-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCraniofacial Ciliopathies and the Interpretation of Hedgehog Signal Transduction
article has not abstract
Published in the journal: . PLoS Genet 12(12): e32767. doi:10.1371/journal.pgen.1006460
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1006460Summary
article has not abstract
An emerging body of literature has shown that cilia-dependent Hedgehog (HH) signaling is crucial to the patterning of the face. Ciliopathic mutations are frequently associated with craniofacial anomalies, and while the links are clear, the observed phenotypes can vary widely, leading to confusion about how these mutations affect processing of HH effectors. In November 2016’s issue of PLOS Genetics, Chang et al. uncover an important role for HH-dependent transcriptional repression during facial development.
Cilia and Developmental Roles for HH Signaling
Cilia are tiny, hair-like projections found on the surface of eukaryotic cells. These projections can be motile, aiding in the movement of cells and surrounding liquids. There are also immotile cilia, which are notable in vertebrates for the interpretation of many extracellular signals [1]. Cilia are complex organelles, requiring a suite of proteins involved in cellular functions as disparate as protein synthesis, microtubule organization, vesicular trafficking, and intraflagellar transport. Therefore, it is no surprise that numerous studies in animal models have demonstrated that cilia proteins are crucial for the patterning of developing organs. Because the majority of cells have cilia, affected organ systems are diverse, and altered function could potentially lead to a wide range of human diseases. Recent advances in genetic analysis confirm this theory, as multiple mutations affecting cilium structure and function have been implicated in congenital anomalies. The craniofacial complex is one of the systems most commonly affected by cilia dysfunction.
Of the many external cues, HH signal transduction has emerged as a key molecular pathway reliant on functional cilia [2,3]. However, despite the many ciliopathic animal models that now exist, a great deal of confusion arises when studying the consequences of cilia mutations on HH. In some studies, mutations mimic a loss of HH signaling, while in other contexts, HH signaling appears to increase. This has been particularly evident in craniofacial structures, where loss of cilia can lead to both narrowing of the head with failure of separation of the forebrain hemispheres (holoprosencephaly, associated with loss of HH) and widening of the mid-face (associated with gain of HH) [4,5]. Altogether, this suggests that the phenotypic interpretation of HH signaling changes is more complicated than a simple on/off mechanism. In this issue, Chang et al. mutate the intraflagellar transport proteins Ift88 and Kif3a in the mouse neural crest, comparing the phenotypes to those associated with functional mutations in the HH effectors Gli2 and Gli3 [6]. In doing so, they clarify the molecular basis for facial widening in ciliopathies and uncover a novel in vivo requirement for GLI-mediated transcriptional repression.
Cilia and the Balance of GLI Functions
In vertebrates, the intracellular components of the HH pathway are sequestered in the cilium (Fig 1) [1]. Upon binding of the HH ligand to its receptor Patched (PTC), the transmembrane receptor Smoothened (SMO) translocates to the cilium, where it triggers the processing of the downstream transcriptional effectors GLI2 and GLI3 (Fig 1B). (A third GLI, GLI1, lacks a repressor domain and appears dispensable for embryonic development.) In the cilium, GLI2 and GLI3 associate with Suppressor of Fused (SUFU), and a SMO-triggered release of SUFU is necessary for subsequent GLI activator (GLIA) function. Both GLI2 and GLI3 can be modified to a long activator form or proteolytically cleaved to a truncated repressor form (GLIR). In vitro, both GLI2R and GLI3R are able to inhibit HH target genes; however, to date, the majority of evidence has suggested that GLI3 is the primary repressor in vivo, while GLI2 mediates the bulk of HH activation. In vivo, the difficulty in understanding HH signaling lies in the unique and overlapping functions of the GLI proteins. Until this report, an in vivo role for proteolytic processing of GLI2 had been elusive, particularly as previous reports demonstrated that the constitutively active Gli1 could substitute for Gli2 in a genetic knock-in [7].
Fig. 1. Hedgehog (HH) signaling in the cilium. 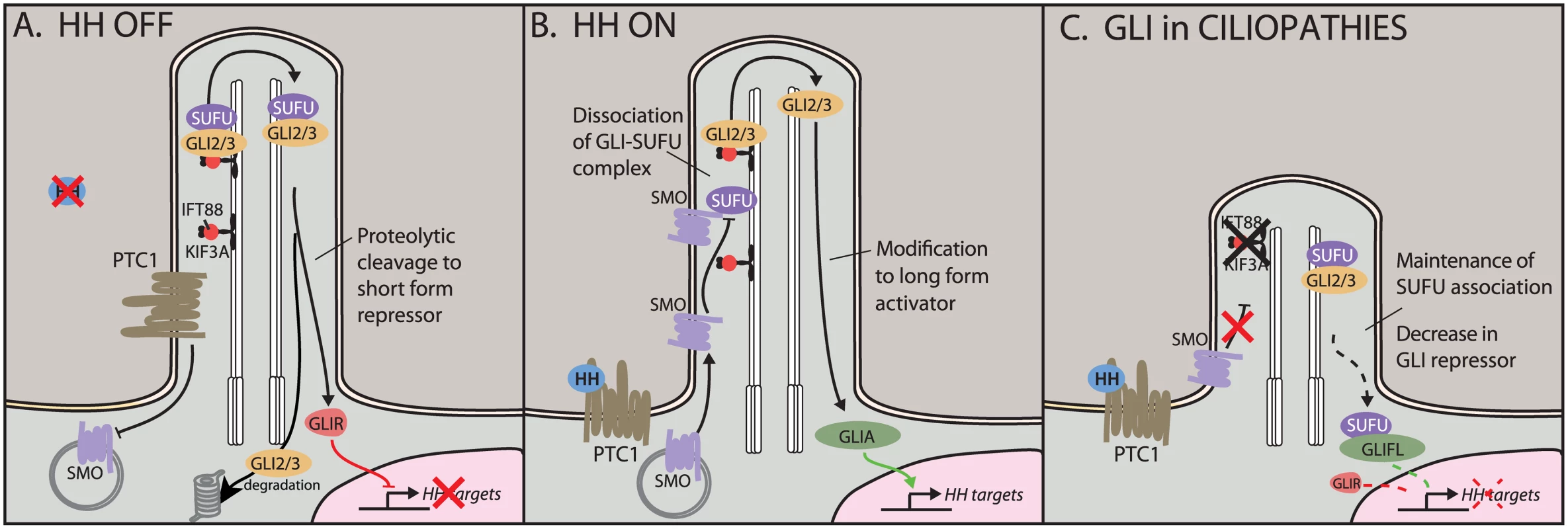
A) In the off state, the HH receptor Patched 1 (PTC1) represses Smoothened (SMO), keeping it out of the cilium. The HH effectors GLI2 and GLI3 localize to the cilium with the help of transport proteins KIF3A and IFT88. Here, they can be proteolytically cleaved to short repressor forms. Full-length GLIs are targeted for degradation. B) In the presence of HH ligand, SMO translocates to the cilium, where it antagonizes the SUFU–GLI association, leading to production and nuclear translocation of GLI activators. C) In ciliopathic Kif3a and Ift88 mutants, HH signaling is unable to disrupt the SUFU–GLI association. This shifts the nuclear ratio of GLIA:GLIR, leading to an increase in full-length GLI (GLIFL) and a reduction in levels of the shorter GLIR. Our current understanding of GLI processing in the cilium suggested that recruitment of SMO to the cilium leads to an increase in the ratio of GLI activator to GLI repressor (GLIA:GLIR) [8,9]. Therefore, tissue-specific interpretation of the GLIA:GLIR ratio could be due to different combinations of binding partners or varying accessibility of target promoters. Furthermore, we could postulate a dedicated set of GLIR targets that are entirely immune to GLIA binding and vice versa. Crucially, different tissues appear to use different ratios of GLIA:GLIR. Together, this suggests a complex interplay of three classes of GLI target genes. For example, HH signaling in dorsal–ventral patterning of the neural tube appears to balance GLI3 repression in the dorsal domain with GLI2 activation in the ventral domain [10]. In contrast, loss of GLI3R function in rhombomere 1 in the hindbrain is sufficient to de-repress expression of fibroblast growth factor-8 (Fgf8) [11]. Similarly, in the limbs, loss of GLI3R leads to severe polydactyly; here, the role of the ligand appears to be to limit the repressor, as loss of sonic hedgehog (Shh) skews the ratio toward GLI3R, resulting in a single digit forming [12,13].
In this paper, Chang et al. demonstrate that disruption of cilia function leads to an increase in the amount of full-length GLI2 and GLI3 in the nucleus, thus shifting the ratio of GLIA:GLIR toward the activator form [6]. This was partially rectified by increasing the amounts of GLI3R. However, complete genetic loss of all Gli3 in the neural crest did not elicit the same phenotype, suggesting that GLI3 repressor did not encompass the full extent of the HH readout. Indeed, the midfacial phenotype was only seen when both Gli2 and Gli3 were eliminated, demonstrating that both GLIs contributed to the phenotype and raising the possibility of a compensatory role for GLI2R that is only uncovered in the absence of GLI3R. By systematically comparing a suite of ciliopathy mutations with different functional mutants of Gli2 and Gli3, Chang et al. provide novel structure–function associations with distinct pathologies.
This paper also highlights the importance of tissue specificity in the interpretation of developmental phenotypes, especially with cues such as HH that are used reiteratively. By limiting mutations to the neural crest tissues, the authors were able to determine that a key function for HH in the neural crest mesenchyme is controlling the levels of transcriptional repression. Future experiments should examine more lineage-restricted tissues in the face as well as temporal requirements.
Of course, a number of open questions remain: Is the continued association with SUFU truly preventing GLI activator function? Is there a role for the cilium in GLI interactions with other regulators or post-translational modifiers? We also need a more accurate way to follow changing GLIA:GLIR ratios in vivo and to compare these to GLI-dependent responses. Finally, we must note that cilia are likely to coordinate multiple other signaling pathways; in the long term, how these pathways intersect will be crucial to our understanding of cilia and human disease.
Zdroje
1. Eggenschwiler JT, Anderson KV (2007) Cilia and developmental signaling. Annu Rev Cell Dev Biol 23 : 345–373. doi: 10.1146/annurev.cellbio.23.090506.123249 17506691
2. Huangfu D, Anderson KV (2005) Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A 102 : 11325–11330. doi: 10.1073/pnas.0505328102 16061793
3. Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426 : 83–87. doi: 10.1038/nature02061 14603322
4. Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA (2004) Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest 114 : 485–494. doi: 10.1172/JCI19596 15314685
5. Brugmann SA, Allen NC, James AW, Mekonnen Z, Madan E, Helms JA (2010) A primary cilia-dependent etiology for midline facial disorders. Hum Mol Genet 19 : 1577–1592. doi: 10.1093/hmg/ddq030 20106874
6. Chang C-F, Chang Y-T, Millington G, Brugmann SA (2016) Craniofacial ciliopathies reveal specific requirements for GLI proteins during development of the facial midline. PLoS Genet 12: e1006351. doi: 10.1371/journal.pgen.1006351 27802276
7. Bai CB, Joyner AL (2001) Gli1 can rescue the in vivo function of Gli2. Development 128 : 5161–5172. 11748151
8. Goetz SC, Anderson KV (2010) The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11 : 331–344. doi: 10.1038/nrg2774 20395968
9. Hui CC, Angers S (2011) Gli proteins in development and disease. Annu Rev Cell Dev Biol 27 : 513–537. doi: 10.1146/annurev-cellbio-092910-154048 21801010
10. Jacob J, Briscoe J (2003) Gli proteins and the control of spinal-cord patterning. EMBO Rep 4 : 761–765. doi: 10.1038/sj.embor.embor896 12897799
11. Blaess S, Corrales JD, Joyner AL (2006) Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development 133 : 1799–1809. doi: 10.1242/dev.02339 16571630
12. Hui CC, Joyner AL (1993) A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet 3 : 241–246. doi: 10.1038/ng0393-241 8387379
13. Riddle RD, Johnson RL, Laufer E, Tabin C (1993) Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75 : 1401–1416. 8269518
Štítky
Genetika Reprodukční medicína
Článek Epistasis and Entropy
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2016 Číslo 12
-
Všechny články tohoto čísla
- Bringing Editors to Preprint Servers
- Epistasis and Entropy
- Middle Age Has Its Advantages
- Craniofacial Ciliopathies and the Interpretation of Hedgehog Signal Transduction
- Comment on " Is Dispensable for Mouse Development"
- Shared Information between Residues Is Sufficient to Detect Pairwise Epistasis in a Protein
- A Hox-Embedded Long Noncoding RNA: Is It All Hot Air?
- Deep Reads: Favorites from a Few Different Shelves
- Buffers Inherent Left/Right Asymmetry Ensuring Symmetric Forelimb Formation
- Intracellular Zn(II) Intoxication Leads to Dysregulation of the PerR Regulon Resulting in Heme Toxicity in
- Mutations in the Heme Exporter Cause Sensory Neurodegeneration with Loss of Pain Perception
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Intracellular Zn(II) Intoxication Leads to Dysregulation of the PerR Regulon Resulting in Heme Toxicity in
- Craniofacial Ciliopathies and the Interpretation of Hedgehog Signal Transduction
- Mutations in the Heme Exporter Cause Sensory Neurodegeneration with Loss of Pain Perception
- Shared Information between Residues Is Sufficient to Detect Pairwise Epistasis in a Protein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



