-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRisk of a Second Primary Cancer after Non-melanoma Skin Cancer in White Men and Women: A Prospective Cohort Study
Background:
Previous studies suggest a positive association between history of non-melanoma skin cancer (NMSC) and risk of subsequent cancer at other sites. The purpose of this study is to prospectively examine the risk of primary cancer according to personal history of NMSC.Methods and Findings:
In two large US cohorts, the Health Professionals Follow-up Study (HPFS) and the Nurses' Health Study (NHS), we prospectively investigated this association in self-identified white men and women. In the HPFS, we followed 46,237 men from June 1986 to June 2008 (833,496 person-years). In the NHS, we followed 107,339 women from June 1984 to June 2008 (2,116,178 person-years). We documented 29,447 incident cancer cases other than NMSC. Cox proportional hazard models were used to calculate relative risks (RRs) and 95% confidence intervals (CIs). A personal history of NMSC was significantly associated with a higher risk of other primary cancers excluding melanoma in men (RR = 1.11; 95% CI 1.05–1.18), and in women (RR = 1.20; 95% CI 1.15–1.25). Age-standardized absolute risk (AR) was 176 in men and 182 in women per 100,000 person-years. For individual cancer sites, after the Bonferroni correction for multiple comparisons (n = 28), in men, a personal history of NMSC was significantly associated with an increased risk of melanoma (RR = 1.99, AR = 116 per 100,000 person-years). In women, a personal history of NMSC was significantly associated with an increased risk of breast (RR = 1.19, AR = 87 per 100,000 person-years), lung (RR = 1.32, AR = 22 per 100,000 person-years), and melanoma (RR = 2.58, AR = 79 per 100,000 person-years).Conclusion:
This prospective study found a modestly increased risk of subsequent malignancies among individuals with a history of NMSC, specifically breast and lung cancer in women and melanoma in both men and women.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(4): e32767. doi:10.1371/journal.pmed.1001433
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001433Summary
Background:
Previous studies suggest a positive association between history of non-melanoma skin cancer (NMSC) and risk of subsequent cancer at other sites. The purpose of this study is to prospectively examine the risk of primary cancer according to personal history of NMSC.Methods and Findings:
In two large US cohorts, the Health Professionals Follow-up Study (HPFS) and the Nurses' Health Study (NHS), we prospectively investigated this association in self-identified white men and women. In the HPFS, we followed 46,237 men from June 1986 to June 2008 (833,496 person-years). In the NHS, we followed 107,339 women from June 1984 to June 2008 (2,116,178 person-years). We documented 29,447 incident cancer cases other than NMSC. Cox proportional hazard models were used to calculate relative risks (RRs) and 95% confidence intervals (CIs). A personal history of NMSC was significantly associated with a higher risk of other primary cancers excluding melanoma in men (RR = 1.11; 95% CI 1.05–1.18), and in women (RR = 1.20; 95% CI 1.15–1.25). Age-standardized absolute risk (AR) was 176 in men and 182 in women per 100,000 person-years. For individual cancer sites, after the Bonferroni correction for multiple comparisons (n = 28), in men, a personal history of NMSC was significantly associated with an increased risk of melanoma (RR = 1.99, AR = 116 per 100,000 person-years). In women, a personal history of NMSC was significantly associated with an increased risk of breast (RR = 1.19, AR = 87 per 100,000 person-years), lung (RR = 1.32, AR = 22 per 100,000 person-years), and melanoma (RR = 2.58, AR = 79 per 100,000 person-years).Conclusion:
This prospective study found a modestly increased risk of subsequent malignancies among individuals with a history of NMSC, specifically breast and lung cancer in women and melanoma in both men and women.
Please see later in the article for the Editors' SummaryIntroduction
Non-melanoma skin cancer (NMSC) is the most common cancer in the United States. It consists mainly of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). Its incidence has been rapidly increasing over the past several decades and the incidence rate was about 6,000/100,000 in the year 2006 [1]. NMSC has a low mortality rate of 1/100,000 [2], but its high prevalence and the expense of related treatment make NMSC a major public health problem and place it among the costliest cancers in the United States [3]. Individuals with personal history of NMSC may be at an altered risk for developing other primary cancers [4]–[11]. One view is that sunlight causes NMSC but also produces vitamin D, which in turn may reduce the risk of other cancers [12]. Another view is that NMSC and other cancers may share common carcinogenic exposures or molecular mechanisms in their etiology, such as DNA repair deficiency and immune suppression, and thus the history of NMSC may indicate an increased risk of subsequent cancer development.
Previous studies suggest a positive association between personal history of NMSC and risk of subsequent cancer at other sites [4]–[11]. Most previous reports, however, were based on cancer registry data without adjustment for potential confounding lifestyle factors [4]–[10]. The only cohort study was limited by its sample size and lacked adequate power to assess individual cancer sites [11]. We carried out a cohort analysis to evaluate the association between personal history of NMSC and subsequent malignancy in the Nurses' Health Study (NHS) and the Health Professionals' Follow-up Study (HPFS).
Methods
Ethics Statement
Our study was approved by the Human Research Committee at the Brigham and Women's Hospital with written informed consent from all participants.
Study Population
The NHS was established in 1976, when 121,700 registered nurses aged 30–55 y in 11 US states responded to a baseline questionnaire regarding risk factors for cancer. Participants completed self-administered, mailed follow-up questionnaires biennially with updated information on their lifestyle, diet, and medical history. The HPFS began in 1986 when 51,529 US male health professionals, including dentists, veterinarians, pharmacists, and optometrists aged 40–75 y, completed a baseline questionnaire on lifestyle, diet, and newly diagnosed diseases. The information was updated biennially with follow-up questionnaires. The follow-up rates of the participants in both cohorts exceed 90%. These studies were approved by the Human Research Committee at Brigham and Women's Hospital. Race was self-identified in this study as White, Asian, African American, and others. Only white participants were included in this study, accounting for 95.6% of the total population in the two cohorts. The rationale for focusing the primary hypothesis on white participants only was that the patterns of incidence (and likely the risk factors) for NMSC differ widely by race.
Identification of NMSC and Other Primary Cancers
We have routinely identified cases of NMSC and other primary cancers in both cohorts (from 1984 in the NHS and from 1986 in the HPFS). Participants reported new diagnoses biennially. With their permission, participants' medical records were obtained and reviewed by physicians to confirm their self-reported diagnosis. Medical records were not obtained for self-reported cases of BCC, because the validity of BCC self-reports was more than 90% in validation studies in our cohorts in early years [13],[14]. The personal history of pathologically confirmed invasive SCC and self-reported BCC was the exposure in this analysis. The study outcome was the occurrence of the first confirmed primary cancer other than NMSC. All other cancer cases were documented by medical records or death certificates, and only confirmed cases were included in the analysis.
Assessment of Covariates
Covariates in this analysis included age (continuous variable), body mass index (BMI) (<21, 21–23, 23–25, 25–27, 27–29, 29–31, >31), physical activity (quintiles), smoking status (never, past 1–14 cigarettes per day, past 15+ cigarettes per day, current 1–14 cigarettes per day, current 15+ cigarettes per day), multi-vitamin use (yes or no), menopause status and hormone replacement therapy use in women (pre-menopause, post-menopause non-user, post-menopause past user, and post-menopause current user), and physical examination in the last 2 y (yes or no). We asked about the location of residence (US states) at birth and at age of 15 and 30. The 50 states (and the District of Columbia) were divided into three ultraviolet (UV) index groups: 5 or less (low UV index); 6 (medium UV index); and 7 or more (high UV index) [15]. We defined participants in these three groups if they resided in the same UV-index region at birth, age of 15 and 30.
Statistical Analysis
Follow-up began in 1984 for the NHS and 1986 for the HPFS when the diagnosis of NMSC was first routinely collected, and follow-up ended in 2008 for both cohorts. Participants who reported a history of cancer (including NMSC) prior to baseline were excluded. Participants contributed person-time from the date of return of the baseline questionnaire (1984 in NHS and 1986 in HPFS) until date of diagnosis of confirmed primary cancer, date of death, or the end of follow-up (May 31, 2008), whichever came first. For those who were lost to follow-up, we censored them at the return date of the last questionnaire. Cox regression analysis with time-dependent covariates was used to determine the relative risks (RRs) and 95% CIs of second primary malignancies associated with a previous NMSC diagnosis. We calculated age-standardized absolute risks (ARs) of second primary malignancies associated with a previous NMSC diagnosis. NMSC diagnosis could change during the follow-up period. For individuals with no personal history of cancer at baseline who went on to be diagnosed with NMSC as a first cancer diagnosis during follow-up, the follow-up period before the NMSC diagnosis contributed person-time to the non-exposure group, and the follow-up period after the NMSC diagnosis contributed person-time to the exposure group. Age was coded as a continuous variable in all the analyses. We showed overall cancer risk with and without melanoma. We performed several secondary analyses. We excluded those diagnosed with other primary cancers within the first 4 y of NMSC diagnosis to minimize the detection bias. We examined BCC and SCC history separately. We performed stratified analysis according to age (≤60 y, >60 y), UV-index of residence at birth, age 15, and age 30 (≤5, = 6, ≥7), smoking status (never smoker, past smoker, current smoker), and BMI (<25, 25–30, ≥30). We coded these factors as dummy variables and tested their interactions with the history of NMSC individually. We tested multiplicative interaction terms by the likelihood ratio test comparing the model with the cross-product terms with the model containing just the main effects of these factors and the history of NMSC along with the same covariates.
We assessed the association between NMSC diagnosis and risk of developing site-specific cancers that were diagnosed in more than 100 patients in each cohort. For individual cancer sites, the Cox models additionally included risk factors specific for some cancer sites. We included additional covariates in the multivariate models for breast, ovarian, endometrial, prostate cancers, and melanoma. The Bonferroni correction for p-value was applied for multiple comparisons for individual cancer sites among men and women, calculated as 0.05/n (n = 28). Statistical analyses were conducted using SAS software (version 9, SAS Institute). All statistical tests were two-sided.
Results
Characteristics of our study population according to a personal history of NMSC in mid-point of the follow-up (1998) are shown in Table 1. Participants with a history of NMSC were more likely to be older and tended to burn and have more severe sunburns. Participants with history of NMSC diagnosis were more likely to have red or blonde hair and to reside in high UV-index states. Other characteristics were similar between the exposure group and the non-exposure group.
Tab. 1. Characteristics according to personal history of non-melanoma skin cancer in 1998. 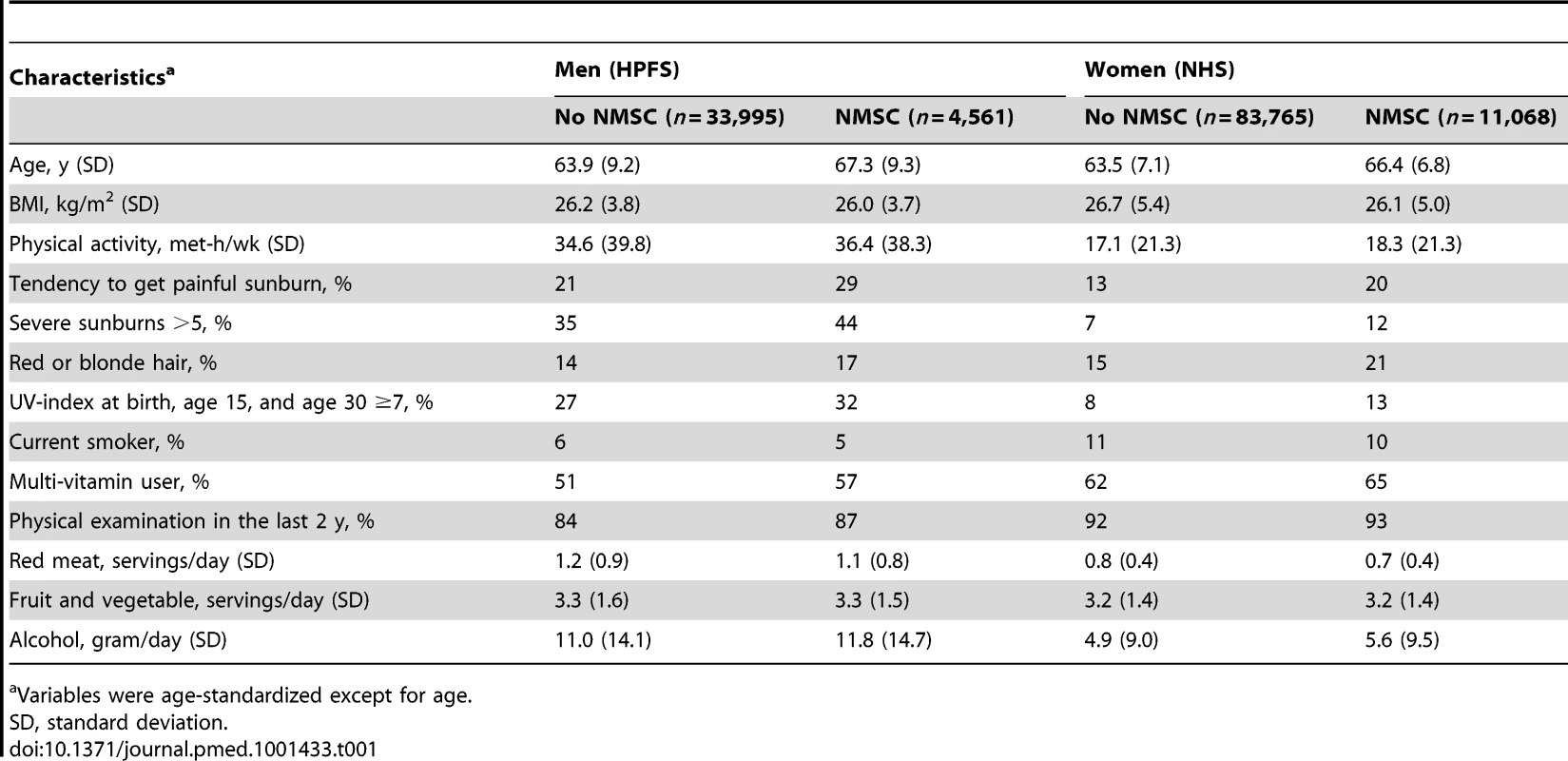
Variables were age-standardized except for age. We followed the HPFS participants from 1986 to 2008, for a total of 833,496 person-years. During this period, 1,577 cases of SCC, 10,422 cases of BCC, and 10,590 primary cancer cases other than NMSC were recorded. The mean time for the development of a primary cancer after NMSC was 116±47 mo. We followed the NHS from 1984 to 2008 (2,116,178 person-years) during which 2,322 cases of SCC, 21,781 cases of BCC, and 18,857 primary cancer cases other than NMSC were recorded. The mean time for the development of a primary cancer after NMSC was 156±71 mo.
A personal history of NMSC was associated with a higher risk of other primary cancers in men (RR = 1.15; 95% CI 1.09–1.22, p<0.0001) and women (RR = 1.26; 95% CI 1.21–1.31, p<0.0001) (Table 2). The association attenuated slightly when melanoma was excluded from the outcome in the analysis, but remained significant in men (RR = 1.11; 95% CI 1.05–1.18, p = 0.0007) and in women (RR = 1.20; 95% CI 1.15–1.25, p<0.0001). Age-standardized AR was 176 in men and 182 in women per 100,000 person-years. The association remained significant after we excluded those diagnosed with other primary cancers within the first 4 y of NMSC diagnosis in men (RR = 1.15; 95% CI 1.05–1.25) and women (RR = 1.19; 95% CI 1.11–1.28). In men, the association was significant according to BCC diagnosis (RR = 1.17; 95% CI 1.10–1.24) but not SCC diagnosis (RR = 1.01; 95% CI 0.87–1.17). In women, the association was significant for both BCC (RR = 1.25; 95% CI 1.20–1.30) and SCC diagnosis (RR = 1.24; 95% CI 1.10–1.39). We compared people with personal history of SCC with people with personal history of BCC on their risk of developing subsequent cancer, and no significant differences were found (Table S1). In addition, we have compared SCC in situ group with invasive SCC group and the group of SCC or BCC; the results are shown in Table S2. Compared to those with history of invasive SCC or those with history of SCC or BCC, individuals with history of SCC in situ were less likely to develop subsequent cancers. Such risk reduction was significant among women.
Tab. 2. Overall and stratified analysis of risks of total subsequent primary cancers according to personal history of non-melanoma skin cancer in men and women. 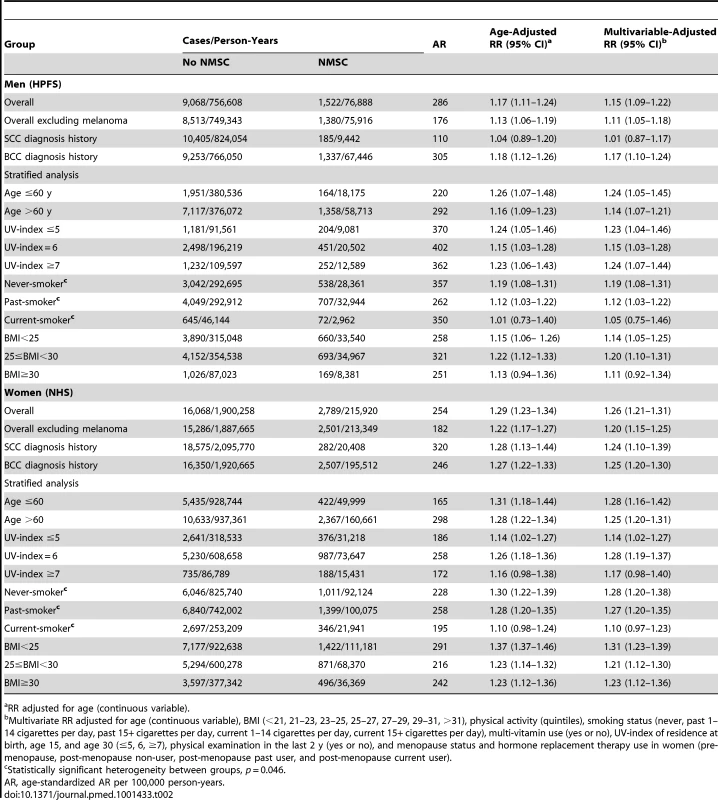
RR adjusted for age (continuous variable). No substantial differences were found in the stratified analysis according to age, UV-index, or BMI. When stratified by smoking status, significant associations were found in never-smokers (RR = 1.19; 95% CI 1.08–1.31 in men, and RR = 1.28; 95% CI 1.20–1.38 in women) and past smokers (RR = 1.12; 95% CI 1.03–1.22 in men, and RR = 1.27; 95% CI 1.20–1.35 in women), but not in current smokers (RR = 1.05; 95% CI 0.75–1.46 in men, and RR = 1.10; 95% CI 0.97–1.23 in women). The p-value for interaction was 0.046 for men and women combined.
For individual cancer sites (Table 3), a history of NMSC was associated with an increased risk of prostate cancer in men (RR = 1.11; 95% CI 1.02–1.20, p = 0.01). The age-standardized AR was 137 per 100,000 person-years. The RR was similar for fatal prostate cancer (RR = 1.17; 95% CI 0.89–1.53). A history of NMSC was also associated with an increased risk of melanoma in men (RR = 1.99; 95% CI 1.63–2.43, p<0.0001). The age-standardized AR was 116 per 100,000 person-years. In women, a history of NMSC was associated with an increased risk of breast cancer (RR = 1.19; 95% CI 1.11–1.28, p<0.0001; AR = 87 per 100,000 person-years), lung cancer (RR = 1.32; 95% CI 1.14–1.52, p = 0.0002; AR = 22 per 100,000 person-years), leukemia (RR = 1.30; 95% CI 1.00–1.69, p = 0.05; AR = 7 per 100,000 person-years), kidney cancer (RR = 1.48; 95% CI 1.10–1.99, p = 0.01; AR = 8 per 100,000 person-years), and melanoma (RR = 2.58; 95% CI 2.34 –2.98, p<0.0001; AR = 79 per 100,000 person-years). After taking into account the multiple comparisons for individual cancers (n = 28), the associations with breast cancer, lung cancer in women, and melanoma in both men and women remained significant. We analyzed SCC and BCC history separately for individual cancer sites. We observed different rates for second cancer development according to personal history of SCC and BCC. However, no statistically significant heterogeneity (p for heterogeneity ranged from 0.16 to 0.88) was found for any cancer site between BCC and SCC due to the limited power (Table S3).
Tab. 3. Risks of subsequent primary cancers at different sites according to personal history of non-melanoma skin cancer in men and women. 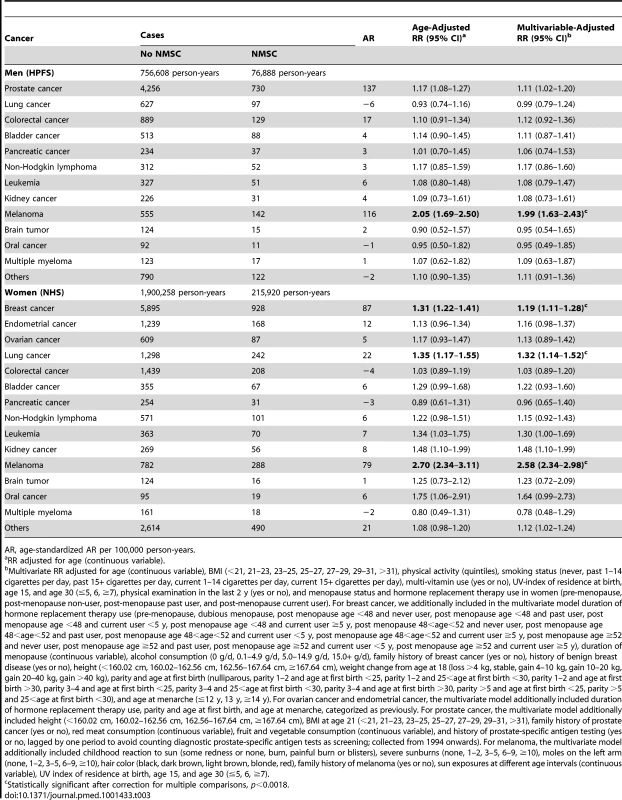
AR, age-standardized AR per 100,000 person-years. Discussion
To the best of our knowledge, this is the largest prospective study on this topic. In this study, a total of 36,102 cases of NMSC and 29,447 cases of cancers other than NMSC were documented. Among those with a personal history of NMSC, we found a 15% increased risk in men and a 26% increased risk in women of developing a second primary cancer, compared with those who had no such history. A systematic review summarizing previous studies revealed that NMSC is associated with more than 10% increased risk of subsequent primary cancer in registry-based studies and nearly 50% increased risk in cohort studies [16]. Our study has extended previous findings by adding a prospective analysis in two large US cohorts with more than two decades of follow-up. The unique aspects of our study included stratified analyses by other risk factors, disentanglement of surveillance bias, and comprehensive adjustment for potential confounders.
It was speculated that the association between NMSC and subsequent cancer risk may be different among people living in locations with different UV-indexes. Specifically, southern regions have solar UV-B radiation levels that provide sufficient vitamin D to reduce the risk of cancer incidence, and thus inverse associations were more likely to be found. On the contrary, studies that found positive associations were mostly conducted in northern regions where UV-B radiation levels do not provide sufficient vitamin D [17],[18]. In our analysis stratified by UV-index, no substantial differences were found for the associations between NMSC and cancer risk among locations with different UV-indexes. To the extent that some cancers have been suggested to be reduced with higher vitamin D [19], it might be worth noting the fact that while colorectal cancer was not increased in this study, it was not decreased as would be expected if NMSC were a marker for sunlight and thus vitamin D exposure.
Intensified medical surveillance of persons with a history of NMSC is unlikely to explain the increased cancer incidence observed in our study. In our analysis, we adjusted for physical examination in the last 2 y, and the result changed little. After we excluded those diagnosed with other primary cancers within the first 4 y of NMSC diagnosis, the association remained significant. Compared to those with history of invasive SCC or with history of SCC or BCC, individuals with history of SCC in situ were less likely to develop subsequent cancers. These results suggested that the patients who carried NMSC precursors but did not develop skin cancers might be either less genetically susceptible or have lower exposure. In addition, for prostate cancer, the association remained significant for fatal prostate cancer. Furthermore, several cancers that we observed associations with are not ones that would be detected on routine screening. Moreover, at least one study that assessed deaths rather than cancer incidence also found increased cancer mortality in people with history of NMSC [20].
Several studies have observed an increased risk of NMSC after other cancers. In one study, chronic lymphocytic leukemia patients had an increased risk of death due to NMSC (RR = 17.0, 95% CI 14.4–19.8) [21]. In another study, among 14 different sites for first primary malignancies, 11 of these sites including prostate, breast, and leukemia were followed by an increased risk of skin cancer (for SCC, RR of 14.1 for males and 14.6 for females) [22]. However, while treatment of these primary cancers may predispose to subsequent skin cancers, most of the NMSC cases are cured by surgical excision without any systemic chemotherapy, and radiation, and their concomitant side effects, including possible carcinogenicity. In addition, the similarity between the age-adjusted and multivariate-adjusted RRs demonstrated that the observed association between NMSC and subsequent cancers is unlikely to be explained by confounding from smoking, obesity, vitamin use, exercise, or any of the other measured risk factors that we controlled for.
The link between NMSC and risk of other cancers is likely to represent an etiologic association. For melanoma especially, the link may be due to sun exposure. For other cancers, while there are several explanations [23]–[28] of the association between NMSC and the risk of subsequent cancer, studies have found that certain genetic markers underlying skin cancer are also associated with other cancer types [29]. It is biologically plausible that deficiencies of pathways responsible for protecting against cellular transformation in multiple tissues, such as DNA repair or immune responses, may act systemically and play a role in cutaneous and internal carcinogenesis.
Humans have evolved several DNA repair pathways dealing with damage [30]. The nucleotide excision repair (NER) pathway is responsible for the repair of a wide variety of DNA damage that leads to distortion of the DNA helix. Such bulky DNA adducts include UV-induced photoproducts, smoking-related benzo(a)pyrene diolepoxide (BPDE)-DNA adducts, and other DNA damage induced by chemical carcinogens. Reduced capacity of the NER has been shown to confer susceptibility to certain cancers in the general population, including melanoma, BCC, SCC, SCC of head and neck, lung cancer, breast cancer, and bladder cancer [31]–[34]. Personal history of NMSC may be a marker of susceptibility due to reduced DNA repair capacity and it may predict the risk of subsequent cancer development.
The NER activity has been shown to be tissue-specific. For example, relatively low NER efficiency was observed in oral tissues [35]. Both rapidly proliferating tissues (e.g., kidney) and slowly proliferating tissues (e.g., lung) exhibit higher demand for NER capacity upon stimulation to proliferation [36]. The DNA repair system consists of several distinct pathways with many subcomponents, each interacting and overlapping with one another in order to achieve genomic stability and high fidelity. Some tissues, such as breast, lack redundant systems of DNA repair that are present in other tissues [37],[38]. Defects in DNA repair would be expected to have greater impact in such tissues without extensive DNA repair redundancy. In addition, a number of studies have suggested a role of sex hormone (e.g., estrogen and androgen) in the regulation of DNA repair activity in breast and prostate cancers [39]. After correction for multiple comparisons for individual types of cancers, the significant association remained for breast cancer, lung cancer in women, and melanoma in both men and women. Even though the positive associations are biologically plausible, we cannot rule out the possibility of chance findings for each individual cancer site.
In our analysis stratified by smoking status, significant association was found among never and past smokers. Because the NER enzymes recognize bulky DNA adducts including both UV-induced photoproducts and smoking-related BPDE-DNA adducts, the interaction between smoking status and history of NMSC highlights the potential role of the NER pathway in the development of second cancers. However, the effect of inherited insufficient capacity of the NER indicated by history of NMSC is only apparent among non-current smokers and for lung cancer in women. Further mechanistic investigation is warranted.
Sub-optimal immune surveillance could be another common susceptibility factor for both cutaneous and internal cancers. Malignant progression is accompanied by profound immune suppression that interferes with an effective antitumor response and tumor elimination [40]. Impaired immunity has been implicated as a non-site-specific determinant of cancer risk [41]. In addition, UV radiation can also cause immunosuppression. UV exposure adversely affects the skin immune system by diminishing antigen-presenting cell function, inducing immunosuppressive cytokine production, and modulating contact and delayed-type hypersensitivity reactions [42],[43], all of which can reduce the body's surveillance for tumor cells [44],[45]. UV suppresses immune reactions locally, but can also affect the immune system in a systemic fashion when higher UV doses are given [46],[47]. UV radiation affects immune surveillance by modulating the balance between an effective immune response and immune tolerance of an emerging cancer [41]. We did not observe an association between UV-index and the risk of cancer except for skin cancer in our study, which makes this explanation less likely for our findings.
The identification of BCC cases in this study was based on self-report without pathological confirmation. However, the participants in the two cohorts were nurses and health professionals. The validity of their reports was expected to be high, and it has been proven in our validation studies [13],[14]. In addition, previous studies of BCC in the NHS using the self-reported cases identified both constitutional and sun exposure risk factors as expected, such as lighter pigmentation, less childhood and adolescent tanning tendency, higher tendency to sunburn, and tanning salon attendance [48],[49]. We recently confirmed the MC1R gene as the top BCC risk locus using the NHS and HPFS samples [50]. These data together suggest that the bias due to self-report of BCC is likely to be minimal in our study. Moreover, the potential under-report of BCC diagnosis would be expected to bias observed associations toward the null, and such bias would not explain the positive associations that we found.
The strengths of our study included the prospective cohort design and updated assessment of cancer diagnosis and other risk factors every 2 y, more than two decades of follow-up, and a large number of incident skin cancer cases. We had detailed data on related covariates for stratified analyses and comprehensive adjustment for potential confounders. All the participants were health professionals, minimizing potential confounding by educational attainment or differential access to health care. Nevertheless, we cannot completely exclude residual confounding, and our findings may not assign causality. Although the observed significant associations in breast cancer, lung cancer in women, and melanoma in both men and women remained significant after correction for multiple comparisons, we cannot absolutely rule out chance findings for individual cancer sites, and the underlying mechanism for the associations found in specific cancer sites is not entirely clear. In addition, the significant associations for some individual cancers did not meet the adjusted p-value threshold because of their limited sample size.
We cannot estimate the recurrence rate of NMSC or subsequent cancer risk among people with multiple NMSC because we only recorded the first report of each type of skin cancer in both cohorts. We do not have data for non-whites in this study, and our results cannot be generalized to non-whites owing to the dramatic difference in skin cancer incidence among different races. In summary, we observed a modestly increased risk of other cancers among individuals with a history of NMSC. Because our study was observational, these results should be interpreted cautiously and are insufficient evidence to alter current clinical recommendations. Nevertheless, these data support a need for continued investigation of the potential mechanisms underlying this relationship.
Supporting Information
Zdroje
1. RogersHW, WeinstockMA, HarrisAR, HinckleyMR, FeldmanSR, et al. (2010) Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol 146 : 283–287.
2. LewisKG, WeinstockMA (2007) Trends in nonmelanoma skin cancer mortality rates in the United States, 1969 through 2000. J Invest Dermatol 127 : 2323–2327.
3. ChenJG, FleischerABJr, SmithED, KanclerC, GoldmanND, et al. (2001) Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg 27 : 1035–1038.
4. FrischM, HjalgrimH, OlsenJH, MelbyeM (1996) Risk for subsequent cancer after diagnosis of basal-cell carcinoma. A population-based, epidemiologic study. Ann Intern Med 125 : 815–821.
5. HemminkiK, DongC (2000) Subsequent cancers after in situ and invasive squamous cell carcinoma of the skin. Arch Dermatol 136 : 647–651.
6. MilanT, PukkalaE, VerkasaloPK, KaprioJ, JansenCT, et al. (2000) Subsequent primary cancers after basal-cell carcinoma: a nationwide study in Finland from 1953 to 1995. Int J Cancer 87 : 283–288.
7. FrischM, MelbyeM (1995) New primary cancers after squamous cell skin cancer. Am J Epidemiol 141 : 916–922.
8. WassbergC, ThornM, YuenJ, RingborgU, HakulinenT (1999) Second primary cancers in patients with squamous cell carcinoma of the skin: a population-based study in Sweden. Int J Cancer 80 : 511–515.
9. TroyanovaP, DanonS, IvanovaT (2002) Nonmelanoma skin cancers and risk of subsequent malignancies: a cancer registry-based study in Bulgaria. Neoplasma 49 : 81–85.
10. NugentZ, DemersAA, WisemanMC, MihalcioiuC, KliewerEV (2005) Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev 14 : 2584–2590.
11. ChenJ, RuczinskiI, JorgensenTJ, YenokyanG, YaoY, et al. (2008) Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst 100 : 1215–1222.
12. GarlandCF, GarlandFC, GorhamED, LipkinM, NewmarkH, et al. (2006) The role of vitamin D in cancer prevention. Am J Public Health 96 : 252–261.
13. ColditzGA, MartinP, StampferMJ, WillettWC, SampsonL, et al. (1986) Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 123 : 894–900.
14. HunterDJ, ColditzGA, StampferMJ, RosnerB, WillettWC, et al. (1990) Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol 1 : 13–23.
15. QureshiAA, LadenF, ColditzGA, HunterDJ (2008) Geographic variation and risk of skin cancer in US women. Differences between melanoma, squamous cell carcinoma, and basal cell carcinoma. Arch Intern Med 168 : 501–507.
16. WhelessL, BlackJ, AlbergAJ (2010) Nonmelanoma skin cancer and the risk of second primary cancers: a systematic review. Cancer Epidemiol Biomarkers Prev 19 : 1686–1695.
17. TuohimaaP, PukkalaE, SceloG, OlsenJH, BrewsterDH, et al. (2007) Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer 43 : 1701–1712.
18. GrantWB (2009) Re: Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst 101 : 210; author reply 210–211.
19. MaY, ZhangP, WangF, YangJ, LiuZ, et al. (2011) Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol 29 : 3775–3782.
20. KahnHS, TathamLM, PatelAV, ThunMJ, HeathCWJr (1998) Increased cancer mortality following a history of nonmelanoma skin cancer. JAMA 280 : 910–912.
21. RoyleJA, BaadePD, JoskeD, GirschikJ, FritschiL (2011) Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer 105 : 1076–1081.
22. HemminkiK, JiangY, SteineckG (2003) Skin cancer and non-Hodgkin's lymphoma as second malignancies. markers of impaired immune function? Eur J Cancer 39 : 223–229.
23. LeighIM, Newton BishopJA, KripkeML (1996) Skin cancer. Introduction. Cancer Surv 26 : 1–6.
24. KripkeML (1994) Ultraviolet radiation and immunology: something new under the sun–presidential address. Cancer Res 54 : 6102–6105.
25. EnglishDR, ArmstrongBK, KrickerA, FlemingC (1997) Sunlight and cancer. Cancer Causes Control 8 : 271–283.
26. FrankelS, GunnellDJ, PetersTJ, MaynardM, Davey SmithG (1998) Childhood energy intake and adult mortality from cancer: the Boyd Orr Cohort Study. Bmj 316 : 499–504.
27. SpitzMR, TilleyBC, BatsakisJG, GibeauJM, NewellGR (1984) Risk factors for major salivary gland carcinoma. A case-comparison study. Cancer 54 : 1854–1859.
28. AuWW, WilkinsonGS, TyringSK, LegatorMS, el ZeinR, et al. (1996) Monitoring populations for DNA repair deficiency and for cancer susceptibility. Environ Health Perspect 104 Suppl 3 : 579–584.
29. StaceySN, SulemP, JonasdottirA, MassonG, GudmundssonJ, et al. (2011) A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat Genet 43 : 1098–1103.
30. RastogiRP, Richa, KumarA, TyagiMB, SinhaRP (2010) Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids 2010 : 592980.
31. BerwickM, VineisP (2000) Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. J Natl Cancer Inst 92 : 874–897.
32. WeiQ, MatanoskiGM, FarmerER, HedayatiMA, GrossmanL (1993) DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci U S A 90 : 1614–1618.
33. GrossmanL, WeiQ (1995) DNA repair and epidemiology of basal cell carcinoma. Clin Chem 41 : 1854–1863.
34. LiC, WangLE, WeiQ (2009) DNA repair phenotype and cancer susceptibility–a mini review. Int J Cancer 124 : 999–1007.
35. MitchellD, PanikerL, GodarD (2012) Nucleotide excision repair is reduced in oral epithelial tissues compared with skin. Photochem Photobiol 88 : 1027–1032.
36. GospodinovA, IvanovR, AnachkovaB, RussevG (2003) Nucleotide excision repair rates in rat tissues. Eur J Biochem 270 : 1000–1005.
37. WelcshPL, SchubertEL, KingMC (1998) Inherited breast cancer: an emerging picture. Clin Genet 54 : 447–458.
38. ScullyR, LivingstonDM (2000) In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature 408 : 429–432.
39. KitagishiY, KobayashiM, MatsudaS (2012) Defective DNA repair systems and the development of breast and prostate cancer (Review). Int J Oncol
40. FinnOJ (2012) Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 23 Suppl 8: viii6–9.
41. CramerDW, FinnOJ (2011) Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol 23 : 265–271.
42. BeissertS, SchwarzT (1999) Mechanisms involved in ultraviolet light-induced immunosuppression. J Investig Dermatol Symp Proc 4 : 61–64.
43. NarayananDL, SaladiRN, FoxJL (2010) Ultraviolet radiation and skin cancer. Int J Dermatol 49 : 978–986.
44. WelshMM, KaragasMR, ApplebaumKM, SpencerSK, PerryAE, et al. (2008) A role for ultraviolet radiation immunosuppression in non-melanoma skin cancer as evidenced by gene-environment interactions. Carcinogenesis 29 : 1950–1954.
45. MurphyGM (2009) Ultraviolet radiation and immunosuppression. Br J Dermatol 161 Suppl 3 : 90–95.
46. SchwarzT, SchwarzA (2011) Molecular mechanisms of ultraviolet radiation-induced immunosuppression. Eur J Cell Biol 90 : 560–564.
47. de GruijlFR (2008) UV-induced immunosuppression in the balance. Photochem Photobiol 84 : 2–9.
48. van DamRM, HuangZ, RimmEB, WeinstockMA, SpiegelmanD, et al. (1999) Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol 150 : 459–468.
49. HanJ, ColditzGA, HunterDJ (2006) Risk factors for skin cancers: a nested case-control study within the Nurses' Health Study. Int J Epidemiol 35 : 1514–1521.
50. NanH, XuM, KraftP, QureshiAA, ChenC, et al. (2011) Genome-wide association study identifies novel alleles associated with risk of cutaneous basal cell carcinoma and squamous cell carcinoma. Hum Mol Genet 20 : 3718–3724.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts
- Supervised and Unsupervised Self-Testing for HIV in High- and Low-Risk Populations: A Systematic Review
- Preeclampsia as a Risk Factor for Diabetes: A Population-Based Cohort Study
- Cinacalcet in Patients with Chronic Kidney Disease: A Cumulative Meta-Analysis of Randomized Controlled Trials
- Excess Long-Term Mortality following Non-Variceal Upper Gastrointestinal Bleeding: A Population-Based Cohort Study
- Air Pollution and Atherosclerosis: New Evidence to Support Air Quality Policies
- Industry Use of Evidence to Influence Alcohol Policy: A Case Study of Submissions to the 2008 Scottish Government Consultation
- Herpes Zoster Vaccine Effectiveness against Incident Herpes Zoster and Post-herpetic Neuralgia in an Older US Population: A Cohort Study
- Implementation and Scale-Up of Psycho-Trauma Centers in a Post-Conflict Area: A Case Study of a Private–Public Partnership in Northern Uganda
- Risk of a Second Primary Cancer after Non-melanoma Skin Cancer in White Men and Women: A Prospective Cohort Study
- Fine Particulate Air Pollution and the Progression of Carotid Intima-Medial Thickness: A Prospective Cohort Study from the Multi-Ethnic Study of Atherosclerosis and Air Pollution
- Prophylactic Perioperative Sodium Bicarbonate to Prevent Acute Kidney Injury Following Open Heart Surgery: A Multicenter Double-Blinded Randomized Controlled Trial
- Life Expectancies of South African Adults Starting Antiretroviral Treatment: Collaborative Analysis of Cohort Studies
- Focusing the Spotlight on Lack of Access to Health Information
- Big Data Opportunities for Global Infectious Disease Surveillance
- Grand Challenges in Global Mental Health: Integration in Research, Policy, and Practice
- Intimate Partner Femicide in South Africa in 1999 and 2009
- Untreated Pain, Narcotics Regulation, and Global Health Ideologies
- Is It Time to Screen Women with History of Hypertensive Pregnancy Disorders for Diabetes?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts
- Excess Long-Term Mortality following Non-Variceal Upper Gastrointestinal Bleeding: A Population-Based Cohort Study
- Untreated Pain, Narcotics Regulation, and Global Health Ideologies
- Herpes Zoster Vaccine Effectiveness against Incident Herpes Zoster and Post-herpetic Neuralgia in an Older US Population: A Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



