 : 1020–1022.
3. SirovichB, WelchG (2004) The frequency of pap smear screening in the United States. J Gen Intern Med 19 : 243–250.
4. SirovichB, GottliebDJ, FisherES (2003) The burden of prevention: downstream consequences of PAP smear testing in the elderly. J Med Screen 10 : 189–195.
5. SaslowD, SolomonD, LawsonHW, KillackeyM, KulasingamSL, et al. (2012) American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 62 : 147–172.
6. Committee on Practice Bulletins–Gynecology (2012) ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 120 : 1222–1238.
7. Van WijngaardenWJ, DuncanID (1993) Rationale for stopping cervical screening in women over 50. BMJ 306 : 967–971.
8. ReboljM, van BallegooijenM, LyngeE, LoomanC, Essink-BotML, et al. (2009) Incidence of cervical cancer after several negative smear results by age 50: prospective observational study. BMJ 338: b1354.
9. DowlingEC, KlabundeC, PatnickJ, Ballard-BarbashR (2010) Breast and cervical cancer screening programme implementation in 16 countries. J Med Screen 17 : 139–146.
10. Health Research Authority (2009, rev April 2013) Defining research. Available: http://www.hra.nhs.uk/documents/2013/09/defining-research.pdf. Accessed 11 December 2013.
11. SasieniP, AdamsJ, CuzickJ (2003) Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 89 : 88–93.
12. SasieniP, CastanonA, CuzickJ (2009) Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 339: b2968.
13. NHS Cervical Screening Programme (2006) Audit of invasive cervical cancers. Available: http://cancerscreening.org.uk/cervical/publications/nhscsp28.html. Accessed 15 July 2013.
14. Sasieni P, Castanon A, editors (2012) NHSCSP Audit of invasive cervical cancer: national report 2007–2011. Available: http://www.cancerscreening.nhs.uk/cervical/publications/nhscsp-audit-invasive-cervical-cancer.html. Accessed 15 July 2013.
15. NHS Cervical Screening Programme (2000) Achievable standards, benchmarks for reporting, and criteria for evaluating cervical cytopathology. Available: http://www.cancerscreening.nhs.uk/cervical/publications/cc-02.html. Accessed 4 December 2013.
16. Office for National Statistics (2013) Cancer statistics registrations, England (series MB1). Available: http://www.ons.gov.uk/ons/search/index.html?newquery=seriesmb1. Accessed 27 July 2013.
17. Welsh Cancer Intelligence and Surveillance Unit (2012) Cancer incidence in Wales, 2006–2010. Available: http://www.wales.nhs.uk/sites3/page.cfm?orgid=242&pid=59080. Accessed 4 December 2013.
18. International Collaboration of Epidemiological Studies of Cervical Cancer (2007) Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer 120 : 885–891.
19. Office for National Statistics (2013) Cancer statistics registrations. Registrations of cancer diagnosed in 2011, England. Available: http://www.ons.gov.uk/ons/rel/vsob1/cancer-statistics-registrations-england-series-mb1-/no-42-2011/stb-cancer-statistics-registrations-2011.html. Accessed 27 July 2013.
20. Health and Social Care Information Centre (2010) Cervical Screening Programme—England: 2009–2010. Available: https://catalogue.ic.nhs.uk/publications/screening/cervical/cerv-scre-prog-eng-2009-10/cerv_scre_prog_eng_2009-10_rep_v3.pdf. Accessed 4 December 2013.
21. Health and Social Care Information Centre (2012) Cervical screening programme, England—2011–12. Available: http://www.cancerscreening.nhs.uk/cervical/statistics-bulletin.html. Accessed 15 July 2013.
22. KamineniA, WeinmannS, ShyKK, GlassAG, WeissNS (2013) Efficacy of screening in preventing cervical cancer among older women. Cancer Causes Control 24 : 1653–1660.
23. LarsenLP, OlesenF (1996) Characteristics of subgroups of attenders and non-attenders in an organised screening programme for cervical cancer. J Med Screen 3 : 133–139.
24. DinkelspielH, FettermanB, PoitrasN, KinneyW, CoxJT, et al. (2012) Screening history preceding a diagnosis of cervical cancer in women age 65 and older. Gynecol Oncol 126 : 203–206.
25. DattaSD, KoutskyLA, RatelleS, UngerER, ShlayJ, et al. (2008) Human papillomavirus infection and cervical cytology in women screened for cervical cancer in the United States, 2003–2005. Ann Intern Med 148 : 493–500.
26. PetoJ, GilhamC, FletcherO, MatthewsFE, PetoJ, et al. (2004) The cervical cancer epidemic that screening has prevented in the UK. Lancet 364 : 249–256.
27. SasieniP, AdamsJ (1999) Effect of screening on cervical cancer mortality in England and Wales: analysis of trends with an age period cohort model. BMJ 318 : 1244–1245.
28. BeralV (1974) Cancer of the cervix: a sexually transmitted infection? Lancet 1 : 1037–1040.
29. International Agency for Research on Cancer, World Health Organization (2005) Cervix cancer screening. IARC Handbooks of Cancer Prevention. Lyon (France): IARC Press. 302 p.
30. MurphyS, XuJ, KochanekK (2012) Division of Vital Statistics (2012) Deaths: preliminary data for 2010. Natl Vital Stat Rep 60 : 1–52 Available: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed 27 July 2013.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Soon Will Be Ten: A Call for Papers on the Health of Pre-adolescent Children
- Home Blood Pressure Monitoring: New Evidence for an Expanded Role
- Cervical Cancer Screening in Older Women: New Evidence and Knowledge Gaps
- Taxes on Sugar-Sweetened Beverages to Curb Future Obesity and Diabetes Epidemics
- The Failure of Screening and Treating as a Malaria Elimination Strategy
- Averting Obesity and Type 2 Diabetes in India through Sugar-Sweetened Beverage Taxation: An Economic-Epidemiologic Modeling Study
- Managing Incidental Genomic Findings in Clinical Trials: Fulfillment of the Principle of Justice
- Associations between Intimate Partner Violence and Termination of Pregnancy: A Systematic Review and Meta-Analysis
- Developing a Sustainable Nutrition Research Agenda in Sub-Saharan Africa—Findings from the SUNRAY Project
- Scale-up of Malaria Rapid Diagnostic Tests and Artemisinin-Based Combination Therapy: Challenges and Perspectives in Sub-Saharan Africa
- Preterm Birth and Childhood Wheezing Disorders: A Systematic Review and Meta-Analysis
- Cervical Screening at Age 50–64 Years and the Risk of Cervical Cancer at Age 65 Years and Older: Population-Based Case Control Study
- Improving Women's Health through Universal Health Coverage
- A Risk Prediction Model for the Assessment and Triage of Women with Hypertensive Disorders of Pregnancy in Low-Resourced Settings: The miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) Multi-country Prospective Cohort Study
- Risk Stratification by Self-Measured Home Blood Pressure across Categories of Conventional Blood Pressure: A Participant-Level Meta-Analysis
- Impact of Intermittent Screening and Treatment for Malaria among School Children in Kenya: A Cluster Randomised Trial
- Muscle-Strengthening and Conditioning Activities and Risk of Type 2 Diabetes: A Prospective Study in Two Cohorts of US Women
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Associations between Intimate Partner Violence and Termination of Pregnancy: A Systematic Review and Meta-Analysis
- Risk Stratification by Self-Measured Home Blood Pressure across Categories of Conventional Blood Pressure: A Participant-Level Meta-Analysis
- A Risk Prediction Model for the Assessment and Triage of Women with Hypertensive Disorders of Pregnancy in Low-Resourced Settings: The miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) Multi-country Prospective Cohort Study
- Scale-up of Malaria Rapid Diagnostic Tests and Artemisinin-Based Combination Therapy: Challenges and Perspectives in Sub-Saharan Africa
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




