-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Global Overview of the Genetic and Functional Diversity in the Pathogenicity Island
The Helicobacter pylori cag pathogenicity island (cagPAI) encodes a type IV secretion system. Humans infected with cagPAI–carrying H. pylori are at increased risk for sequelae such as gastric cancer. Housekeeping genes in H. pylori show considerable genetic diversity; but the diversity of virulence factors such as the cagPAI, which transports the bacterial oncogene CagA into host cells, has not been systematically investigated. Here we compared the complete cagPAI sequences for 38 representative isolates from all known H. pylori biogeographic populations. Their gene content and gene order were highly conserved. The phylogeny of most cagPAI genes was similar to that of housekeeping genes, indicating that the cagPAI was probably acquired only once by H. pylori, and its genetic diversity reflects the isolation by distance that has shaped this bacterial species since modern humans migrated out of Africa. Most isolates induced IL-8 release in gastric epithelial cells, indicating that the function of the Cag secretion system has been conserved despite some genetic rearrangements. More than one third of cagPAI genes, in particular those encoding cell-surface exposed proteins, showed signatures of diversifying (Darwinian) selection at more than 5% of codons. Several unknown gene products predicted to be under Darwinian selection are also likely to be secreted proteins (e.g. HP0522, HP0535). One of these, HP0535, is predicted to code for either a new secreted candidate effector protein or a protein which interacts with CagA because it contains two genetic lineages, similar to cagA. Our study provides a resource that can guide future research on the biological roles and host interactions of cagPAI proteins, including several whose function is still unknown.
Published in the journal: . PLoS Genet 6(8): e32767. doi:10.1371/journal.pgen.1001069
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001069Summary
The Helicobacter pylori cag pathogenicity island (cagPAI) encodes a type IV secretion system. Humans infected with cagPAI–carrying H. pylori are at increased risk for sequelae such as gastric cancer. Housekeeping genes in H. pylori show considerable genetic diversity; but the diversity of virulence factors such as the cagPAI, which transports the bacterial oncogene CagA into host cells, has not been systematically investigated. Here we compared the complete cagPAI sequences for 38 representative isolates from all known H. pylori biogeographic populations. Their gene content and gene order were highly conserved. The phylogeny of most cagPAI genes was similar to that of housekeeping genes, indicating that the cagPAI was probably acquired only once by H. pylori, and its genetic diversity reflects the isolation by distance that has shaped this bacterial species since modern humans migrated out of Africa. Most isolates induced IL-8 release in gastric epithelial cells, indicating that the function of the Cag secretion system has been conserved despite some genetic rearrangements. More than one third of cagPAI genes, in particular those encoding cell-surface exposed proteins, showed signatures of diversifying (Darwinian) selection at more than 5% of codons. Several unknown gene products predicted to be under Darwinian selection are also likely to be secreted proteins (e.g. HP0522, HP0535). One of these, HP0535, is predicted to code for either a new secreted candidate effector protein or a protein which interacts with CagA because it contains two genetic lineages, similar to cagA. Our study provides a resource that can guide future research on the biological roles and host interactions of cagPAI proteins, including several whose function is still unknown.
Introduction
Helicobacter pylori persistently infects more than one half of all humans, and can cause ulcer disease, gastric cancer, and MALT lymphoma [1]. The H. pylori cag pathogenicity island (cagPAI) is an intriguing virulence module of this obligate host-associated bacterium [2]–[4]. H. pylori strains that possess a functional cagPAI are particularly frequently associated with severe sequelae, notably gastric atrophy and cancer [4]–[7]. The cagPAI is ∼37 kb long, and contains ∼28 genes [3]. These genes encode multiple structural components of a bacterial type IV secretion system (t4ss) as well as the 128 kDa effector protein, CagA [7]. After H. pylori has adhered to a host cell, the Cag t4ss translocates CagA into that cell. CagA is subsequently phosphorylated by host cell kinases and interacts with multiple targets (e.g. SHP-2, Grb2, FAK), profoundly altering host cellular functions [8], [9]. The alterations induced by the cagPAI are thought to ultimately contribute to malignant transformation [4], [10], and CagA has been designated a bacterial oncoprotein [11].
H. pylori has a high mutation rate, which has resulted in extensive genetic diversity [12], and also recombines frequently with other H. pylori [13]. H. pylori isolates have been subdivided into distinct biogeographic populations and subpopulations with specific geographical distributions that reflect ancient human migrations [14]–[16]. The global population structure of H. pylori is now well understood based on multilocus haplotypes from seven housekeeping genes. However, very little is known about the biogeographic variation of virulence factors, such as the cagPAI, nor has the impact of genetic variation on disease outcome and host adaptation been adequately addressed. Previous analyses on the basis of comparative genome hybridization have demonstrated marked differences between biogeographic populations with respect to the cagPAI [17]. Microarray analysis of 56 globally representative strains of H. pylori revealed that the cagPAI was present in almost all strains from some biogeographic populations and subpopulations in Africa and Asia, while it was variably present in other populations [17]. The cagPAI was lacking in all isolates of hpAfrica2, which is distantly related to the other populations [17]. Currently, nine complete cagPAI sequences are publicly available [2], [18]–[22], whose isolates belong to hpEurope (7 sequences), hspWAfrica (1) and hspEAsia (1) (see Results), and no sequence data is available for the cagPAI in the other six populations and subpopulations where the cagPAI is present.
Here we analyze complete cagPAI sequences from 38 isolates representing all known H. pylori populations and subpopulations and compare their genetic polymorphisms with measures of functional expression. Our data show that the cagPAI has shared a long evolutionary history with the H. pylori core genome, and displays a remarkable global conservation of gene content, structure and function, with minor exceptions. We provide evidence that the cagPAI was acquired by ancestral H. pylori in a single event that occurred before modern humans migrated out of Africa. Sequence comparisons identified domains in multiple components of the t4ss that are likely to be under diversifying selection, and these findings can guide future research into the function of t4ss components.
Results
Distribution of the cagPAI in a global collection of H. pylori
In order to define the occurrence of the cagPAI in H. pylori, we screened a globally representative collection of H. pylori isolates from 53 different geographical or ethnic sources [15], [16] (Figure 1). 877 isolates were tested for the presence of the cagPAI by a PCR approach. Strains were classified as cagPAI-positive if we succeeded in separate PCR amplifications for the 5′ and 3′ ends of the cagPAI, or as cagPAI-negative if we succeeded in amplifying an empty site with primers from the flanking regions. The cagPAI was present in at least 95% of strains assigned to the hpAfrica1 (hspWAfrica plus hspSAfrica), hpEastAsia (hspEAsia, hspMaori) and hpAsia2 populations. In contrast, none of the hpAfrica2 strains possessed the cagPAI, and it was only variably present in strains from the populations hpEurope (225/330 strains; 58%), hpNEAfrica (58/72 : 81%), and hpSahul (32/49; 65%) or the hspAmerind subpopulation of hpEastAsia (5/18; 28%).
Fig. 1. Distribution of the cag pathogenicity island in a global collection of H. pylori strains from different populations. 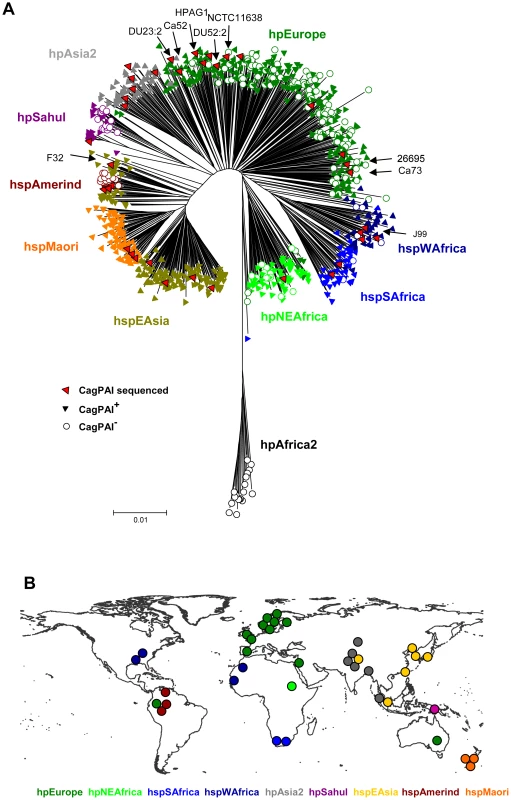
(A) Neighbor joining (NJ) tree of neutral genetic relatedness of H. pylori strains, including information about the presence or absence of the cagPAI. The NJ tree was calculated from concatenated sequences of seven housekeeping genes (length 3406 bp) from 877 isolates of H. pylori [16] plus 9 additional isolates from which either cagPAI sequences [20] or whole genome sequences had been published (indicated by arrows; [2], [18]–[22]. Each strain was scored for presence (filled triangles) or absence (empty circles) of the cagPAI based on the results of PCR reactions that span the ends of the cagPAI. Population assignments based on Bayesian analyses [15], [16] are indicated by the color coding of symbols that correspond to the labels next to the tree; red symbols indicate all strains whose cagPAI sequences are now available, including the 29 strains that have been newly selected for cagPAI sequence analysis. (B) Geographic sources of strains whose cagPAI sequences are now available. Each dot indicates the source of isolation of one of the 38 cagPAI sequences that were analyzed. The dots are color-coded by population or subpopulation as in (A). Based on their multilocus sequence typing (MLST) haplotypes, seven strains with published cagPAI sequences belong to the hpEurope population (NCTC11638 from Australia [2]; 26695 from England [18]; and DU23, DU52, Ca52, Ca73 [20] and HPAG1 [21] from Sweden). J99 from the U.S.A. [22] belongs to hpAfrica1, and F32 [19] from Japan belongs to the hspEAsia population of hpEastAsia. None of these published cagPAI sequences were from strains of the hpNEAfrica, hpSahul, or hpAsia2 populations, from the hpEastAsia subpopulations hspAmerind or hspMaori, or from the hpAfrica1 subpopulation hspSAfrica, although those populations are also potentially important for our understanding of the evolutionary history of H. pylori. We therefore selected 29 strains from our global strain collection to supplement these nine published cagPAI sequences and provide a globally representative sample of cagPAI diversity (Figure 1). These strains included all known biogeographic populations, except for the cag-negative hpAfrica2. The entire cagPAI, approximately 37 kilobasepairs in length, was sequenced and annotated from each of the 29 strains, either after shot-gun cloning of overlapping long-range PCR products or via direct amplification of multiple, smaller PCR products.
Conserved synteny and low macrodiversity in the cagPAI
The 38 complete cagPAI sequences were compared by pairwise sequence alignments and by a multiple alignment in Kodon relative to the cagPAI from J99 used as a scaffold sequence (Figure 2). The general pattern of gene content and gene order (signifying macrodiversity) was similar in most sequences, with only limited variation due to changed synteny or deletions. Synteny changes resulted from genomic rearrangements, horizontal genetic exchange (e.g. replacement of HP0521 by HP0521b), possibly in conjunction with IS (insertion sequence) element insertion, or gene inversions, such as for HP0535. Insertions, deletions, point mutations, frameshift mutations or disruption through insertion elements (Figure S1) were also observed in some of the cagPAI sequences, some of which should have resulted in pseudogenes. We therefore tested all strains for their ability to induce interleukin-8 (IL-8) in gastric epithelial cells (Figure 2, Figure 3), as an indicator of PAI function [23]. Most of the strains containing a cagPAI were able to induce IL-8, indicating that many of the mutations did not drastically reduce the general function of the cagPAI (Table 1).
Fig. 2. Conservation of the cagPAI genetic organization across H. pylori biogeographic populations. 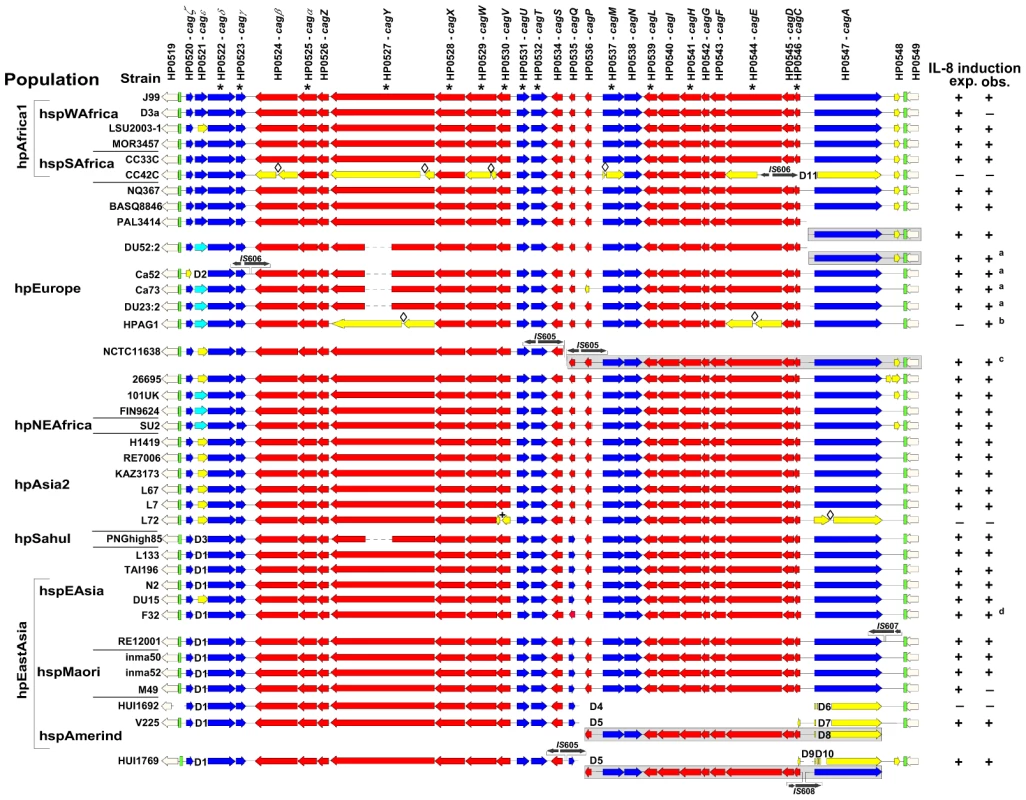
The sequences were aligned in KODON using the cagPAI of strain J99 as a scaffold sequence. Individual isolates are grouped according to biogeographic (sub-)populations. The continuity of the cagPAI was disrupted in isolates PAL3414, V225 and HUI1769, and fragments found in secondary locations are displayed in grey-shaded boxes on separate lines. The two cagPAI sequences from reference strains J99 and 26695 were extracted from whole genomes. Genes essential for a basic function of the cagPAI type IV secretion system (IL-8 induction; [3]) are labeled with an asterisk*. Activity of the Cag t4ss (IL-8 secretion; + or −) was monitored during experimental infection of AGS cells with H. pylori. Obs., observed IL-8 secretion; exp., IL-8 secretion expected from the cagPAI sequence; red, genes in forward orientation; blue, genes in reverse orientation; light blue, shorter gene version; white, different gene HP521B [20] in this locus; yellow, pseudogenes; black, IS elements; green, cagPAI insertion sites. Diamonds: frameshift mutations leading to pseudogenes. Δ followed by numbers 1 through 10 indicate different deletions (manifestation of macrodiversity) and are consecutively numbered as mentioned in the text and Table 1. a,b,c,d: strains not functionally tested in this study possess functional cagPAIs according to the following references: a [20]; b [21]; c [2]; d [19]. Fig. 3. Variability of Cag t4ss function in H. pylori strains from different biogeographic populations. 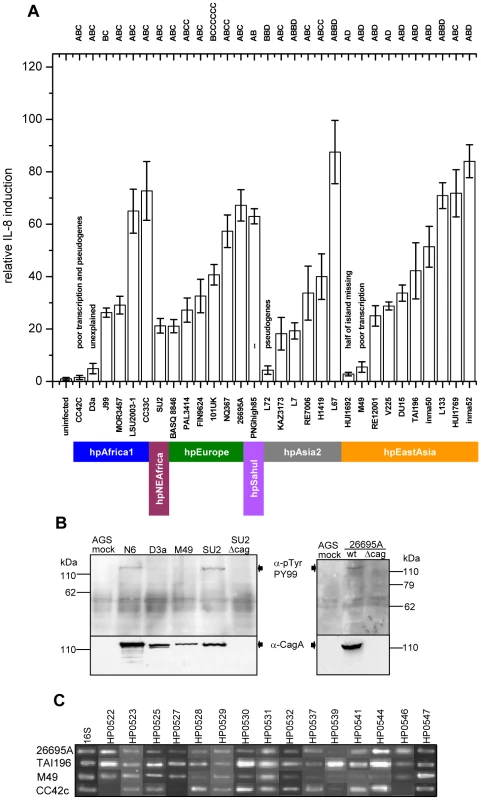
(A) IL-8 induction in human gastric epithelial cells by diverse H. pylori strains from different biogeographic populations. IL-8 secretion induced at 20 h post infection by live H. pylori in gastric epithelial cells (AGS, shown here, and MKN28, data not shown) was determined as a read-out for Cag t4ss activity. The two strains J99 and 26695A, for which entire genome sequences are available, were included as positive controls. CagA EPIYA motifs for each strain are indicated on top of the graph. Exceptions in the genetic integrity of some of the islands and other explanations for an observed loss of functionality are indicated above the single bars. Colored bars designate the population assignments of strains. Coincubation experiments were performed independently at least three times for each strain, with similar results, and one representative experiment, performed in triplicates for each strain, is shown. IL-8 secretion is depicted in relative values, as a multiple of the negative control (mock), which was set to 1. (B,C) Assessing underlying causes of loss of function of cagPAIs in some H. pylori strains. (B) CagA translocation assays performed after infection of AGS cells with the two selected H. pylori strains D3A and M49. These displayed loss of cagPAI-related activity in IL-8 release assays. Both strains were unable to translocate CagA into human gastric epithelial cells. Strains SU2, N6, and 26695A wild type (wt) were used as positive controls for CagA translocation. Strains SU2Δcag and 26695AΔcag (isogenic cagPAI deletion mutants to SU2 and 26695A) were included as negative controls. (C) transcript amounts of single cagPAI genes. 30 strains (4 strains shown here – for complete results see Table S3) were studied using semiquantitative RT PCR for each gene with known function in the Cag t4ss (refer to Table 2 for gene names). Two strains with loss of t4ss function, CC72C, and M49, are shown. TAI196 and 26695A are depicted as positive controls. TAI196, a strain with a high propensity to induce IL-8, shows relatively high transcript amounts for the majority of genes. Strains CC42C and L72 (not shown) which have pseudogenes and lost the ability to induce IL-8, showed low or undetectable transcript amounts for some genes including the pseudogenes. M49 displayed low transcript amounts for a number of essential genes of the t4ss located predominantly in the right half of the cagPAI (genes HP0528, and HP0537 to HP0544). Tab. 1. Genetic macro- and minidiversity variants (gene order and orientation, gene identity, insertion elements) within the H. pylori cagPAI with regard to population assignments. 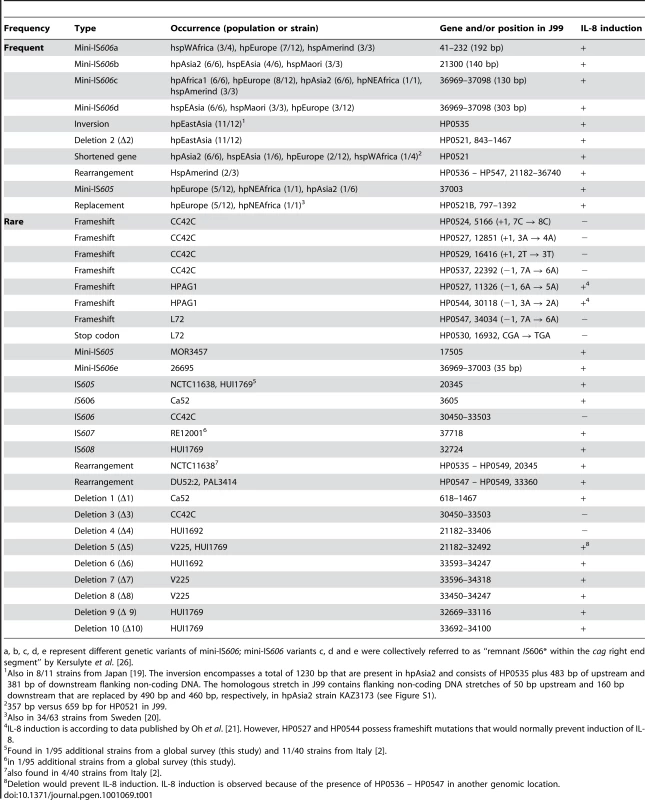
a, b, c, d, e represent different genetic variants of mini-IS606; mini-IS606 variants c, d and e were collectively referred to as “remnant IS606* within the cag right end segment” by Kersulyte et al. [26]. Fixed and transient variants in cagPAI sequence organization
Most new mutations are deleterious, whether associated with single nucleotide polymorphisms, mobile elements or genomic rearrangements, and will be removed by purifying selection. However, mutations without a drastic effect on fitness, so-called neutral or nearly neutral mutations, can remain as rare variants within a population for long time periods. The vast majority of such mutations remain at low frequency until they are (usually) lost due to genetic drift. Rare neutral mutations can become more frequent over time, or even become fixed, also due to genetic drift [24]. Still other mutations are under positive selection. These rapidly become frequent or fixed due to Darwinian selection. In isolated clonal populations, Muller's ratchet can even result in some deleterious mutations rising to high frequency [25] and the same is true of extreme bottlenecks, which can fix deleterious mutations immediately. These basic evolutionary principles indicate that the demographies of rare versus frequent mutations differ and should be examined separately.
Frequent variants
A number of frequent cagPAI macrodiversity variants were found, some of which were present in all isolates of at least one sub-population, or almost all isolates (Table 1). These included insertion events due to one of three variants of IS606 [26] or of a mini-IS605 insertion [27], [28], an inversion of gene HP0535 plus its flanking non-coding DNA, a deletion of either the complete HP0521 ORF (Δ2; Figure 2) or part of that ORF, or the replacement of HP0521 by the unrelated ORF HP0521B (Figure 2, Table 1). Additionally, most of the 3′ (right) half of the cagPAI is lacking in all three hspAmerind strains due to one of two similar 11.2 kb deletions with distinct 3′ ends (Δ4, Δ5; Figure 2). These large deletions terminate within HP0546, and are associated with a second (intergenic) deletion of 410 bp or a 620 bp deletion that terminates within the N-terminal part of HP0547 (cagA). In strains V225 and HUI1769, a copy of the deleted segment plus the HP0546 and HP0547 ORFs have translocated to a separate, currently unidentified, location of the chromosome, leaving a shortened version of HP0546 at the original location (Figure 2). It is interesting to note that IL-8 induction was not eliminated by any of these frequent mutations (Figure 2, Figure 3, Table 1), suggesting that they are not deleterious to cagPAI function, and might be neutral or even under positive selection.
Rare variants
Rare variants were present in only one or two strains, are probably transient, and will tend to disappear during genetic drift [29]. The rare variants included frameshift mutations in multiple ORFs within three single isolates (CC42C, HPAG1 and L72) and IS elements (mini-IS605, IS605, IS606, IS607 or IS608 [26]) that have integrated at distinct locations in 7 other isolates (Table 1; Figure S1). Our dataset consisted of only 38 isolates, and it was possible that these rare mutations might be more widely distributed. We therefore screened 95 other globally representative strains for the presence of IS605, IS606, IS607 or IS608 at those locations, but only identified two additional strains with IS element insertions, one each for IS605 (MOR3055 – hspWAfrica) and IS607 (BASQ9523 – hpEurope) (data not shown). Thus, strains carrying these particular insertion mutations really are rare.
We also found two rare, distinct genomic rearrangements (Table 1). One of these was in strain NCTC11638 from Australia and has been reported previously [2]. It splits the cagPAI between ORFs HP0534 and HP0535 into two segments, one of which is translocated elsewhere in the genome, and is distinct from the split of the cagPAI in the hspAmerind strains. Previous analyses identified the same rearrangement in 4/40 strains from Italy [2], but it was not found in any of the other 38 cagPAI sequences analyzed here nor in any of the 95 other, globally representative strains that we investigated by PCR. The other rearrangement separated HP0547 (cagA) through HP0549 plus flanking DNA from the rest of the cagPAI. It has been previously described for two hpEurope strains from Sweden and one from Australia [20]. We found the same pattern in a fourth hpEurope strain isolated in Palestine (PAL3414). Both of these rearrangements were present in less than 5% of isolates.
The 17 rare mutations were identified in a total of 12 isolates. Only three of those, CC42C, HUI1692 and L72, did not induce IL-8, indicating that the majority of the rare sequence changes also did not cause a severe loss of cagPAI function. This observation is compatible with most of the rare mutations being selective neutral or near-neutral.
Genomic decay
Three overlapping small deletions (Δ1, Δ2, Δ3) that removed the HP521 ORF were found in all but one hpEastAsia isolate, one hpEurope isolate and the hpSahul strain (Figure 2; Table 1), but those did not abolish cagPAI function (see above). Eight other deletions were found in four individual strains (Figure 2). Two of these isolates were unable to induce IL-8: CC42C (hspSAfrica) contains multiple frameshift mutations and an insertion of IS606 as well as deletion Δ11, which removes part of cagA (HP547). Δ4 and Δ6 deleted half of the cagPAI in hspAmerind strain HUI1692. The cagPAI is clearly decaying in both CC42C and HUI1692. In contrast, although deletions Δ5 and Δ7–Δ10 also removed large parts of the cagPAI in hspAmerind strains V225 and HUI1769, these deletions occurred in a segment that has been duplicated to a separate location (see above) and these two isolates remain able to induce IL-8. Thus, with one exception (Δ1), these deletions are rare and seem to be associated with accelerated decay of non-functional cagPAI genes. In addition, the cagPAI in non IL-8-inducing strain L72 also contained one frameshift and one premature stop codon in a coding region, and seems to be undergoing decay.
Signatures of selection within individual cagPAI genes
Darwinian selection for variation in coding regions can also be exerted at the nucleotide or protein level. We therefore analyzed sequence polymorphisms (microdiversity) in individual cagPAI genes for traces of such selection (Materials and Methods). Similar to housekeeping genes [30], almost all alleles of each cagPAI ORF were unique to one isolate among the 38 strains. Exceptionally, we identified duplicates of a single allelic sequence in six genes; in each case, the strains possessing the duplicate alleles were from a common population (Table S4). Occasional duplicate alleles within populations have also been described for housekeeping genes [30] and are considered to represent homologous recombination. Again, similar to housekeeping genes, most cagPAI genes seemed to be under purifying selection because their Ka/Ks ratios were ≤0.2 (Table 2). However, five genes (HP0534-0535, HP0538, HP0546-0547) showed signs of positive or diversifying selection because their overall Ka/Ks ratios were greater than 0.2; of these, cagA (HP0547) had the highest proportion of non-synonymous polymorphisms (Ka/Ks = 0.45). However, Ka/Ks ratios are relatively insensitive indicators of Darwinian selection, which can act at the level of single protein epitopes or conformational domains. We therefore used a Bayesian method (PAML/CODEML [31]) to search MLST and cagPAI genes for codons that might be under diversifying selection (indicated by ω >1). Only two of the seven MLST housekeeping genes (trpC, yphC) contained an appreciable frequency (3.9%; 5.3%) of codons with posterior probabilities of ω >1 being above 0.95 (Table 2). In contrast, >5.3% of the codons matched this criterion in 10 of the 28 cagPAI ORFs (Table 2), including four of the five ORFs with high overall Ka/Ks ratios (HP0535, HP0538, HP0546, HP0547).
Tab. 2. Sequence diversity, Ks/Ka ratios, and codons under diversifying selection in cagPAI and housekeeping genes (37 strains). 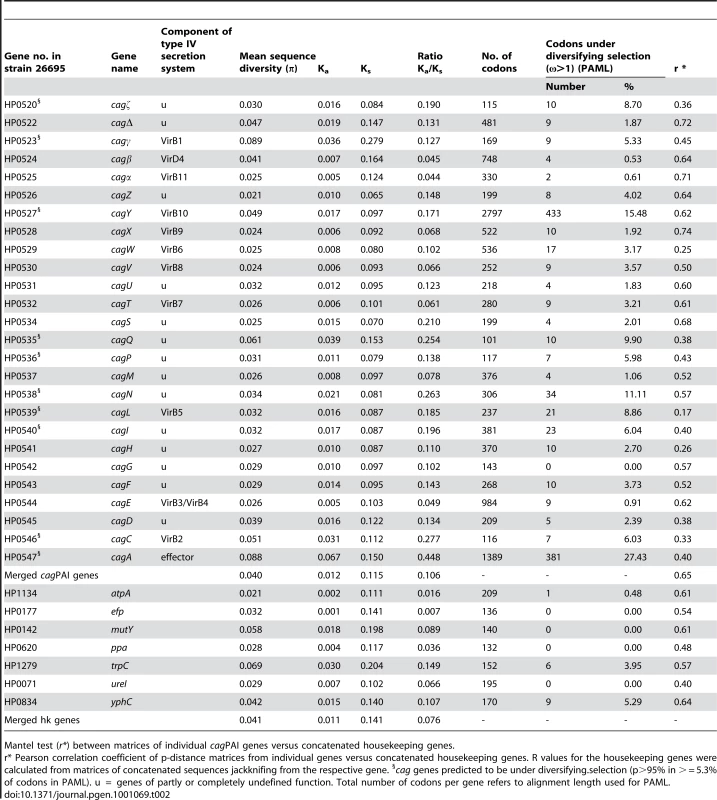
Mantel test (r*) between matrices of individual cagPAI genes versus concatenated housekeeping genes. We also tested eleven cagPAI ORFs, including nine with high frequencies of codons under selection according to PAML, and two with lower frequencies (HP0524, HP0525) with a second Bayesian program, OmegaMap [32], [33], which unlike PAML also takes into account the occurrence of recombination (ρ) between different alleles (Table S5). OmegaMap detected fewer codons with high probabilities of positive selection, but the codons that it identified often overlapped with codons that had been identified as being under positive selection by PAML (Table S5). Finally, we employed a sliding window along codons of PAML posterior probabilities of ω to identify clusters of sites with signs of diversifying selection (Figure 4). The combination of three forms of analysis (criteria: Ka/Ks >0.2, or likelihood of at least 95% for ω >1 in ≥5.3% of codons, or at least two clusters of two or more adjacent amino acids (aa) predicted under diversifying selection in PAML) identified 13 cagPAI genes that are likely to have evolved under diversifying selection: HP0520, HP0522, HP0523, HP0527, HP0528, HP0534, HP0535, HP0536, HP0538, HP0539, HP0540, HP0546 and HP0547. Of these, functions or structural contributions are known only for HP0523 (virB1), HP0527 (virB10), HP0539 (virB5), HP0546 (virB2) and HP0547 (cagA) [7], [34]–[38]. The percentage of codons with high likelihood of positive selection was highest in cagA (26.9%), followed by cagY (15.5%) and a gene of unknown function, cagQ (HP0535; 9.9%) (Table 2).
In addition to a high frequency of putative codons under diversifying selection, HP0527 (cagY) and HP0547 (cagA) also exhibited variable gene lengths. This was due to variable numbers of repetitive modules within the genes, as previously reported [35], [39]. In the CagA protein, the number of phosphorylation sites (C-terminal EPIYA repeat motifs) differed, as did the types of these repeats (Figure 3). As previously described [39], the third EPIYA motif of CagA was type D in most (13/17) Asian strains whereas type D was not found in isolates from any other population. This reflected the preponderance of type D EPIYA in isolates assigned to the hpEastAsia and hpAsia2 populations. If the EPIYA type D motif were ancestral in Asian populations, this finding might reflect horizontal acquisition of cagA by the four exceptional Asian strains from Western strains. Homologous recombination involving the cagPAI has also been reported in isolates from Mestizos in Peru [40] and might reflect selection due to functional differences that are related to ethnic specificity.
Fig. 4. Sliding window map of maximum likelihood analysis of codons to be under diversifying selection for complete cagPAIs and housekeeping genes. 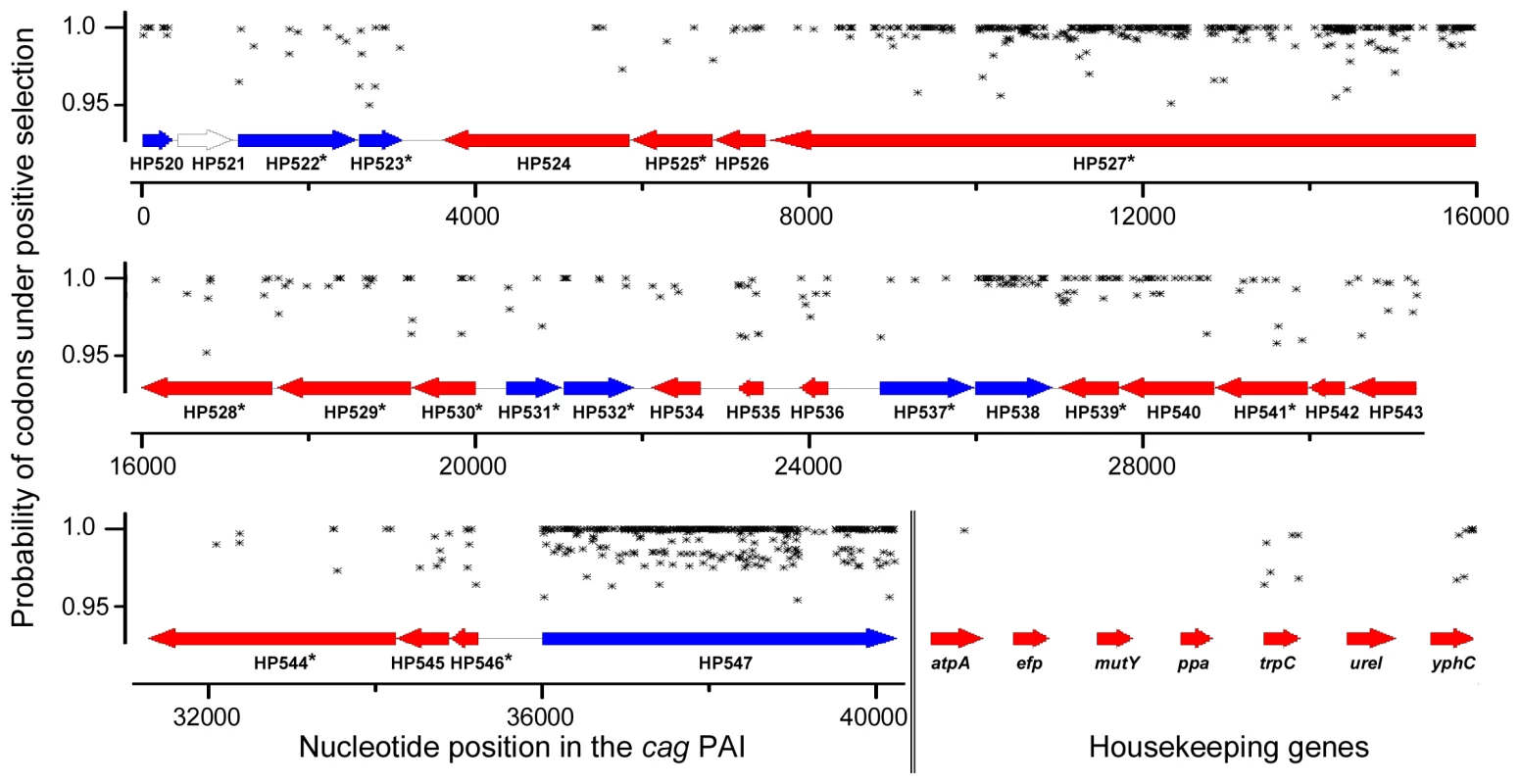
Codons calculated by CODEML (model M3) to have a high likelihood p>95% of being under diversifying selection in each gene of the cagPAI or housekeeping genes of all analyzed strains are highlighted by black symbols. Comparison of cagPAI and housekeeping gene phylogeny
We next asked whether the phylogeny of cagPAI genes was similar to that of housekeeping genes. Concatenated sequences of the cagPAI genes yielded a tree (Figure 5B) that is very similar to the tree based on a concatenate of the seven MLST housekeeping genes (Figure 5A). Similarly, matrices of pairwise genetic distances of the concatenated cagPAI genes were highly correlated with corresponding matrices of pairwise distances of concatenated housekeeping genes (R = 0.65, p<0.001) (Figure 5C). These data show that 42% of the variance among cagPAI genes can be attributed to a linear relationship with housekeeping genes. The correlations for individual cagPAI genes ranged from R = 0.17 to R = 0.74 (Table 2). While most cagPAI genes thus fell into the range observed for the individual housekeeping genes (0.46 to 0.69), the correlations were lower for particular cagPAI genes (e.g. cagL, R = 0.17), which might reflect selection and/or recombination between cagPAIs from different bacterial populations. These observations indicate a generally similar genealogy of cagPAI and housekeeping genes, which would imply that the cagPAI has accompanied H. pylori since before human migrations out of Africa some 60,000 years ago [17]. In agreement, the genetic diversity of the cagPAI genes per population decreased significantly with distance from Northeast Africa (data not shown).
Fig. 5. Pairwise correlation of genetic distances and phylogeographic diversity between H. pylori housekeeping genes and concatenated cagPAI genes. 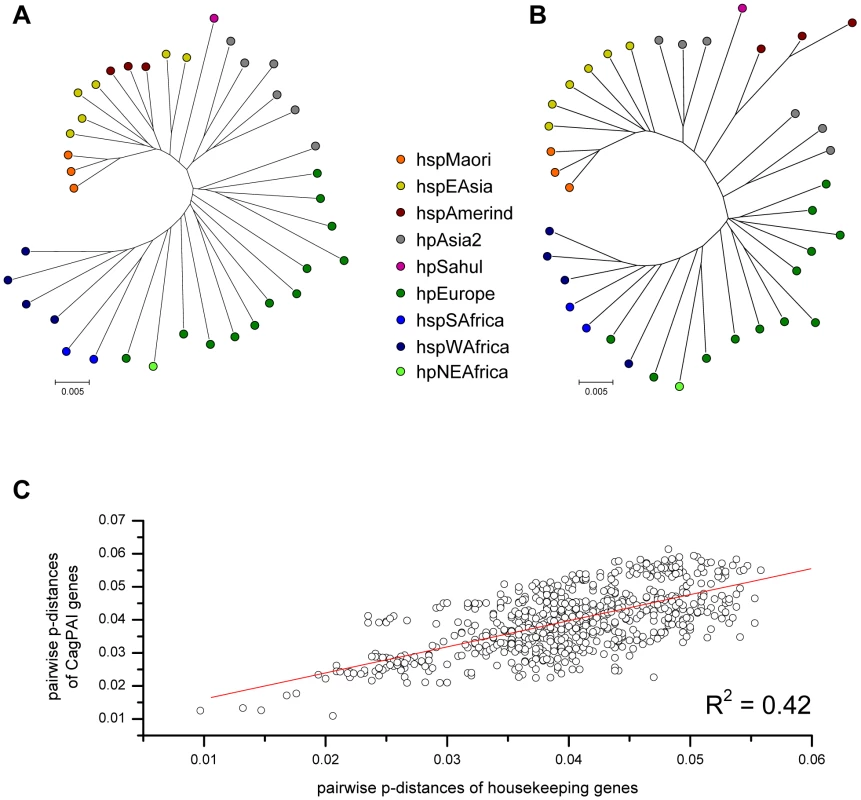
(A) neighbor-joining (NJ) tree analysis of concatenated housekeeping genes for all strains, whose complete cagPAIs were analyzed. (B) NJ tree analysis of concatenated cagPAI genes for all strains. (C) Mantel comparison of pairwise genetic distances in housekeeping genes and cagPAI genes. cagPAI sequence variation and type IV secretion system function
Only five of the strains tested here were not able to induce IL-8 (Figure 3). The same five strains did not translocate CagA into AGS cells, a second marker of t4ss function (Figure 3B). For three of the five strains (CC42, L72 and HUI1692), a lack of function can be explained by sequence features of coding sequence (CDS) decay. The cagPAI of CC42C contains multiple pseudogenes, some of which are crucial for t4ss function [3]. Half of the cagPAI including numerous essential t4ss genes is lacking in strain HUI1692. For strain L72, a point mutation results in a premature stop codon in gene HP0530, which is essential for t4ss function. In contrast, the cagPAI sequences did not offer obvious explanations for the lack of induction of IL-8 by strains M49 and D3a. We therefore investigated the transcript abundance of all 14 genes involved in IL-8 induction and of cagA for 28 sequenced strains as well as for the reference strains 26695A and J99 (Figure 3C; Table S3). The inability of strain M49 to induce IL-8 can be accounted for by very low transcript levels for 7/15 cagPAI genes (Figure 3C; Table S3); the cause of this low transcription is unknown. However, we are unable to explain the inability of strain D3a to induce IL-8, because it was not impaired in cagPAI transcription (Table S3). We are also not readily able to explain the considerable variation of transcript levels among the other strains that did induce IL-8 (Table S3), except that it did not correlate with the macrodiversity patterns described above (data not shown).
Similar to the variable transcript levels, the levels of IL-8 induction also varied dramatically (Figure 3). This variation did not correlate with strain assignments to biogeographic populations or with the type and number of EPIYA motifs within CagA (Figure 3A; [39]). Nor did they correlate with quantitative values for adhesion of the strains to AGS or MKN28 gastric epithelial cells (data not shown).
Discussion
Since its discovery in 1996 [2], the cagPAI has probably been the most intensively studied segment of the H. pylori genome. The virulence functions of the Cag t4ss and its translocated effector, CagA, have been investigated in great detail, and numerous studies have correlated cagPAI-associated polymorphic markers with disease risk. However, all these studies focused on one or only few genes within the cagPAI (such as cagA), and were performed with strains from one or few geographic regions. We therefore anticipated that a comparative analysis of complete cagPAI sequences from a globally representative and well characterized collection of strains would provide valuable information about the evolutionary history of the cagPAI and its variability within a phylogeographic context. The complete cagPAI sequences of 29 strains were determined and combined with 9 published complete sequences to yield a large and comprehensive dataset of cagPAI diversity, which was analysed at the levels of both macrodiversity (differences in gene content, synteny and function), and microdiversity (sequence polymorphisms).
Phylogeographic implications of H. pylori cagPAI diversity
It has previously been noted from limited samples that different populations of H. pylori differ in the frequency of possession of the cagPAI [14], [17]. Our data on 877 isolates from all known H. pylori populations and subpopulations provide unambiguous evidence for this variability. Carriage of the cagPAI varies from almost universal presence in hpEastAsia and hpAfrica1 through intermediate presence (hpEurope) to complete absence (hpAfrica2) (Figure 1). The cagPAI is also absent in the related species H. acinonychis [17], which resulted from a host jump from humans to large felines [41]. The absence of the cagPAI from hpAfrica2 and H. acinonychis has been interpreted as the ancestral state, i.e. H. pylori acquired this genomic island by horizontal gene transfer from an unknown source after H. pylori had established itself in humans [17]. But when was it acquired, and on how many occasions?
The data presented here indicate that the cagPAI was only acquired once because its microdiversity correlated with microdiversity within housekeeping genes (Figure 5). That acquisition was prior to 60,000 years ago, the time when H. pylori accompanied modern humans during their migrations “out of Africa” [16], because cagPAI sequence microdiversity diminished with distance from North East Africa. An important implication of this conclusion is that, with the exception of hpAfrica2, the variable presence of the cagPAI in H. pylori populations usually reflects secondary loss, rather than inheritance of the ancestral virgin state.
Macrodiversity versus fitness and function
Previous analyses have shown that strains that circulate within the same communities, and even within the same stomach, can be mixed in respect to possession of the cagPAI [30]. This observation indicates that cag positive bacteria do not outcompete cag negative bacteria in all environments. Nevertheless, our data support the inference [17] that a functional cagPAI provides a fitness advantage to H. pylori in most human populations: macrodiversity variants that inactivated t4ss function through deletions or insertion of IS elements were rare, whereas macrodiversity variants that were frequent did not affect t4ss function. For instance, shortening, complete loss or replacement (by HP0521b) of gene HP0521 was observed in almost all populations but this did not reduce cagPAI functionality, suggesting that this gene is not important for t4ss functions. Similarly, the genetic organization of the cagPAI was in general strongly conserved, and insertion elements did not play a decisive evolutionary role for the cagPAIs, unlike previous conclusions [2]. Even separation of the cagPAI in two parts did not lead to loss of function, except when a deletion was involved.
cagPAI t4ss microdiversity and signatures of positive selection
High variation at the level of sequence microdiversity was found along the cagPAI, but this is also true of housekeeping genes, and might possibly result from the high frequencies of mutation and recombination in H. pylori [14], [16]. However, unlike most housekeeping genes, multiple cagPAI ORFs showed signs of Darwinian diversifying selection, as indicated by higher Ka/Ks values and codon-based analyses, which identified specific amino acids or regions of particularly high non-synonymous diversity in 13 cagPAI genes (Figure 4, Table 2). In the following we attempt to interpret these measures of selection by mapping them onto known components including structural features of the t4ss encoded by the cagPAI.
Seventeen of the cagPAI genes are essential for the known t4ss functions (IL-8 induction, CagA translocation [3]), of which 12 have been characterized in structural or functional terms (virB1,2,4,5,6,7,8,9,10,11 and virD4 orthologs, cagA). In Figure 6, we present a schematic structural model of the cagPAI t4ss apparatus including all known structural Cag proteins plus the effector CagA. Different shades of grey indicate the proportion of amino acids which are likely to have undergone diversifying selection according to PAML.
Fig. 6. Model of the Cag t4ss of H. pylori, highlighting diversifying selection on outer and secreted components of the t4ss apparatus. 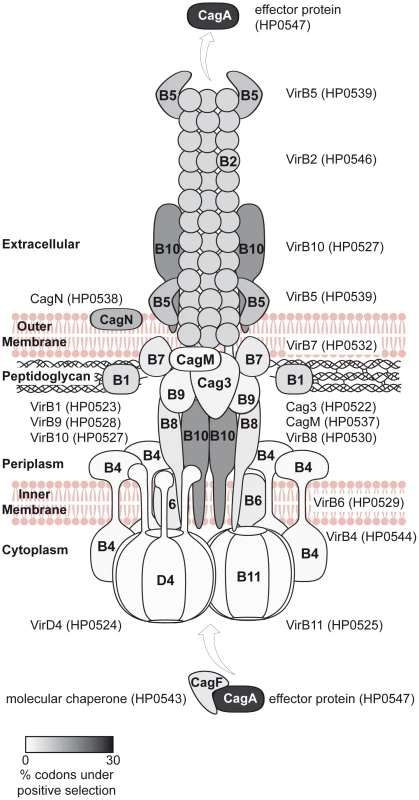
Each defined component of the cagPAI-encoded secretion system was shaded in grey according to averaged probability values, indicating the proportion of amino acids likely to be under diversifying selection for each individual protein; the probability values were calculated for each gene by the software CODEML (Table 2). 10 cagPAI genes which do not participate in the structure or are of unknown function are not included in the model. The model of the Cag t4ss is based on [3], [48]–[50], [61], [62]. Fig. 7. Diversifying selection in the MARK2 kinase binding domain of CagA. 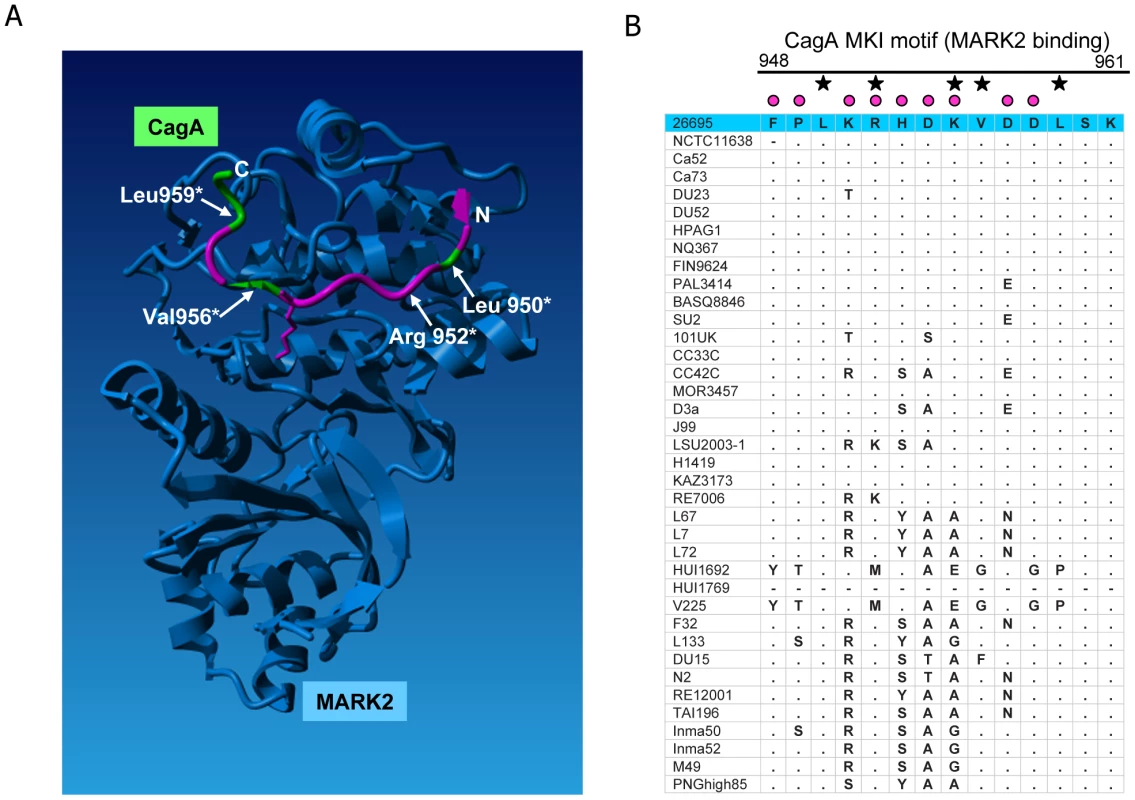
(A) amino acids (aa) in CagA predicted to be under diversifying selection (PAML, Model 3) were mapped onto the crystal structure of a short peptide within the CagA C-terminal domain (aa 948 to aa 961, MK1 peptide; aa under positive selection colored in pink, aa not predicted to be under positive selection colored in green), in complex with its interaction partner, the human kinase MARK2 [44]. Four residues critically involved in this interaction (Leu950, Arg952, Val954, Leu959) are labelled. Several amino acids involved in this interaction (e.g. Arg952 and Lys955, Ref. 44) are predicted to be under diversifying selection and are highly variable in our global strain collection. (B) amino acid alignment of the MARK2 binding region in the analyzed global strain collection. Black asterisks: amino acids involved in MARK2 binding. Pink dots: amino acids predicted to be under positive selection. Small dots in alignment: residue identical with reference strain 26695 (blue line on top). Hyphen: aa missing in respective strain. CagA
The translocated effector protein CagA (HP0547), which interacts with various host proteins [42], had the highest proportion of such amino acids of the entire cagPAI. These were distributed along its entire length, suggesting functional adaptation or modulation. CagA binds to host cell integrins [42] and is translocated into host cells by the cagPAI t4ss. Within the host cell, individual domains of CagA interact with intracellular proteins such as SH-2 proteins and protein kinases (e.g. Src, Abl [19], MARK2/PAR1b kinase family [7], [9]). These interactions render it potentially subject to diversifying or positive selection due to host polymorphisms which could even result in modified host protein interactions. A prominent example of amino acid diversity noted previously are the EPIYA motifs in the C-terminal half of CagA, which differ between Asian (hpAsia2; hpEastAsia) (type D) and all other populations [43]. The D type EPIYA repeat binds SHP-2 phosphatase more avidly than other types [19]. A clear bipartite “Eastern”/“Western” separation in the present global dataset was not only observed in phylogenetic trees based on the C-terminal half of CagA containing the divergent EPIYA repeat motifs, but also in its less well-characterized N-terminal moiety. Interestingly, CagA from the ancient and isolated hpSahul population [15] localised in between the Eastern and Western type CagA clusters (not shown).
The global strain selection provided further evidence of functional adaptation in a different CagA motif. Recently, structural analyses of a second CagA subdomain (CM domain, aa 885 to 1005) in complex with its interaction partner from the human host, the cellular kinase MARK2, were performed [44]. This analysis revealed the crucial contribution of specific residues in CagA (MKI motif; [44]) to the physical interaction with the kinase. The short CagA peptide that could be mapped in the cocrystal (Phe948–Lys961) is characterized in our strain collection by high amino acid variability (Figure 7A and 7B). Superposition of the amino acids under selection (according to PAML) onto the structure of the peptide [44] revealed that all but five of the 14 amino acids in this MARK2 binding domain of CagA have a high posterior probability of being under diversifying selection (Figure 7A). Interestingly, Arg952 and Val956, which both strongly influence MARK2 binding [44], have a likelihood of 1.0 and 0.81, respectively, of being under positive selection whereas two other MARK2 binding residues, Leu950 and Leu959, were not under diversifying selection. This result suggests that, although some specific MARK2 binding sites in CagA do have a lower propensity of being under positive selection, the binding strength of CagA to MARK2 can still be influenced by H. pylori protein variation, indicative of functional fine-tuning. These predicted functional implications of global variation in the MKI motif are in agreement with an earlier study by Lu et al. [9] who observed differences in CagA PAR1b binding and function when they exchanged two Western and Eastern phylogeographic variants of the CagA MARK2/PAR1b binding region within CagA chimeras. We therefore expect that other regions of CagA that are under selection (Figure 4) also warrant detailed structural and functional analyses. The observed CagA diversity, which is proposed to allow functional fine-tuning, may not only be associated with different host ethnicities but also with niche-dependent intrahost diversification during long-term colonization (e.g. stomach antrum versus corpus) [45], [46].
Other cag genes
A prior general comparison of component diversity in type III and IV secretion systems from different bacterial species [47] found that core structural proteins located in the bacterial cytoplasm or the inner membrane exhibit significantly lower diversity than do structural proteins exposed on the surface of the bacteria or secreted effector proteins [47]. Two well-characterized cag genes whose gene products are exposed on the cell surface have experienced strong selection: cagY (HP0527), which encodes a VirB10 ortholog that is a structural component of the cagPAI t4ss [36], and cagC (HP0546), which encodes a VirB2 pilin subunit ortholog [35], [38]. CagY is under selection due to host antibodies and/or direct host interactions [35], [36]. In cagC, those codons with the highest likelihood of diversifying selection (amino acids 21 to 42; Table S5) overlap with codons forming surface-exposed and highly strain-specific epitopes in the N-terminus of mature CagC [38]. The virB2 (HP0546) and virB5 (HP0539) orthologs of the cagPAI show signatures of diversifying selection in the present study; they encode surface-exposed pilin and pilus tip structural components of the Cag apparatus [48] and their sequence homology with functionally related VirB2 and VirB5 proteins from other bacteria is so low that they had to be identified by non-sequence-based approaches [37], [38]. We also find that 9 other cagPAI genes are under diversifying selection but their function is largely unclear. These include HP0520, HP0522 (part of the Cag outer membrane subcomplex [49]), HP0523 (cagγ; proposed to code for a virB1 orthologous peptidyglycan hydrolase [34], [50]), HP0528 (virB9), HP0534, HP0535, HP0536, HP0538 (encodes a membrane protein [50], [51]), and HP0540 [52]. Of these, HP0535 exhibits extensive non-synonymous variation and a clear bipartite Eastern-Western subdivision, similar to cagA. This gene is not involved in IL-8 induction or CagA translocation and is not predicted to possess a signal peptide. It may be a non-canonical secreted protein (score of 0.48 by SecretomeP). Based on the signs of selection and high diversity, we hypothesize that the HP0535-encoded protein interacts closely with CagA or is a novel effector protein that is translocated into host cells by the Cag t4ss. Of the other genes under diversifying selection whose function is unknown, HP0520 might be a non-canonical secreted protein because its SecretomeP score was also high (0.92).
In contrast to the genes just described, genes encoding cagPAI proteins that are not thought to be exposed on the bacterial surface [3] should be subject to purifying selection. In agreement with this expectation, other cagPAI genes including virD4 (HP0524) and virB11 (HP0525) orthologs [36], [50], displayed lower non-synonymous diversity and fewer codons under positive selection (Figure 6; Table S5).
In conclusion, the present work reports a genetic and functional approach within a global population genetic perspective to study diversity in a complex secretion system. This comprehensive library of data allowed the identification of genes with a high probability of having undergone diversifying selection. cagPAI genetic diversity is accompanied by modulations in functionality, but rarely by complete loss of function. Functional modulation of the t4ss appears to be an important feature in vivo and is predicted to rely not only on protein diversification but also on strain-dependent transcript level diversity in the cagPAI. These data will be a resource for future research on the biological roles and variable host interactions of individual cagPAI proteins. It will also foster research on the phylogeographic variability and evolution of determinants of host interaction in other microbes. The diversity in this dataset will also be useful to evaluating predictions by recent evolutionary models based on the structure of proteins, such as neutral networks of protein folds [53], [54]), which might be able to distinguish selection processes that favor structural versus functional conservation.
Materials and Methods
Bacterial isolates, sequencing, and RT–PCR
Bacterial isolates and sequences of seven housekeeping gene fragments (atpA, efp, mutY, ppa, trpC, ureI, yphC) have been described previously [13], [16], [55]. Strains were checked for the presence of the cagPAI by PCR, amplifying the 5′ (Primers O2872 + O2902) and 3′ (O2899 + O3326) flanking regions, or for absence (empty site) (primers O2872 + O3326). Primer sequences are provided in Table S1. Strains were chosen to represent all currently defined H. pylori populations possessing the cagPAI (Figure 1, Figure 2). The complete cagPAI was amplified for sequencing as two overlapping long range PCR products of ∼20 kb each with primers O2903 + O3048 and O3047 + O2904 (Table S1), respectively in 50 µl reactions with the EXL long range polymerase kit (Stratagene) using the following conditions: bacterial DNA 20 ng, Primers 20 µM each, 6 µl of 2mM dNTPs, 5 µl Buffer 1, 1 µl stabilizing solution, 1 µl EXL Polymerase, H2O to 50 µl. An initial denaturation for 1 min at 94°C was followed by 30 cycles of 45 sec at 94°C, 1 min at 65°C and 17 min 30 sec at 68°C. Long range PCR fragments were subjected to shotgun cloning. DNA fragments ranging from 0.8 to 1.2 kb were end repaired and cloned into the pGEM T-Easy vector (Promega), inserts were sequenced to 10-fold coverage by MWG Biotech. Alternatively, the cagPAIs were amplified as overlapping PCR products of ∼5 kb each with additional primers listed in Table S1 (primer combinations available on request) and sequenced with an extended set of primers (Table S1) by gene walking. The cagPAI sequence of strain PNGhigh85 was obtained by shotgun 454 sequencing of the whole genome (unpublished). Sequences were assembled with Gap4 (Staden Package, GCG Wisconsin). The individual cagPAI sequences have been submitted to the EMBL Nucleotide Sequence Database (accession numbers FR666825 - FR666857). Details for RNA preparation and RT-PCR are given in Text S1. RT-PCR primers and cycling conditions for transcript analyses of the cagPAIs are listed in Table S2.
Multiple sequence alignment, sequence analysis, annotation, and phylogenetic analyses
CDSs were annotated in ACT and in KODON (Applied Maths BVBA, Sint-Martens-Latem, Belgium), automatic multiple sequence alignment of individual cagPAI genes was performed in BIONUMERICS (Applied Maths BVBA, Sint-Martens-Latem, Belgium) and corrected manually after visual inspection, where necessary. Sequence comparison and graphical output of multiple complete cagPAI sequences was performed in KODON. We only included one of eleven cagPAI sequences (F32) available from Japanese strains [19] because information is lacking on the phylogeographic population assignment of the remaining 10 strains. Pairwise genetic distances, phylogenetic trees and FST were calculated in MEGA3 [56] and in Arlequin [57], respectively. Pairwise geographic distances and distance from North East Africa (Addis Ababa, Ethiopia), as well as confidence intervals were calculated as previously described [16]. For analyses of increasing diversity with geographic distance from East Africa, the dataset was stripped of recent migrants [16] which resulted in the use of 33 out of the 37 cagPAI sequences. Pseudogenes were excluded from the dataset in all phylogenetic analyses.
Evolutionary analyses
Ks/Ka ratios were determined in DnaSP4.0 [58] and SWAAP, including a sliding window analysis. The number and location of potential codons under selection (ω) in each cagPAI gene were determined using the program CODEML in PAML 3.15 [59], implementing a sliding windows graphic representation. This software calculates the ratio of maximum likelihood of different evolutionary algorithms (models) for each codon (site) of a coding sequence to be under positive selection (ω>1), followed by Naive Empirical Bayes (NEB) and Bayes Empirical Bayes (BEB) analyses of posterior probabilities. Sites with a posterior probability P>0.95 by the CODEML codon substitution models M3 (discrete) or M8 (beta and ω) of ω>1 were considered as being under positive or diversifying selection. The likelihood of codons under diversifying selection in the presence of recombination was further analyzed using OmegaMap (V 0.5; [32]). This software uses a Bayesian modeling algorithm to calculate the probability of codons to evolve under diversifying selection (ω>1) in the presence of recombination (ρ). By explicitly modeling recombination, this method has a low rate to detect false positives. The settings used in the program were: norders = 100, thinning = 100, rhoprior = inverse, omegaprior = inverse, block length = 3 and 100,000 or 250,000 iterations. 5,000 iterations were deduced after each calculation as the burn-in phase. The model type used for both ω and ρ was “variable”. Three repetitions of the calculations with different settings were initially performed for control genes of defined structural properties and where some information is available about their function (e.g. HP0546), to exclude high variations in the calculations due to inadequate settings. Pseudogenes were excluded from the dataset.
Housekeeping genes and population structure
Fragments of the housekeeping genes atpA, efp, mutY, ppa, trpC, ureI, and yphC were amplified and both strands were sequenced from independent PCR products as described [55]. Alternatively, comparable sequences were extracted from the published genomes (26695, HPAG1, J99). These sequences were assigned to populations and subpopulations by STRUCTURE [14].
Functional assays of the cagPAI t4ss
IL-8 induction assay using the human gastric epithelial carcinoma cell line AGS (isolated from adenocarcinoma from a Caucasian patient) was performed for all strains of the sequencing project. Strain 26695A [60] was used as a reference. Cells were cultured in RPMI 1640 medium (buffered with 25 mM HEPES, supplemented with 10% heat-inactivated fetal bovine serum (medium and serum: Biochrom, Berlin, Germany). Details for bacterial culture conditions are given in Text S1. Cell infection experiments for IL-8 secretion measurement were performed on subconfluent cell layers (70%–90% confluence) in 24-well tissue culture plates. Cells were washed three times and preincubated in fresh medium with serum for 30 min prior to infection. By the addition of exponentially growing bacteria that were resuspended in cell culture medium (RPMI 1640, 25 mM HEPES, 10% heat-inactivated serum), the infection was started (MOI of 50). To synchronize the infection, the incubation plates were centrifuged at 500 x g, 20°C, for 3 min. The coincubation was carried out for 20 h. Non-infected cells (mock coincubated) were used as negative control. Supernatants were harvested, cleared of cell debris by centifugation, immediately frozen and stored at −20°C until use. Release of IL-8 into the cell supernatants was quantified by using BD OptEIA IL-8 enzyme-linked immunosorbent assay kit (BD Pharmingen; San Diego, USA) according to the company's instructions, using appropriate dilutions. The assays were performed in triplicate and the means and standard deviations of at least six independent coincubations were calculated. Adherence of the strains was tested in a high throughput assay, but no correlation was found between adherence and the IL-8 induction (data not shown).
To study CagA translocation, AGS cells were cultured in six-well plates and infected with H. pylori at a multiplicity of infection (MOI) of 100. After 4 h of coincubaction, non-adherent bacteria were removed by washing twice with PBS-Dulbecco (pH 7.4; Biochrom, Berlin, Germany). Cells were harvested with a cell scraper and resuspended in 1 ml PBS (pH = 7.4; Biochrom, Berlin, Germany). After centrifugation (250 x g, 4°C, 5 min), cells were resuspended in 300 µl of modified RIPA buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 1 protease inhibitor tablet per 10 ml buffer (Complete, Roche, Mannheim, Germany), 1 mM PMSF). During lysis, cells were incubated on ice for 30 min. Lysates were cleared by centrifugation (10 min, 21,900 x g, 4°) and the pellets were carefully separated from the supernatants. The pellet fraction was resuspended in 100 µl RIPA buffer and the fractions were immediately frozen at −80°C. To determine the amount of protein, a BCA protein assay was performed using the BCA Protein Assay kit (Pierce, Rockford, IL, USA) according to the manufacturer's instructions.
Western blot analysis of CagA translocation
Equal amounts of cleared cell lysates (see above; corresponding to 10 µg of protein) of infected cells were resuspended in 5 x SDS loading buffer (0.31M Tris-HCl, pH6.8, 37.5% glycerol, 10% SDS, 0.05% bromophenol blue, 20% β-mercaptoethanol) and boiled for 10 min. For determination of molecular mass, BenchMark pre-stained Protein Ladder (Invitrogen, Karlsruhe, Germany) was used. Samples were separated on 10.4% denaturing SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Protran BA 85, Whatman, Dassel, Germany) by semi-dry blotting. Membranes were blocked with 5% non-fat dried milk in TBS-T (20 mM Tris-HCl, 13.7 mM NaCl, 0.1% Tween 20, pH 7.4) for 1 h and subsequently incubated with specific primary antibody. Anti-CagA-antibody (Rabbit anti-H. pylori Cag antigen IgG fraction [polyclonal], Austral Biologicals, San Ramon, USA) was used at a dilution of 1/1,000 for the detection of CagA protein. To detect phosphorylated CagA, PY99-antibody (Santa Cruz Biotechnology, Heidelberg, Germany) was used (dilution 1/250). Goat-anti-Rabbit-HRP antibody (dilution 1/10,000, Jackson Immunoresearch Laboratories, Suffolk, Great Britain) or Goat-anti-mouse-HRP-antibody (dilution 1/5,000, Dianova, Hamburg, Germany) were used as secondary antibodies. Signal detection was performed with Enhanced SuperSignal West chemiluminescence substrate (Pierce, Rockford, IL, USA), and detection was on X-ray film (Hyperfilm, Amersham Biosciences, Buckinghamshire, UK).
Supporting Information
Zdroje
1. SuerbaumS
MichettiP
2002 Helicobacter pylori infection. N Engl J Med 347 1175 1186
2. CensiniS
LangeC
XiangZ
CrabtreeJE
GhiaraP
1996 cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A 93 14648 14653
3. FischerW
PulsJ
BuhrdorfR
GebertB
OdenbreitS
2001 Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol 42 1337 1348
4. WiedemannT
LoellE
MuellerS
StoeckelhuberM
StolteM
2009 Helicobacter pylori cag-Pathogenicity island-dependent early immunological response triggers later precancerous gastric changes in Mongolian gerbils. PLoS ONE 4 e4754 doi:10.1371/journal.pone.0004754
5. FigueiredoC
MachadoJC
PharoahP
SerucaR
SousaS
2002 Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst 94 1680 1687
6. AmievaMR
El OmarEM
2008 Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 134 306 323
7. HatakeyamaM
2008 SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol 11 30 37
8. HatakeyamaM
2009 Helicobacter pylori and gastric carcinogenesis. J Gastroenterol 44 239 248
9. LuHS
SaitoY
UmedaM
Murata-KamiyaN
ZhangHM
2008 Structural and functional diversity in the PAR1b/MARK2-binding region of Helicobacter pylori CagA. Cancer Sci 99 2004 2011
10. PeekRMJr
CrabtreeJE
2006 Helicobacter infection and gastric neoplasia. J Pathol 208 233 248
11. OhnishiN
YuasaH
TanakaS
SawaH
MiuraM
2008 Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A 105 1003 1008
12. BjorkholmB
SjolundM
FalkPG
BergOG
EngstrandL
2001 Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori. Proc Natl Acad Sci U S A 98 14607 14612
13. SuerbaumS
Maynard SmithJ
BapumiaK
MorelliG
SmithNH
1998 Free recombination within Helicobacter pylori. Proc Natl Acad Sci U S A 95 12619 12624
14. FalushD
WirthT
LinzB
PritchardJK
StephensM
2003 Traces of human migrations in Helicobacter pylori populations. Science 299 1582 1585
15. MoodleyY
LinzB
YamaokaY
WindsorHM
BreurecS
2009 The peopling of the Pacific from a bacterial perspective. Science 323 527 530
16. LinzB
BallouxF
MoodleyY
HuaL
ManicaA
2007 An African origin for the intimate association between humans and Helicobacter pylori. Nature 445 915 918
17. GressmannH
LinzB
GhaiR
PleissnerK-P
SchlapbachR
2005 Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet 1 e43 doi:10.1371/journal.pgen.0010043
18. TombJ-F
WhiteO
KerlavageAR
ClaytonRA
SuttonGG
1997 The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388 539 547
19. AzumaT
YamakawaA
YamazakiS
OhtaniM
ItoY
2004 Distinct diversity of the cag pathogenicity island among Helicobacter pylori strains in Japan. J Clin Microbiol 42 2508 2517
20. BlomstergrenA
LundinA
NilssonC
EngstrandL
LundebergJ
2004 Comparative analysis of the complete cag pathogenicity island sequence in four Helicobacter pylori isolates. Gene 328 85 93
21. OhJD
Kling-BackhedH
GiannakisM
XuJ
FultonRS
2006 The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc Natl Acad Sci U S A 103 9999 10004
22. AlmRA
LingL-SL
MoirDT
KingBL
BrownED
1999 Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397 176 180
23. ShimoyamaT
CrabtreeJE
1997 Mucosal chemokines in Helicobacter pylori infection. J Physiol Pharmacol 48 315 323
24. KimuraM
1991 The neutral theory of molecular evolution: a review of recent evidence. Jpn J Genet 66 367 386
25. MULLERHJ
1964 THE RELATION OF RECOMBINATION TO MUTATIONAL ADVANCE. Mutat Res 106 2 9 : 2-9
26. KersulyteD
KaliaA
ZhangM
LeeHK
SubramaniamD
2004 Sequence organization and insertion specificity of the novel chimeric ISHp609 transposable element of Helicobacter pylori. J Bacteriol 186 7521 7528
27. KersulyteD
AkopyantsNS
CliftonSW
RoeBA
BergDE
1998 Novel sequence organization and insertion specificity of IS605 and IS606: chimaeric transposable elements of helicobacter pylori [In Process Citation]. Gene 223 175 186
28. AkopyantsNS
CliftonSW
KersulyteD
CrabtreeJE
YoureeBE
1998 Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol 28 37 53
29. OhtaT
2002 Near-neutrality in evolution of genes and gene regulation. Proc Natl Acad Sci U S A 99 16134 16137
30. SchwarzS
MorelliG
KusecekB
ManicaA
BallouxF
2008 Horizontal versus familial transmission of Helicobacter pylori. PLoS Pathog 4 e1000180 doi:10.1371/journal.ppat.1000180
31. YangZ
1997 PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13 555 556
32. WilsonDJ
McVeanG
2006 Estimating diversifying selection and functional constraint in the presence of recombination. Genetics 172 1411 1425
33. MuzziA
MoschioniM
CovacciA
RappuoliR
DonatiC
2008 Pilus operon evolution in Streptococcus pneumoniae is driven by positive selection and recombination. PLoS ONE 3 e3660 doi:10.1371/journal.pone.0003660
34. ZhongQ
ShaoS
MuR
WangH
HuangS
2010 Characterization of peptidoglycan hydrolase in Cag pathogenicity island of Helicobacter pylori. Mol Biol Rep
35. ArasRA
FischerW
Perez-PerezGI
CrosattiM
AndoT
2003 Plasticity of repetitive DNA sequences within a bacterial (Type IV) secretion system component. J Exp Med 198 1349 1360
36. RohdeM
PulsJ
BuhrdorfR
FischerW
HaasR
2003 A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol Microbiol 49 219 234
37. KwokT
ZablerD
UrmanS
RohdeM
HartigR
2007 Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 449 862 866
38. AndrzejewskaJ
LeeSK
OlbermannP
LotzingN
KatzowitschE
2006 Characterization of the pilin ortholog of the Helicobacter pylori type IV cag pathogenicity apparatus, a surface-associated protein expressed during infection. J Bacteriol 188 5865 5877
39. HatakeyamaM
2004 Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer 4 688 694
40. AhmedN
DobrindtU
HackerJ
HasnainSE
2008 Genomic fluidity and pathogenic bacteria: applications in diagnostics, epidemiology and intervention. Nat Rev Microbiol 6 387 394
41. EppingerM
BaarC
LinzB
RaddatzG
LanzC
2006 Who ate whom? Adaptive Helicobacter genomic changes that accompanied a host jump from early humans to large felines. PLoS Genet 2 e120 doi:10.1371/journal.pgen.0020120
42. Jimenez-SotoLF
KutterS
SewaldX
ErtlC
WeissE
2009 Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathog 5 e1000684 doi:10.1371/journal.ppat.1000684
43. SuerbaumS
JosenhansC
2007 Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol 5 441 452
44. NesicD
MillerMC
QuinkertZT
SteinM
ChaitBT
2010 Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nat Struct Mol Biol 17 130 132
45. Reyes-LeonA
AthertonJC
ArgentRH
PuenteJL
TorresJ
2007 Heterogeneity in the activity of Mexican Helicobacter pylori strains in gastric epithelial cells and its association with diversity in the cagA gene. Infect Immun 75 3445 3454
46. MatteoMJ
GranadosG
PerezCV
OlmosM
SanchezC
2007 Helicobacter pylori cag pathogenicity island genotype diversity within the gastric niche of a single host. J Med Microbiol 56 664 669
47. MediniD
CovacciA
DonatiC
2006 Protein homology network families reveal step-wise diversification of Type III and Type IV secretion systems. PLoS Comput Biol 2 e173 doi:10.1371/journal.pcbi.0020173
48. BackertS
FronzesR
WaksmanG
2008 VirB2 and VirB5 proteins: specialized adhesins in bacterial type-IV secretion systems? Trends Microbiol 16 409 413
49. Pinto-SantiniDM
SalamaNR
2009 Cag3 is a novel essential component of the Helicobacter pylori Cag type IV secretion system outer membrane subcomplex. J Bacteriol 191 7343 7352
50. KutterS
BuhrdorfR
HaasJ
Schneider-BrachertW
HaasR
2008 Protein subassemblies of the Helicobacter pylori Cag type IV secretion system revealed by localization and interaction studies. J Bacteriol 190 2161 2171
51. BourzacKM
SatkampLA
GuilleminK
2006 The Helicobacter pylori cag pathogenicity island protein CagN is a bacterial membrane-associated protein that is processed at its C terminus. Infect Immun 74 2537 2543
52. PattisI
WeissE
LaugksR
HaasR
FischerW
2007 The Helicobacter pylori CagF protein is a type IV secretion chaperone-like molecule that binds close to the C-terminal secretion signal of the CagA effector protein. Microbiology 153 2896 2909
53. BastollaU
PortoM
Eduardo RomanMH
VendruscoloMH
2003 Connectivity of neutral networks, overdispersion, and structural conservation in protein evolution. J Mol Evol 56 243 254
54. NoirelJ
SimonsonT
2008 Neutral evolution of proteins: The superfunnel in sequence space and its relation to mutational robustness. J Chem Phys 129 185104
55. AchtmanM
AzumaT
BergDE
ItoY
MorelliG
1999 Recombination and clonal groupings within Helicobacter pylori from different geographic regions. Mol Microbiol 32 459 470
56. KumarS
NeiM
DudleyJ
TamuraK
2008 MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9 299 306
57. ExcoffierL
LavalG
SchneiderS
2005 Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol Bioinform Online 1 47 50 : 47-50
58. RozasJ
RozasR
1999 DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15 174 175
59. YangZ
2007 PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24 1586 1591
60. JosenhansC
EatonKA
ThevenotT
SuerbaumS
2000 Switching of flagellar motility in Helicobacter pylori by reversible length variation of a short homopolymeric sequence repeat in fliP, a gene encoding a basal body protein. Infect Immun 68 4598 4603
61. CouturierMR
TascaE
MontecuccoC
SteinM
2006 Interaction with CagF is required for translocation of CagA into the host via the Helicobacter pylori type IV secretion system. Infect Immun 74 273 281
62. BuslerVJ
TorresVJ
McClainMS
TiradoO
FriedmanDB
2006 Protein-protein interactions among Helicobacter pylori cag proteins. J Bacteriol 188 4787 4800
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 8
-
Všechny články tohoto čísla
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Mutation in the Gene Encoding Ubiquitin Ligase LRSAM1 in Patients with Charcot-Marie-Tooth Disease
- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Did Genetic Drift Drive Increases in Genome Complexity?
- The 5p15.33 Locus Is Associated with Risk of Lung Adenocarcinoma in Never-Smoking Females in Asia
- An Alpha-Catulin Homologue Controls Neuromuscular Function through Localization of the Dystrophin Complex and BK Channels in
- Epigenetically-Inherited Centromere and Neocentromere DNA Replicates Earliest in S-Phase
- Survival and Growth of Yeast without Telomere Capping by Cdc13 in the Absence of Sgs1, Exo1, and Rad9
- Tuberous Sclerosis Complex 1 Regulates dE2F1 Expression during Development and Cooperates with RBF1 to Control Proliferation and Survival
- Disease-Associated Mutations That Alter the RNA Structural Ensemble
- The Transcriptomes of Two Heritable Cell Types Illuminate the Circuit Governing Their Differentiation
- Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in
- Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD
- Transcriptional Regulation by CHIP/LDB Complexes
- Conserved Role of in Ethanol Responses in Mutant Mice
- A Global Overview of the Genetic and Functional Diversity in the Pathogenicity Island
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3–Dependent Manner in
- Genetic Analysis of Baker's Yeast Msh4-Msh5 Reveals a Threshold Crossover Level for Meiotic Viability
- Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels
- Something New: An Interview with Radoje Drmanac
- The Extinction Dynamics of Bacterial Pseudogenes
- Microtubule Actin Crosslinking Factor 1 Regulates the Balbiani Body and Animal-Vegetal Polarity of the Zebrafish Oocyte
- Consistent Association of Type 2 Diabetes Risk Variants Found in Europeans in Diverse Racial and Ethnic Groups
- Transmission of Mitochondrial DNA Diseases and Ways to Prevent Them
- Telomere Disruption Results in Non-Random Formation of Dicentric Chromosomes Involving Acrocentric Human Chromosomes
- Chromosome Axis Defects Induce a Checkpoint-Mediated Delay and Interchromosomal Effect on Crossing Over during Drosophila Meiosis
- Dynamic Chromatin Organization during Foregut Development Mediated by the Organ Selector Gene PHA-4/FoxA
- Ancient Protostome Origin of Chemosensory Ionotropic Glutamate Receptors and the Evolution of Insect Taste and Olfaction
- A Wnt-Frz/Ror-Dsh Pathway Regulates Neurite Outgrowth in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Did Genetic Drift Drive Increases in Genome Complexity?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



