-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Role of Adiposity in Cardiometabolic Traits: A Mendelian Randomization Analysis
Background:
The association between adiposity and cardiometabolic traits is well known from epidemiological studies. Whilst the causal relationship is clear for some of these traits, for others it is not. We aimed to determine whether adiposity is causally related to various cardiometabolic traits using the Mendelian randomization approach.Methods and Findings:
We used the adiposity-associated variant rs9939609 at the FTO locus as an instrumental variable (IV) for body mass index (BMI) in a Mendelian randomization design. Thirty-six population-based studies of individuals of European descent contributed to the analyses.
Age - and sex-adjusted regression models were fitted to test for association between (i) rs9939609 and BMI (n = 198,502), (ii) rs9939609 and 24 traits, and (iii) BMI and 24 traits. The causal effect of BMI on the outcome measures was quantified by IV estimators. The estimators were compared to the BMI–trait associations derived from the same individuals. In the IV analysis, we demonstrated novel evidence for a causal relationship between adiposity and incident heart failure (hazard ratio, 1.19 per BMI-unit increase; 95% CI, 1.03–1.39) and replicated earlier reports of a causal association with type 2 diabetes, metabolic syndrome, dyslipidemia, and hypertension (odds ratio for IV estimator, 1.1–1.4; all p<0.05). For quantitative traits, our results provide novel evidence for a causal effect of adiposity on the liver enzymes alanine aminotransferase and gamma-glutamyl transferase and confirm previous reports of a causal effect of adiposity on systolic and diastolic blood pressure, fasting insulin, 2-h post-load glucose from the oral glucose tolerance test, C-reactive protein, triglycerides, and high-density lipoprotein cholesterol levels (all p<0.05). The estimated causal effects were in agreement with traditional observational measures in all instances except for type 2 diabetes, where the causal estimate was larger than the observational estimate (p = 0.001).Conclusions:
We provide novel evidence for a causal relationship between adiposity and heart failure as well as between adiposity and increased liver enzymes.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(6): e32767. doi:10.1371/journal.pmed.1001474
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001474Summary
Background:
The association between adiposity and cardiometabolic traits is well known from epidemiological studies. Whilst the causal relationship is clear for some of these traits, for others it is not. We aimed to determine whether adiposity is causally related to various cardiometabolic traits using the Mendelian randomization approach.Methods and Findings:
We used the adiposity-associated variant rs9939609 at the FTO locus as an instrumental variable (IV) for body mass index (BMI) in a Mendelian randomization design. Thirty-six population-based studies of individuals of European descent contributed to the analyses.
Age - and sex-adjusted regression models were fitted to test for association between (i) rs9939609 and BMI (n = 198,502), (ii) rs9939609 and 24 traits, and (iii) BMI and 24 traits. The causal effect of BMI on the outcome measures was quantified by IV estimators. The estimators were compared to the BMI–trait associations derived from the same individuals. In the IV analysis, we demonstrated novel evidence for a causal relationship between adiposity and incident heart failure (hazard ratio, 1.19 per BMI-unit increase; 95% CI, 1.03–1.39) and replicated earlier reports of a causal association with type 2 diabetes, metabolic syndrome, dyslipidemia, and hypertension (odds ratio for IV estimator, 1.1–1.4; all p<0.05). For quantitative traits, our results provide novel evidence for a causal effect of adiposity on the liver enzymes alanine aminotransferase and gamma-glutamyl transferase and confirm previous reports of a causal effect of adiposity on systolic and diastolic blood pressure, fasting insulin, 2-h post-load glucose from the oral glucose tolerance test, C-reactive protein, triglycerides, and high-density lipoprotein cholesterol levels (all p<0.05). The estimated causal effects were in agreement with traditional observational measures in all instances except for type 2 diabetes, where the causal estimate was larger than the observational estimate (p = 0.001).Conclusions:
We provide novel evidence for a causal relationship between adiposity and heart failure as well as between adiposity and increased liver enzymes.
Please see later in the article for the Editors' SummaryIntroduction
The incidence and prevalence of cardiovascular disease (CVD) are continuously increasing in parallel with the increase in obesity and metabolic diseases, especially in low - and middle-income countries [1]. An association between increased body mass index (BMI) and cardiometabolic diseases has been demonstrated by many well-designed epidemiological studies, and has previously been shown to be close to log-linear, at least for BMI>25 kg/m2 [2]. However, confounding, reverse causation, and other issues with conventional observational studies can seriously impair the possibility of making causal inference, and lead to imprecision in estimation of both the direction and magnitude of the effects, as has been shown for the associations between BMI and mortality from respiratory disease and lung cancer [3]. Several randomized clinical trials have found that lifestyle interventions aiming at weight loss decrease the risk of type 2 diabetes (T2D) and metabolic syndrome [4]–[6], whereas the follow-ups of these studies for CVD outcomes have been underpowered [7],[8]. The causal relationships of long-term obesity to disease are difficult to assess within conventional randomized clinical trials, necessitating other study designs.
In the past decade, instrumental variable (IV) analysis has become widely used for assessing causality using genetic variants under the name of “Mendelian randomization” (MR) [9]. MR represents one of the methods to infer causal relationships between epidemiologically relevant phenotypes. In MR study designs, a genetic variant associated with an intermediate phenotype (in the present report, BMI) is used as an IV to evaluate the causal relationship of the intermediate phenotype with the outcome of interest (Figure 1). Since genetic variants are assumed to be randomly distributed within a population, the IV is regarded as independent of confounders affecting the intermediate phenotype (BMI)–outcome relationship [10]. In the presence of confounding and reverse causation, the IV approach is an alternative for statistical estimation of causal relationships, especially within large-scale studies, where classical epidemiological modeling—fully adjusted for a wide range of covariates and across numerous outcomes—would be difficult. While acknowledging the issue of observed and unobserved confounding, we consider MR as a pragmatic tool for elucidating the epidemiological data through utilization of the findings from genetic association studies on intermediate phenotypes. The strength of the causal interpretation depends crucially on the validity of assumptions and caveats within MR experiments, some of which are difficult to evaluate [11]. If the basic assumptions are violated, invalid conclusions would be drawn from the experiments. In the past five years, large-scale collaborative efforts have successfully identified more than 30 loci associated with BMI and obesity [12]. The single nucleotide polymorphism (SNP) rs9939609, within the fat-mass - and obesity-associated gene (FTO) locus, was the first associated with BMI by genome-wide association studies, and the association has been extensively replicated in individuals of European descent and in other ethnic groups [12]. FTO locus variants alone have been reported to explain 0.34% of the phenotypic variability in BMI [13], and the rs9939609 variant is considered a good instrument in MR studies because of its specificity (lack of known pleiotropy) and decent effect size [14],[15].
Fig. 1. In a Mendelian randomization framework, genotype–phenotype association is assumed to be independent of confounding factors. 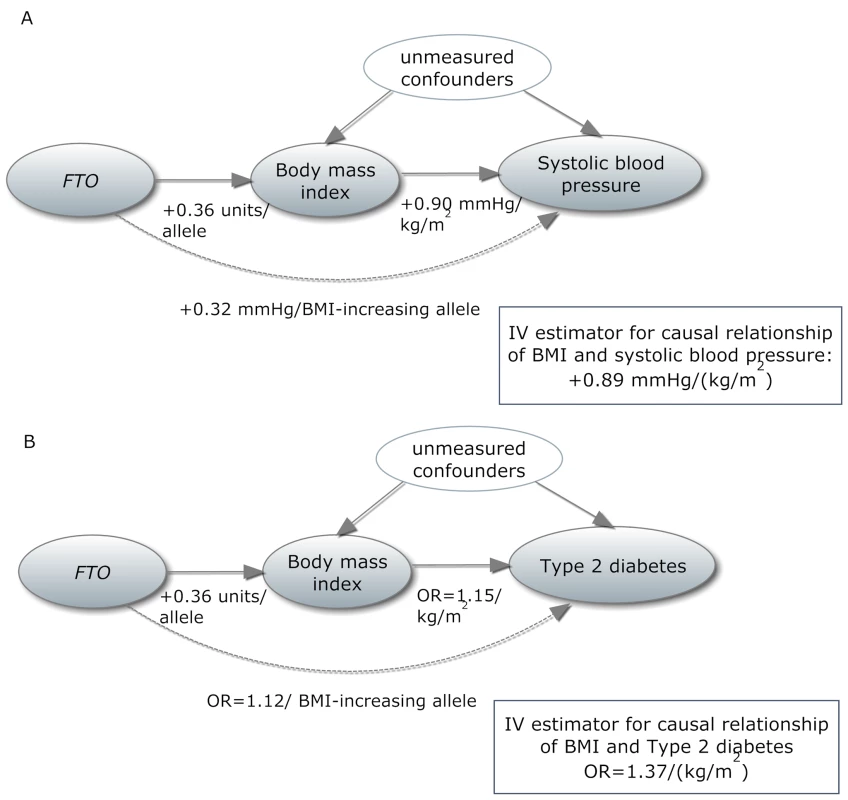
(A) In an example from our study, the IV estimator is calculated as the beta coefficient from the association of FTO with systolic blood pressure divided by the beta coefficient from the association of FTO with BMI (IV estimator = 0.32/0.36 = 0.89 mm Hg/BMI unit). The IV estimator is equivalent to what is seen when systolic blood pressure is regressed on BMI. These results are supportive of a causal, non-confounded relationship. For binary traits, the calculation of the IV estimator is done on the log-odds scale. (B) The relationship of BMI with T2D, where the IV estimator is ln(ORIV) = ln(1.12)/0.36, which equals a causal OR of BMI for T2D of 1.37. This is larger than what is seen in the standard age- and sex-adjusted logistic regression of T2D on BMI (p = 0.001), indicating that confounding or reverse causation may be present or that BMI measured once in adulthood does not fully reflect the effect of lifetime adiposity. Several MR studies using FTO variants have supported the hypothesis of a causal relationship between adiposity and cardiometabolic phenotypes, such as ischemic heart disease, C-reactive protein (CRP), systolic and diastolic blood pressure, fasting insulin, triglycerides, metabolic syndrome, and decreased concentrations of high-density lipoprotein cholesterol (HDL-C) [14]–[19]. However, the causal relationship between obesity and increased risk of other CVD and metabolic phenotypes, such as heart failure, stroke, and non-alcoholic fatty liver disease, is not yet established using these methods, probably because of power issues, as large sample sizes are needed for MR studies [15]. Table 1 shows an overview of previous MR studies of adiposity and cardiometabolic phenotypes, with reported sample sizes and instruments used.
Tab. 1. Comparison of our study with previous Mendelian randomization studies of adiposity on cardiometabolic phenotypes. 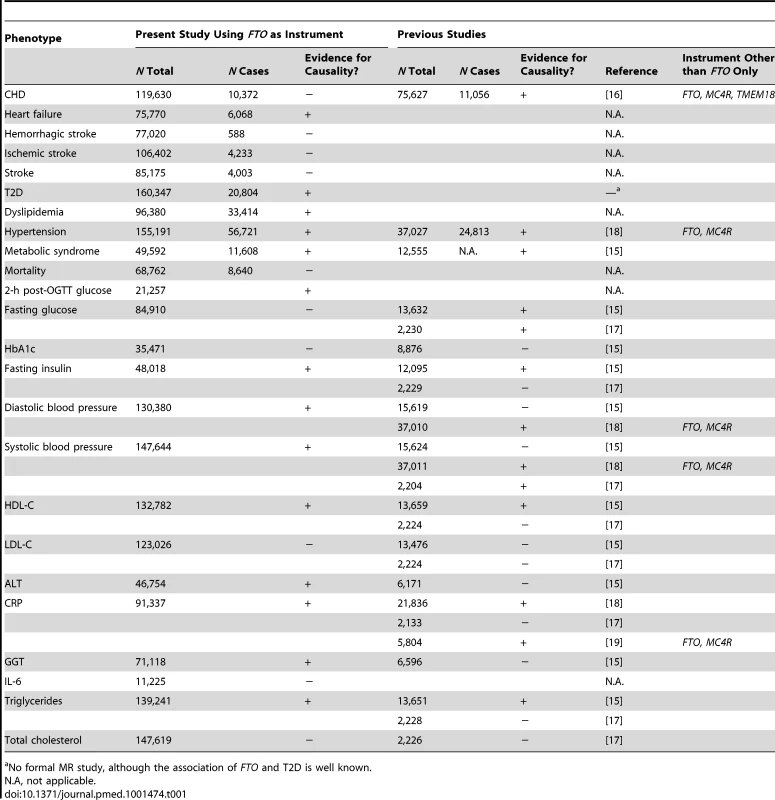
No formal MR study, although the association of FTO and T2D is well known. In the present investigation, which is the largest MR study to date, we aimed to evaluate the evidence for a causal relationship between adiposity, assessed as elevated BMI, and a wide range of cardiometabolic phenotypes including coronary heart disease, stroke, T2D, and heart failure, as well as a number of intermediate phenotypes related to future disease end points.
Methods
The study was conducted within the European Network for Genetic and Genomic Epidemiology (ENGAGE) consortium, represented here by 36 cross-sectional and longitudinal cohort studies and up to 198,502 individuals of European descent (Table S1).
Genotypes
Of the many highly correlated variants within the FTO locus, we chose the widely confirmed and extensively studied variant rs9939609 as the index SNP and IV for this study. Whenever possible, we used direct genotype information for rs9939609 from participating cohorts (n = 21) that had FTO variant genotypes available (Table S2). Eleven out of 36 studies performed de novo genotyping of rs9939609 for the present study, and ten studies used direct genotype information on rs9939609 from previously genotyped array data. Whenever rs9939609 was not genotyped directly, we used either (i) the HapMap II CEU (European) reference panel–imputed genetic information from genome-wide association studies (http://hapmap.ncbi.nlm.nih.gov/downloads/genotypes/2008-10_phaseII/) for rs9939609 (n = 5) or (ii) genotype information from a predefined list of proxies that are in high linkage disequilibrium (LD) with rs9939609 (n = 10, r2>0.9; Table S3). For the remaining studies, we used the directly genotyped proxies rs11075989 (n = 5, r2 = 1.0), rs3751812 (n = 4, r2 = 1.0), and rs1421085 (n = 1, r2 = 0.93). We estimated effects of the BMI-increasing A allele of rs9939609, or for the corresponding alleles from proxies (using HapMap II CEU LD data), on phenotypes. We excluded individuals from analysis when the overall array sample call rate was <95%. All studies reported SNPs with Hardy-Weinberg equilibrium exact p>0.0001, an information content >0.99 for imputed SNPs, and a call rate>0.95 for genotyped SNPs.
Outcomes
We studied nine dichotomous cardiometabolic outcomes in up to 160,347 individuals and 14 quantitative cardiometabolic traits in up to 147,644 individuals. Only individuals with both BMI and FTO genotype information available were included in the study.
The CVD dichotomous outcomes of interest were coronary heart disease (CHD), heart failure, hemorrhagic stroke, ischemic stroke, all-cause stroke, and hypertension diagnosed at any time point (ever) during the life course (Table 2). The metabolic dichotomous outcomes included dyslipidemia, metabolic syndrome, and T2D diagnosed at any time point (ever) during the life course. The diagnoses of CHD, heart failure, hemorrhagic stroke, ischemic stroke, all-cause stroke, and all-cause mortality were based on health registries and/or validated medical records (Table S4). Hypertension, dyslipidemia, and T2D diagnoses could be self-reported or based on biochemical measurement within the study, in addition to health registries and validated medical records (Table S4). The diagnosis of metabolic syndrome was based on a modified National Cholesterol Education Program Adult Treatment Panel III definition [20]. We analyzed a subset of individuals with prospectively collected events available for incident cases of all binary outcomes and for all-cause mortality as outcome.
Tab. 2. Meta-analysis results of Mendelian randomization analyses on effect of FTO-derived adiposity on cardiovascular and metabolic disease: dichotomous outcomes. 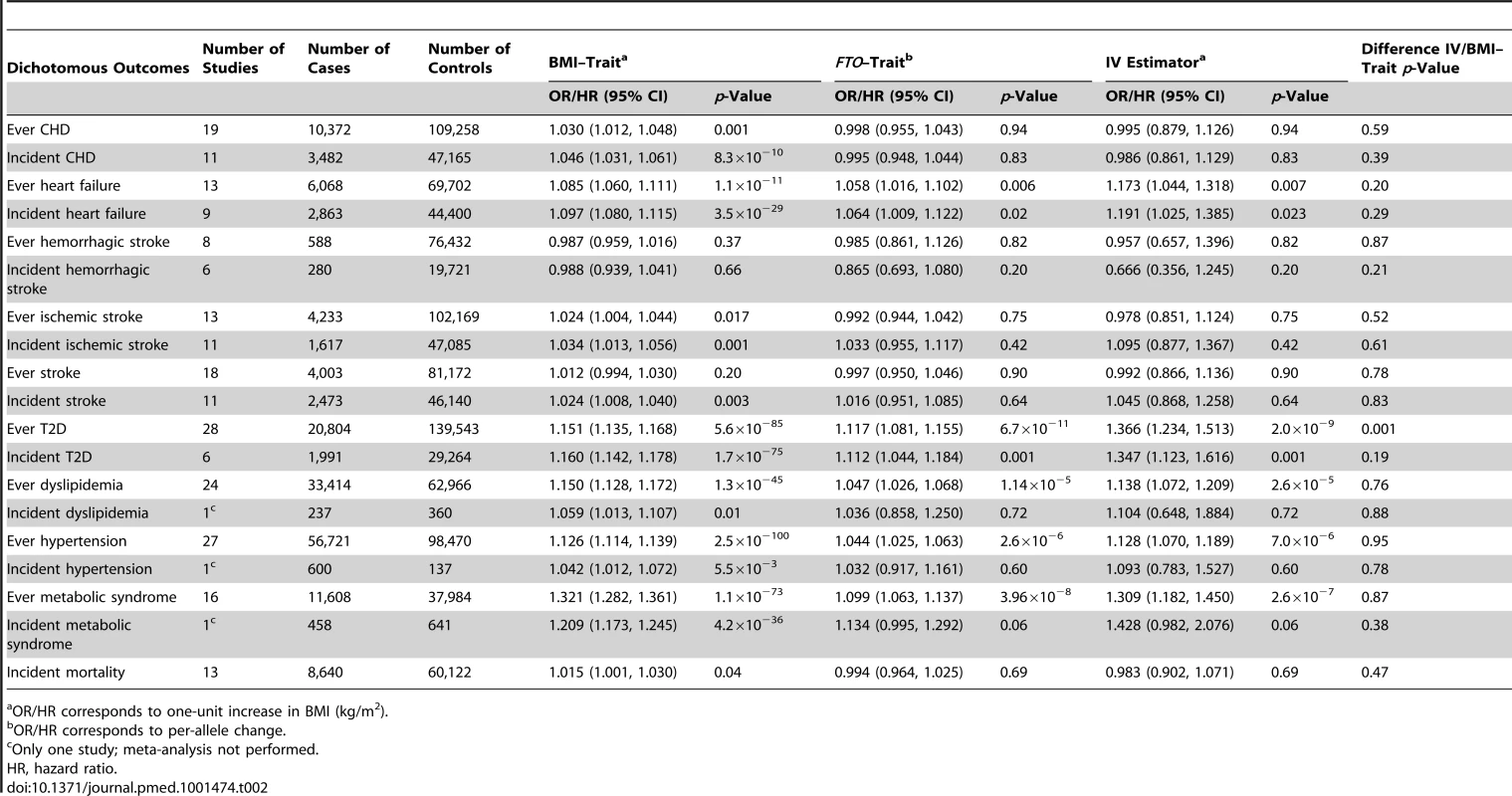
OR/HR corresponds to one-unit increase in BMI (kg/m2). We studied the following quantitative phenotypes (Table 3): (i) measurements of glucose homeostasis in individuals without diabetes: fasting glucose, 2-h post-load glucose from the oral glucose tolerance test (OGTT), hemoglobin A1c (HbA1c), and fasting insulin; (ii) diastolic and systolic blood pressure, with adjustment for blood pressure medication; (iii) lipid metabolism (in individuals without lipid-lowering medication): HDL-C, low-density lipoprotein cholesterol (LDL-C), total cholesterol, and triglycerides; (iv) liver enzyme activity and leakage: alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT); and (v) inflammation markers: CRP and interleukin-6 (IL-6). Prior to analysis the following variables were transformed to the natural logarithmic scale: fasting insulin, ALT, GGT, CRP, IL-6, and triglycerides (assay specifications are reported in Table S5).
Tab. 3. Meta-analysis results of Mendelian randomization analyses on effect of FTO-derived adiposity on cardiovascular and metabolic disease: quantitative phenotypes. 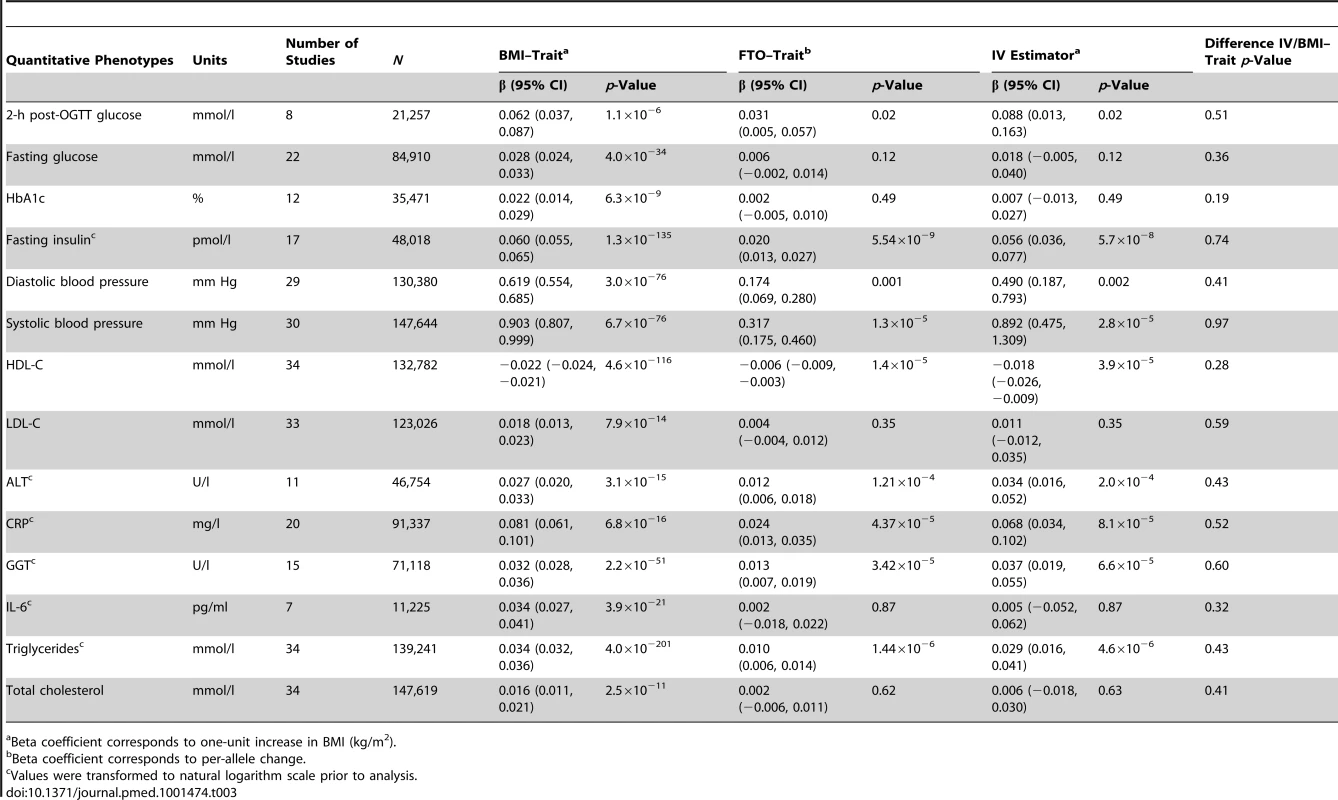
Beta coefficient corresponds to one-unit increase in BMI (kg/m2). Statistical Analyses
Association analyses. We assessed associations between the dichotomous outcomes and (i) FTO and (ii) BMI in each cohort using sex - and age-adjusted logistic regression models. We used Cox proportional hazards models to assess FTO and BMI associations with prospectively collected events [21]. The time origin in the present analysis was set to the date of first BMI measurement available. We assumed log-additive genetic effects on binary traits. We evaluated the associations of (i) FTO and (ii) BMI with the quantitative traits, as well as the association between FTO and BMI, using sex - and age-adjusted linear regression in each cohort, assuming an additive effect of the number of A alleles. The models are described in detail in Text S1. The software used for statistical analysis within each cohort is listed in Table S1.
Meta-analyses. As initial attempts at fixed-effects inverse-variance-weighted meta-analysis indicated considerable between-cohort heterogeneity, we performed random-effects meta-analyses, leading to essentially unchanged effect estimates, but somewhat more conservative confidence intervals (Figure S1). Hence, all results presented are from random-effects meta-analysis. Analyses were run at two centers in parallel using different software packages (GWAMA and R) [22],[23] and yielding identical results.
Instrumental variable analyses. We used the IV estimators to quantify the strength of the causal association between BMI and cardiometabolic traits. The estimate was found as a ratio between the two regression coefficients determined from association meta-analyses (Equation 1): estimated FTO effect on the given trait and estimated FTO effect on BMI in the full study sample (n = 198,502). For binary traits, the formula is identical to the Wald estimator [24].
For quantitative and binary outcomes with only one SNP as instrument, the IV estimator derived by Equation 1 is identical to that derived by the widely used two-stage least squares method [25]. The standard errors for the IV estimators were estimated using the delta method (Equation 2), ignoring correlation, based on a comprehensive sensitivity analysis; see Text S1, Figure S2, and Tables S6 and Table S7 for further details.
For each trait, we tested the null hypothesis of no difference between the respective IV estimator and the conventional regression-based estimator of the effect of BMI on trait via a classical z-test.
We did not apply correction for multiple testing as the associations between BMI and multiple cardiometabolic traits are widely reported [2],[5].
Results
Association between FTO Variant and BMI
Random-effects meta-analysis of the association between FTO variant and BMI in the 36 studies (n = 198,502) showed a positive effect of the A allele on BMI (β = 0.36 per additional A allele; 95% CI, 0.31–0.40; p = 4.3×10−52), with an effect size in line with that of previous studies [13]. The effect estimates ranged between 0.05 and 0.74 BMI units per copy of A allele, yielding an I2 for heterogeneity between studies of 55% (p = 3.6×10−5; Figure 2; Text S1). We assessed potential causes of this heterogeneity in a meta-regression of the study-specific beta coefficient estimates of effect sizes for the association between FTO and BMI—including study-specific mean age and mean BMI as covariates—and whether the study was exclusively of a diabetes case group or not. Effect size estimates decreased non-significantly with increasing cohort age in cohorts with mean age>40 y (n = 31, p = 0.07).
Fig. 2. Association between FTO variant rs9939609 and BMI in 198,502 individuals. 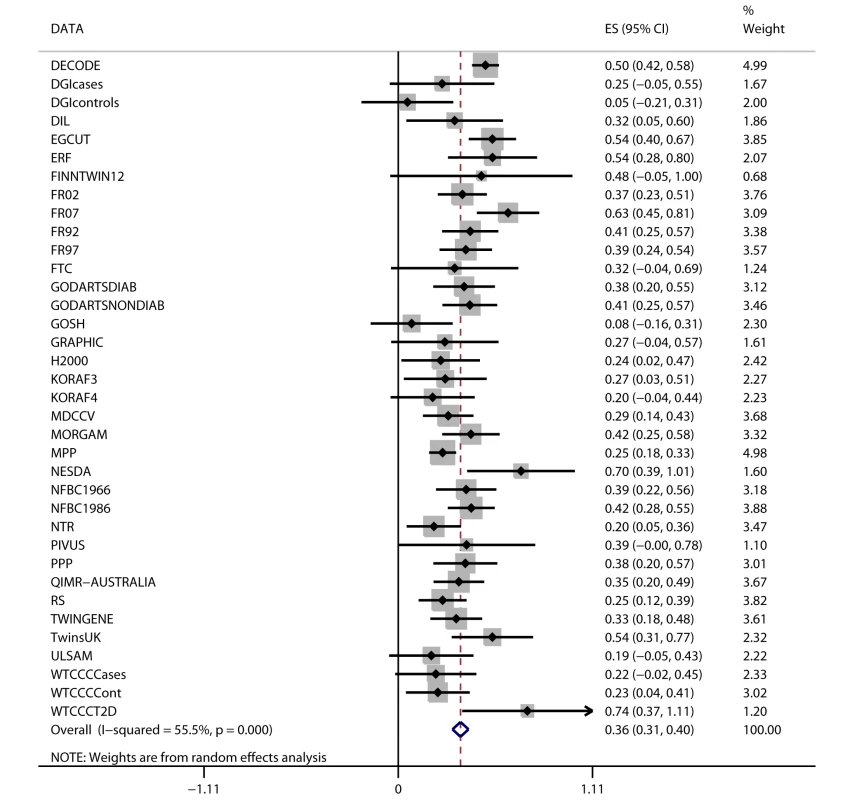
The assigned weight for each study in the meta-analysis is shown in percent (% Weight). ES, estimate. For cohort abbreviations and references, see Table S1. Associations between BMI and Cardiometabolic Traits
We observed positive associations (all p<0.05) between BMI and ever and incident heart failure (Figure 3), ever and incident CHD, ever all-cause stroke, ischemic stroke, hypertension, dyslipidemia, metabolic syndrome, T2D, and mortality (Table 2). We did not observe an association between BMI and ever or incident hemorrhagic stroke, or incident all-cause stroke. BMI was associated (all p<10−6) with all quantitative phenotypes: (i) fasting glucose, fasting insulin, 2-h post-OGTT glucose, and HbA1c; (ii) diastolic and systolic blood pressure; (iii) HDL-C, LDL-C, total cholesterol, and triglycerides; (iv) ALT and GGT; and (v) CRP and IL-6 (Table 3).
Fig. 3. Association between BMI and incident heart failure in 2,863 cases and 44,400 controls. 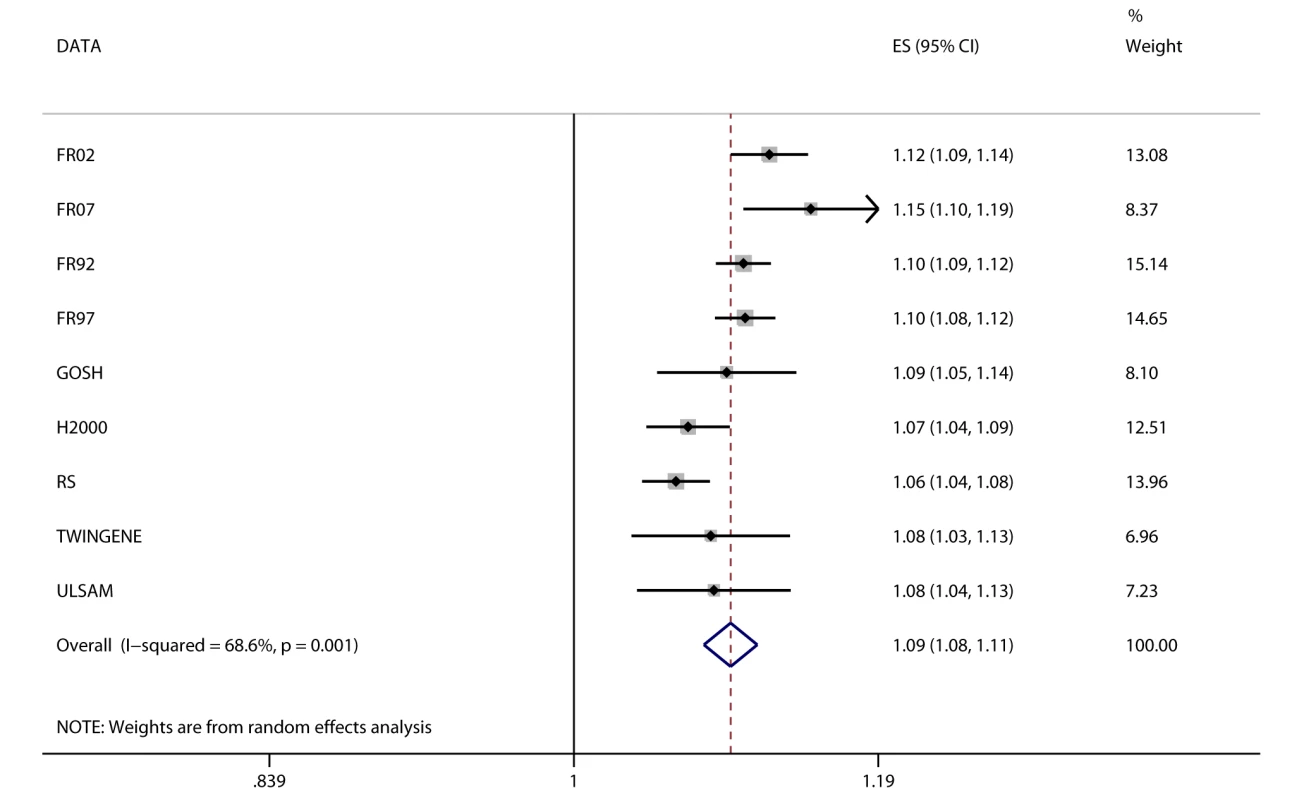
Estimates (ES) are shown on a hazard ratio scale for a one-unit increase in BMI. The assigned weight for each study in the meta-analysis is shown in percent (% Weight). For cohort abbreviations and references, see Table S1. Associations between FTO Variant and Cardiometabolic Traits
We detected a novel association between the BMI-increasing allele of the FTO variant and increased odds/hazard ratios of ever and incident heart failure (Figure 4;Table 2). Associations (all p<0.001) were observed between the FTO variant and increased odds/hazard ratios of ever or incident T2D, ever dyslipidemia, ever metabolic syndrome, and ever hypertension. The FTO variant was associated (all p<0.05) with increased levels of 2-h post-OGTT glucose, fasting insulin, diastolic blood pressure, systolic blood pressure, triglycerides, ALT, GGT, CRP, and decreased HDL-C.
Fig. 4. Association between FTO and incident heart failure in 2,863 cases and 44,400 controls. 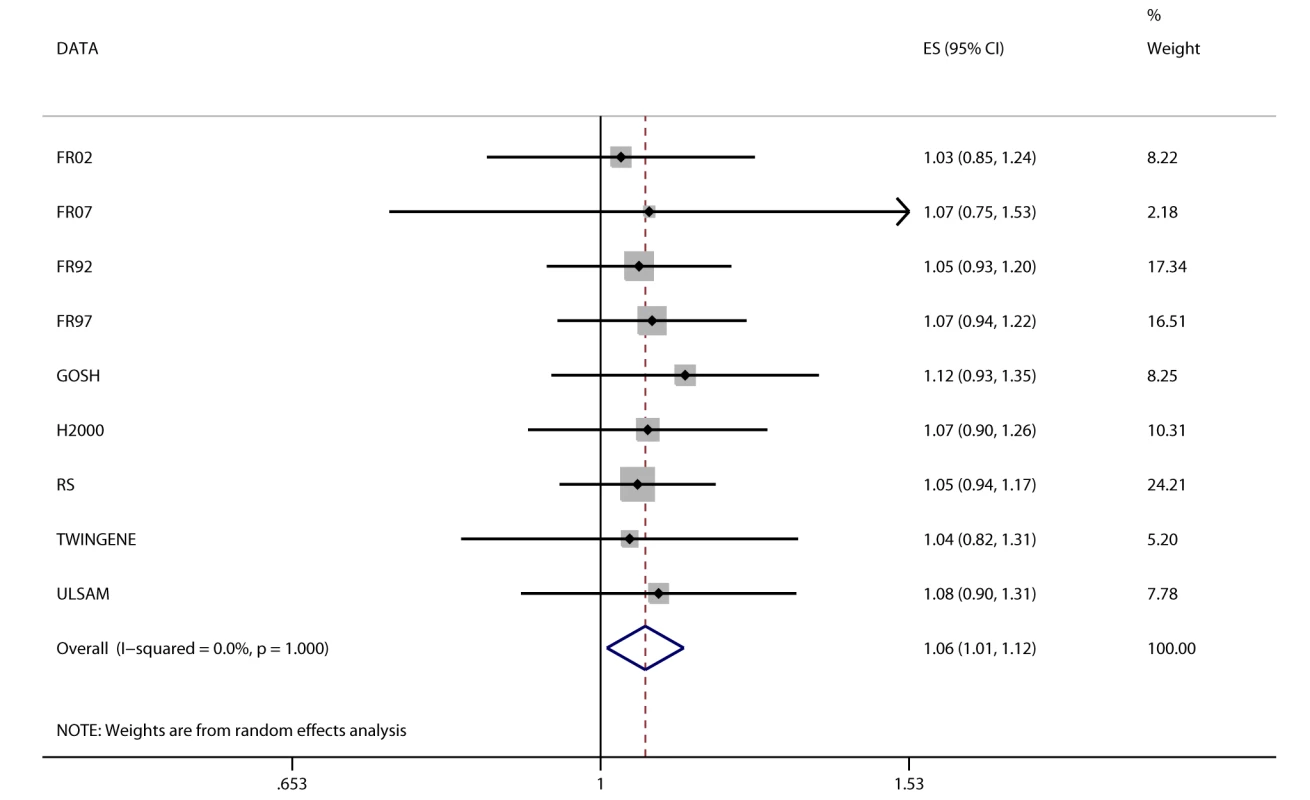
Estimates (ES) are shown on a hazard ratio scale per number of effect alleles. The assigned weight for each study in the meta-analysis is shown in percent (% Weight). For cohort abbreviations and references, see Table S1. Instrumental Variable Analysis
We identified at least nominally significant (p<0.05) causal estimates for the effect of BMI (IV estimators) on ever and incident heart failure, ever hypertension, ever and incident T2D, ever dyslipidemia, and ever metabolic syndrome (Table 2). For other dichotomous outcomes, we were not able to confirm the presence of a causal effect of BMI using the IV approach. The estimates derived from IV analysis based on either logistic regression modeling or Cox proportional hazards models were similar for our significant findings.
The IV estimators pointed to a causal effect of higher BMI on an increase in (i) ALT and GGT levels, a novel finding from the present study; (ii) 2-h post-OGTT glucose and fasting insulin; and (iii) diastolic blood pressure and systolic blood pressure. We also observed an unfavorable effect of BMI on lipid metabolism (in individuals without lipid medication), as indicated by decreased levels of HDL-C and increased levels of triglycerides. The IV estimators pointed to a causal link between BMI and inflammation, as indicated by increased levels of CRP. We did not observe a causal effect of BMI on levels of fasting glucose, HbA1c, LDL-C, IL-6, or total cholesterol (Table 3).
Post hoc power calculation showed that for the binary traits with non-significant IVs (CHD, ischemic stroke, and all-cause stroke), we had an 80% chance of detecting an IV estimator odds ratio (OR) of 1.08–1.09/BMI unit or higher, and a 95% chance of detecting an OR of 1.13–1.15/BMI unit or higher. For fasting glucose, we had a 80% chance of detecting a 0.014 mmol/l change per BMI unit and a 95% chance of detecting a 0.022 mmol/l change, smaller than the effect estimate from ordinary linear regression of BMI on glucose (0.028; Table 3).
The causal estimate of the relationship between BMI and ever T2D derived from the MR analysis (the IV estimator) (OR 1.37; 95% CI, 1.23–1.51) was different from the observed association between BMI and ever T2D (OR 1.15; 95% CI, 1.14–1.17; p = 0.001).
Discussion
Main Findings
In this large-scale meta-analysis, we used a MR design to examine causal associations between adiposity, assessed as elevated BMI, and a number of cardiometabolic outcomes. The present study is, to our knowledge, the most comprehensive MR study published to date, including 24 traits in up to 198,502 individuals with FTO genotype and BMI information available. This analysis has enabled us to provide evidence for many biologically plausible causal relationships, such as those between adiposity and hypertension, and between adiposity and dyslipidemia. Furthermore, we demonstrated evidence for a causal relationship between (i) adiposity and heart failure and (ii) adiposity and increased concentrations of the liver enzymes ALT and GGT. In addition, we showed that traditional cross-sectional estimates of the BMI effect on T2D are smaller than the causal estimates of the BMI–T2D relationship based on FTO-predicted obesity (IV analyses). This difference is probably driven by lifetime changes in BMI affecting T2D risk, and their attenuation introduced by a single measurement of BMI.
Comparison with Previous MR Studies
In the present population-based investigation, we confirm earlier findings that FTO-mediated adiposity increases the risk of metabolic syndrome and of increased CRP, fasting insulin, and triglyceride levels; increased systolic and diastolic blood pressure; and decreased concentrations of HDL-C [14],[15],[17]–[19].
Using standard regression methods for the association between BMI and other cardiovascular traits, we confirmed associations between adiposity and CHD, ischemic stroke, and all-cause stroke, but did not find an association with hemorrhagic stroke, where we had relatively few cases available for analyses. We could not demonstrate a causal relationship via IV methods applied to these cardiovascular outcomes. The same was true for several metabolic traits, such as for fasting glucose, HbA1c, IL-6, total cholesterol, and LDL-C. However, our findings do not exclude causal relationships as such, since despite the large study sample, the IV analyses brought estimators with rather wide confidence intervals, a common feature when only one genotype is used as an IV. Our calculations showed low power to detect ORs of less than 1.05 in the present study, observed for several BMI–trait associations among those with non-significant IV estimators. We could not find evidence for a causal association between adiposity and all-cause mortality. While the causal association between these phenotypes might be absent, nonlinear relationships, potential survival bias, or low power due to a heterogeneous phenotype could have also affected the results.
We were not able to replicate the findings by Nordestgaard et al., who studied the association between adiposity and CHD using a combined allele score based on FTO, MC4R, and TMEM18 variants as an instrument for adiposity, and demonstrated a causal link between BMI and CHD risk [16]. Although the sample sizes and diagnostic criteria were comparable between that study and the present one, Nordestgaard et al. presented more precise estimates, which was probably primarily an effect of the stronger instrument, but the increased precision may also have been influenced by the notion that the ascertainment of CHD events was validated in the three cohorts included, and that results showed low heterogeneity. We found that the IV estimate for the effect of BMI on T2D was higher than that derived from standard logistic regression, which is similar to the finding of Li et al., conducted in east and south Asians [26]. Possible explanations of such an observation include the following: the cross-sectional nature of data that could result in reverse causation (weight loss due to disease or lifestyle interventions), and the notion that the lifelong effect of FTO on adiposity is not entirely captured by a single BMI measurement [27].
Adiposity and Heart Failure
We have provided evidence that the previously suggested association of adiposity with heart failure [28] may indeed be causal. A causal relationship may be mediated through effects of obesity on hypertension, dyslipidemia, and insulin resistance, associations that are also supported by our study. Hypertension, insulin resistance, and T2D have been independently associated with increased risk of heart failure [29],[30]. Hypertension, T2D, dyslipidemia, and insulin resistance are also important risk factors for myocardial infarction, which often results in heart failure [31]. Additionally, increased BMI is associated with cardiac remodeling [32], possibly owing to increased hemodynamic load and increased oxidative stress [33]. Animal models have independently suggested direct apoptotic effects of adiposity on the myocardium [34]. Our study estimates the causal impact of a one-unit increase in BMI as a 17% increase in heart failure incidence. Extrapolating this estimate to the population level based on incidence rates reported by the World Health Organization [35] and the American Heart Association [36], a one-unit increase in BMI corresponds to roughly 220,000 additional heart failure cases in Europe and 113,000 additional cases in the US, at extensive costs for society.
Adiposity and Liver Enzymes
The higher concentrations of liver enzymes observed in the present study caused by an increased BMI are likely to be related to non-alcoholic fatty liver disease, which is characterized by lipid accumulation within hepatocytes as a consequence of increased levels of fatty acids in insulin-resistant individuals. This accumulation predisposes to overproduction of reactive oxygen species, endoplasmic reticulum stress, and lipotoxicity, all of which are harmful to the hepatocytes [37].
Strengths and Limitations
The main strengths of the present investigation include the combination of the very large study sample, prospectively collected events, and a wide range of cardiometabolic phenotypes. The limitations of our study are tied to the validity of the assumptions underlying causal interpretation within MR studies. There are three main assumptions for a MR study: (i) independence between the instrument and confounders, i.e., FTO genotypes are randomized, (ii) a reliable association between the genetic variant and intermediate phenotype, and (iii) conditional independence between the genetic variant and the outcome, given the intermediate phenotype and the confounders, i.e., no pleiotropy [38]. Possible violations of the first and the third assumptions include population stratification, pleiotropic effects, canalization, epigenetic effects, and the presence of genes associated with confounders and outcomes in LD with the FTO variant. Neither the first nor the third assumption can be tested statistically in the observed data using single genotypes as the IV, and conclusions about such assumptions have to be based on previous biological knowledge. There are additional assumptions of MR studies regarding the quantification of the causal effect (as opposed to testing only; see Text S1).
The random distribution of genotypes in the population is the very basis of MR and could be violated if separate ethnic groups with different allele frequencies were analyzed together without accounting for the population substructure. In the present study, all association analysis was done within each study (including individuals from a similar genetic background) separately, and all studies included only individuals of European ancestry. Hence, bias from population stratification is deemed unlikely [39].
With regards to the possibility of pleiotropic effects by FTO or genes in high LD with FTO, we acknowledge that although FTO is one of the most well-studied obesity loci, and there are credible hypotheses for its action on adiposity by increasing the appetite [40],[41], the precise mechanism of the FTO polymorphisms is still unclear, and potential pleiotropy cannot completely be ruled out. It has, however, been demonstrated previously that FTO is not associated with the most obvious potential confounders, such as smoking and drinking habits, income, or education [16]. A suggested way to assess pleiotropy in IV studies using multiple genotypes is to compare IV estimates between variants: if they are similar, it is less plausible that LD or pleiotropy is present [42]. This was done in the study by Nordestgaard and colleagues on the adiposity effect on CHD, and no difference between FTO, MC4R, and TMEM18 was seen in effect on CHD risk [16].
Concerning the reliability of the association (second assumption) between rs9939609 and BMI, this association has been widely replicated in many studies and populations [13],[43],[44]. While having the largest effect on BMI among known common variants, FTO constitutes a relatively weak instrument and thus results in wide confidence intervals for the IV estimators, despite the very large sample size. An approach to increase power in future studies would be to use multiple genetic variants as an instrument. In the present study, there is a possibility of introduction of a bias by using weak instruments in the calculation of the Wald estimator of dichotomous traits [25]. Our sensitivity analysis (Text S1) estimated that in the settings of our study, the estimator is possibly biased towards the null, and the extent of the bias is modest.
Conclusion
The present MR study addressing the role of BMI in 24 traits in up to 198,502 individuals provides novel insights into the causal effect of obesity on heart failure and increased liver enzymes levels. Furthermore, to our knowledge for the first time in a well-powered sample, this study provides robust support for a causal relationship between obesity and a number of cardiometabolic traits reported previously. These results support global public prevention efforts for obesity in order to decrease costs and suffering from T2D and heart failure.
Supporting Information
Zdroje
1. GershBJ, SliwaK, MayosiBM, YusufS (2010) Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J 31 : 642–648.
2. WhitlockG, LewingtonS, SherlikerP, ClarkeR, EmbersonJ, et al. (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373 : 1083–1096.
3. Davey SmithG, SterneJA, FraserA, TyneliusP, LawlorDA, et al. (2009) The association between BMI and mortality using offspring BMI as an indicator of own BMI: large intergenerational mortality study. BMJ 339: b5043.
4. KnowlerWC, Barrett-ConnorE, FowlerSE, HammanRF, LachinJM, et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346 : 393–403.
5. TuomilehtoJ, LindstromJ, ErikssonJG, ValleTT, HamalainenH, et al. (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344 : 1343–1350.
6. PanXR, LiGW, HuYH, WangJX, YangWY, et al. (1997) Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 20 : 537–544.
7. LiG, ZhangP, WangJ, GreggEW, YangW, et al. (2008) The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371 : 1783–1789.
8. UusitupaM, PeltonenM, LindstromJ, AunolaS, Ilanne-ParikkaP, et al. (2009) Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study—secondary analysis of the randomized trial. PLoS ONE 4: e5656 doi:10.1371/journal.pone.0005656
9. Davey SmithG, EbrahimS (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32 : 1–22.
10. DidelezV, SheehanN (2007) Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res 16 : 309–330.
11. Sheehan NA, Meng S, Didelez V (2011) Mendelian randomisation: a tool for assessing causality in observational epidemiology. In: Teare MD, editor. Genetic epidemiology. New York City: Humana Press.
12. DayFR, LoosRJ (2011) Developments in obesity genetics in the era of genome-wide association studies. J Nutrigenet Nutrigenomics 4 : 222–238.
13. SpeliotesEK, WillerCJ, BerndtSI, MondaKL, ThorleifssonG, et al. (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42 : 937–948.
14. TimpsonNJ, NordestgaardBG, HarbordRM, ZachoJ, FraylingTM, et al. (2011) C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes (Lond) 35 : 300–308.
15. FreathyRM, TimpsonNJ, LawlorDA, PoutaA, Ben-ShlomoY, et al. (2008) Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes 57 : 1419–1426.
16. NordestgaardBG, PalmerTM, BennM, ZachoJ, Tybjaerg-HansenA, et al. (2012) The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a mendelian randomisation approach. PLoS Med 9: e1001212 doi:10.1371/journal.pmed.1001212
17. KivimakiM, SmithGD, TimpsonNJ, LawlorDA, BattyGD, et al. (2008) Lifetime body mass index and later atherosclerosis risk in young adults: examining causal links using Mendelian randomization in the Cardiovascular Risk in Young Finns study. Eur Heart J 29 : 2552–2560.
18. TimpsonNJ, HarbordR, Davey SmithG, ZachoJ, Tybjaerg-HansenA, et al. (2009) Does greater adiposity increase blood pressure and hypertension risk?: Mendelian randomization using the FTO/MC4R genotype. Hypertension 54 : 84–90.
19. WelshP, PoliseckiE, RobertsonM, JahnS, BuckleyBM, et al. (2010) Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab 95 : 93–99.
20. GrundySM, CleemanJI, DanielsSR, DonatoKA, EckelRH, et al. (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112 : 2735–2752.
21. CoxD (1972) Regression models and life-tables (with discussion). J R Stat Soc Series B Stat Methodol 34 : 187–220.
22. RossI, RobertG (1996) R: A language for data analysis and graphics. J Comput Graph Stat 5 : 299–314.
23. MagiR, MorrisAP (2010) GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics 11 : 288.
24. WaldA (1940) The fitting of straight lines if both variables are subject to error. Ann Math Stat 11 : 284–300.
25. PalmerTM, SterneJA, HarbordRM, LawlorDA, SheehanNA, et al. (2011) Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epidemiol 173 : 1392–1403.
26. LiH, KilpelainenTO, LiuC, ZhuJ, LiuY, et al. (2012) Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia 55 : 981–995.
27. MeyreD (2012) Is FTO a type 2 diabetes susceptibility gene? Diabetologia 55 : 873–876.
28. KenchaiahS, EvansJC, LevyD, WilsonPW, BenjaminEJ, et al. (2002) Obesity and the risk of heart failure. N Engl J Med 347 : 305–313.
29. IngelssonE, SundstromJ, ArnlovJ, ZetheliusB, LindL (2005) Insulin resistance and risk of congestive heart failure. JAMA 294 : 334–341.
30. WilhelmsenL, RosengrenA, ErikssonH, LappasG (2001) Heart failure in the general population of men—morbidity, risk factors and prognosis. J Intern Med 249 : 253–261.
31. MansonJE, ColditzGA, StampferMJ, WillettWC, RosnerB, et al. (1990) A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med 322 : 882–889.
32. LauerMS, AndersonKM, KannelWB, LevyD (1991) The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA 266 : 231–236.
33. VincentHK, PowersSK, StewartDJ, ShanelyRA, DemirelH, et al. (1999) Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord 23 : 67–74.
34. ZhouYT, GrayburnP, KarimA, ShimabukuroM, HigaM, et al. (2000) Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A 97 : 1784–1789.
35. World Health Organization (2008) The global burden of disease: 2004 update. Geneva: World Health Organization.
36. RogerVL, GoAS, Lloyd-JonesDM, AdamsRJ, BerryJD, et al. (2011) Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123: e18–e209.
37. FuS, YangL, LiP, HofmannO, DickerL, et al. (2011) Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473 : 528–531.
38. SheehanNA, DidelezV, BurtonPR, TobinMD (2008) Mendelian randomisation and causal inference in observational epidemiology. PLoS Med 5: e177 doi:10.1371/journal.pmed.0050177
39. SmithGD, LawlorDA, HarbordR, TimpsonN, DayI, et al. (2007) Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med 4: e352 doi:10.1371/journal.pmed.0040352
40. ChurchC, MoirL, McMurrayF, GirardC, BanksGT, et al. (2010) Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 42 : 1086–1092.
41. CecilJE, TavendaleR, WattP, HetheringtonMM, PalmerCN (2008) An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 359 : 2558–2566.
42. PalmerTM, LawlorDA, HarbordRM, SheehanNA, TobiasJH, et al. (2011) Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res 21 : 223–242.
43. WillerCJ, SpeliotesEK, LoosRJ, LiS, LindgrenCM, et al. (2009) Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41 : 25–34.
44. FraylingTM, TimpsonNJ, WeedonMN, ZegginiE, FreathyRM, et al. (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 : 889–894.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 6- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Uncovering Treatment Burden as a Key Concept for Stroke Care: A Systematic Review of Qualitative Research
- Bigotry and Oppressive Laws in Africa Drive HIV in Men Who Have Sex with Men
- Household Air Pollution in Low- and Middle-Income Countries: Health Risks and Research Priorities
- The Health Effects of Motorization
- The Role of Adiposity in Cardiometabolic Traits: A Mendelian Randomization Analysis
- Patented Drug Extension Strategies on Healthcare Spending: A Cost-Evaluation Analysis
- The Effect of Intermittent Antenatal Iron Supplementation on Maternal and Infant Outcomes in Rural Viet Nam: A Cluster Randomised Trial
- Prevalence of Consensual Male–Male Sex and Sexual Violence, and Associations with HIV in South Africa: A Population-Based Cross-Sectional Study
- Associations between Active Travel to Work and Overweight, Hypertension, and Diabetes in India: A Cross-Sectional Study
- Addressing the Wicked Problem of Obesity through Planning and Policies
- Serum Iron Levels and the Risk of Parkinson Disease: A Mendelian Randomization Study
- Targeting Asymptomatic Malaria Infections: Active Surveillance in Control and Elimination
- Malignant Neglect: The Failure to Address the Need to Prevent Premature Non-communicable Disease Morbidity and Mortality
- Diet and Physical Activity for the Prevention of Noncommunicable Diseases in Low- and Middle-Income Countries: A Systematic Policy Review
- Modern Medicine Is Neglecting Road Traffic Crashes
- Integrating Health Care Delivery and Data Collection in Rural India Using a Rapidly Deployable eHealth Center
- Rising Health Care Costs and Life-Cycle Management in the Pharmaceutical Market
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Diet and Physical Activity for the Prevention of Noncommunicable Diseases in Low- and Middle-Income Countries: A Systematic Policy Review
- Addressing the Wicked Problem of Obesity through Planning and Policies
- Modern Medicine Is Neglecting Road Traffic Crashes
- Uncovering Treatment Burden as a Key Concept for Stroke Care: A Systematic Review of Qualitative Research
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





