-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAn Integration of Genome-Wide Association Study and Gene Expression Profiling to Prioritize the Discovery of Novel Susceptibility Loci for Osteoporosis-Related Traits
Osteoporosis is a complex disorder and commonly leads to fractures in elderly persons. Genome-wide association studies (GWAS) have become an unbiased approach to identify variations in the genome that potentially affect health. However, the genetic variants identified so far only explain a small proportion of the heritability for complex traits. Due to the modest genetic effect size and inadequate power, true association signals may not be revealed based on a stringent genome-wide significance threshold. Here, we take advantage of SNP and transcript arrays and integrate GWAS and expression signature profiling relevant to the skeletal system in cellular and animal models to prioritize the discovery of novel candidate genes for osteoporosis-related traits, including bone mineral density (BMD) at the lumbar spine (LS) and femoral neck (FN), as well as geometric indices of the hip (femoral neck-shaft angle, NSA; femoral neck length, NL; and narrow-neck width, NW). A two-stage meta-analysis of GWAS from 7,633 Caucasian women and 3,657 men, revealed three novel loci associated with osteoporosis-related traits, including chromosome 1p13.2 (RAP1A, p = 3.6×10−8), 2q11.2 (TBC1D8), and 18q11.2 (OSBPL1A), and confirmed a previously reported region near TNFRSF11B/OPG gene. We also prioritized 16 suggestive genome-wide significant candidate genes based on their potential involvement in skeletal metabolism. Among them, 3 candidate genes were associated with BMD in women. Notably, 2 out of these 3 genes (GPR177, p = 2.6×10−13; SOX6, p = 6.4×10−10) associated with BMD in women have been successfully replicated in a large-scale meta-analysis of BMD, but none of the non-prioritized candidates (associated with BMD) did. Our results support the concept of our prioritization strategy. In the absence of direct biological support for identified genes, we highlighted the efficiency of subsequent functional characterization using publicly available expression profiling relevant to the skeletal system in cellular or whole animal models to prioritize candidate genes for further functional validation.
Published in the journal: . PLoS Genet 6(6): e32767. doi:10.1371/journal.pgen.1000977
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000977Summary
Osteoporosis is a complex disorder and commonly leads to fractures in elderly persons. Genome-wide association studies (GWAS) have become an unbiased approach to identify variations in the genome that potentially affect health. However, the genetic variants identified so far only explain a small proportion of the heritability for complex traits. Due to the modest genetic effect size and inadequate power, true association signals may not be revealed based on a stringent genome-wide significance threshold. Here, we take advantage of SNP and transcript arrays and integrate GWAS and expression signature profiling relevant to the skeletal system in cellular and animal models to prioritize the discovery of novel candidate genes for osteoporosis-related traits, including bone mineral density (BMD) at the lumbar spine (LS) and femoral neck (FN), as well as geometric indices of the hip (femoral neck-shaft angle, NSA; femoral neck length, NL; and narrow-neck width, NW). A two-stage meta-analysis of GWAS from 7,633 Caucasian women and 3,657 men, revealed three novel loci associated with osteoporosis-related traits, including chromosome 1p13.2 (RAP1A, p = 3.6×10−8), 2q11.2 (TBC1D8), and 18q11.2 (OSBPL1A), and confirmed a previously reported region near TNFRSF11B/OPG gene. We also prioritized 16 suggestive genome-wide significant candidate genes based on their potential involvement in skeletal metabolism. Among them, 3 candidate genes were associated with BMD in women. Notably, 2 out of these 3 genes (GPR177, p = 2.6×10−13; SOX6, p = 6.4×10−10) associated with BMD in women have been successfully replicated in a large-scale meta-analysis of BMD, but none of the non-prioritized candidates (associated with BMD) did. Our results support the concept of our prioritization strategy. In the absence of direct biological support for identified genes, we highlighted the efficiency of subsequent functional characterization using publicly available expression profiling relevant to the skeletal system in cellular or whole animal models to prioritize candidate genes for further functional validation.
Introduction
The feasibility of carrying out genome-wide association studies (GWAS) has led to the rapid progression of the field of complex-disease genetics over the past few years. Although the GWAS approach has been successful in identifying novel candidate genes leading to new discovery of pathways that are involved in the pathophysiology of diseases, the genetic variants identified so far only explain a small proportion of the heritability for complex traits [1]. Due to the modest genetic effect size and inadequate power to overcome the heterogeneity of genetic effects in meta-analysis, true association signals may not be revealed based on a stringent genome-wide significance threshold alone [2]. In addition, the majority of the GWAS have not provided much information beyond statistical signals to understand the genetic architecture for those usually novel genes that have not been studied for a particular trait/disease before. Thus, the necessity of incorporating additional information when studying the GWAS has become apparent. Expression profiling with gene signatures of cellular models have been used to characterize gene's involvement in bone metabolism and disease processes. One such approach is parathyroid hormone (PTH) stimulated osteoclastogenesis and osteoblast maturation for osteoblastogenesis [3]. PTH indirectly stimulated osteoclastogenesis via its receptors on osteoblasts, which then signal to osteoclast precursors to stimulate osteoclastogenesis. Impaired osteoblastic differentiation reduces bone formation and causes severe osteoporosis in animals [4]. The TNFRSF11B/OPG gene, a well-known candidate gene for osteoporosis, is involved in osteoclastogenesis through the regulation of PTH [5]. Compared to GWAS-identified candidate genes that do not show differential expression in these cellular models, genes like TNFRSF11B/OPG with differential expression are more likely to be involved in skeletal metabolism and thus more likely to be truly associated with osteoporosis. Given that the majority of the reported genome-wide significant SNPs are in the intergenic or noncoding regions [6], it is not clear which SNP/gene might be implicated as a causal SNP/gene. Since intergenic or noncoding SNPs do not appear to affect protein sequence, it is likely that these SNPs either are in linkage disequilibrium with the causal variants or located within the transcription regulation elements of nearby genes. The relative quantification of gene transcripts may act as intermediate phenotypes between genetic loci and the clinical phenotypes. Expression quantitative trait loci (eQTL) analysis in specific tissues is a valuable tool to identify potentially causal SNPs [7]–[10]. By integration of genetic variants, transcriptome, and phenotypic data, investigators have the potential to provide much-needed support to prioritize the candidate susceptibility genes identified from GWAS for further validation [11]–[13].
Previously, we conducted a pilot GWAS for osteoporosis-related phenotypes in a small subset of the Framingham study participants [14]. Osteoporosis is a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture. The heterogeneity of osteoporosis has both an environmental and genetic basis. Although bone mineral density (BMD) is frequently used in the diagnosis and prognosis of osteoporosis [15], a growing body of evidence indicates that femoral geometry also contributes importantly to hip fracture risk [16], [17]. Both BMD and hip geometry are strongly heritable, with heritability estimates between 50% and 85% [18]. In an attempt to identify genes that are involved in the regulation of bone health related phenotypes, genetic linkage analyses [19], [20], candidate gene association studies [21] and recent GWAS [22]–[27] have been used to implicate several loci and candidate genes, such as OPG/RANK/RANKL [22]–[24], [28], LRP5 [22], [23], [29], LRP4 [23], ESR1 [23], [30], VDR [31], and SP7 [24], [25]. However, the majority of genes that contribute to genetic susceptibility to osteoporosis remain to be elucidated.
Seeking to extend these initial observations, in the current study, we first performed a large-scale GWAS analysis for BMD and hip geometry in 2,038 women and 1,531 men from the Framingham Osteoporosis Study using 550,000 SNPs, and then replicated the top findings in 5,595 women and 2,126 men from two independent cohorts of Caucasian individuals. We then prioritized the genome-wide association findings by utilizing publicly available experiments relevant to the skeletal system in cellular or whole animal models, and provided supportive biological information for future functional validation of their involvement in bone metabolism. The expression experiments included (1) gene signatures of a mouse embryo expression atlas and mouse cellular models of osteoblastogenesis and PTH - stimulated osteoblasts; (2) eQTL analysis in human primary osteoblasts, lymphocytes and liver tissues; and (3) likelihood-based causality model selection (LCMS) by integrating genetic variants, gene expression profiling, and skeletal phenotypes in inbred mice to identify candidate genes causally related to bone phenotypes. An overview of the study design is provided in Figure 1.
Fig. 1. Study design. 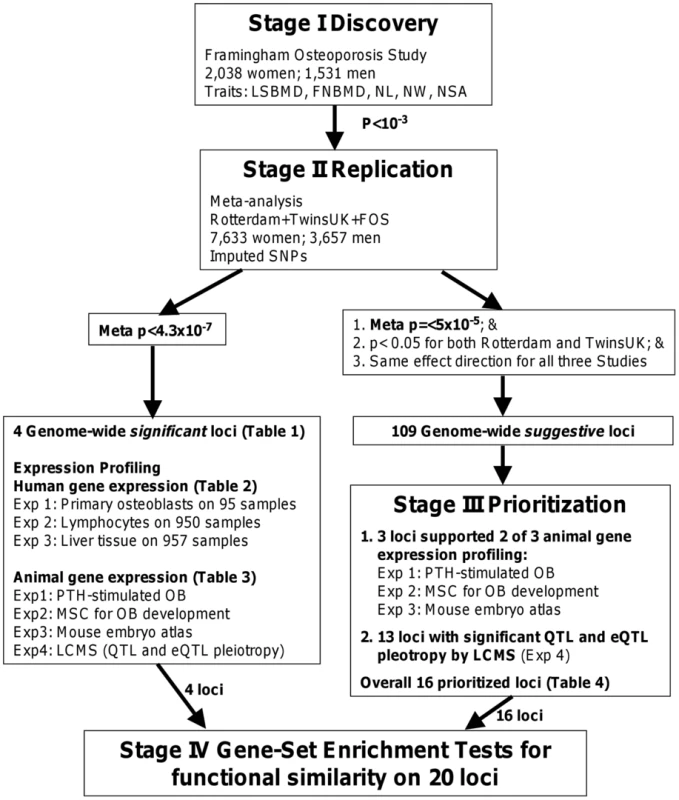
A four-stage approach was applied. We first performed genome-wide association analyses of the BMD and hip geometry traits in the Framingham Osteoporosis Study as a discovery stage (I) and replicated the top findings by meta-analysis (II), with a subsequent assessment of the functional relevance of the replicated findings (III and IV). Results
Stage I: GWAS in Framingham Osteoporosis Study
Significant differences of BMD and geometry indices were found between men and women in the Framingham Study with p-values <0.001 (Table S1). Quantile-quantile plots of observed p-values for single SNP association tests under additive genetic effect models are shown in Figure 2. Except for the tail (likely comprising true associations), the distributions of observed p-values did not deviate from the null distribution, which rules out systematic bias due to bad genotyping or population substructure in our study samples. The estimated genome control λGC for each phenotype ranged from 0.99 to 1.02. The regression coefficients analyzed with and without adjusting for the PCs are highly correlated (r = 0.95–0.98). Thus, we do not expect these principal components to influence our results substantially. SNPs associated with each phenotype at p-values <10−6 are listed in Table S2. For women, the most significant association was found with neck width (NW) for SNP rs16965654 (MAF = 0.01) located 13Kb away from the 5′ upstream region of the WD repeat and SOCS box-containing 1 (WSB1) gene on chromosome 17q11.1 (p = 4.15×10−8). For men, the most significant association was found with neck-shaft angle (NSA) for SNP rs11573709 (MAF = 0.23) located in intron 7 of the RAD23 homolog B (RAD23B) gene on 9q31.2 (p = 2.37×10−7). We also performed association tests by combining men and women together. The most significant association was found with NW for SNP rs16965654 (p = 6.89×10−10).
Fig. 2. Quantile-Quantile plots for BMD and HSA in additive genetic models. 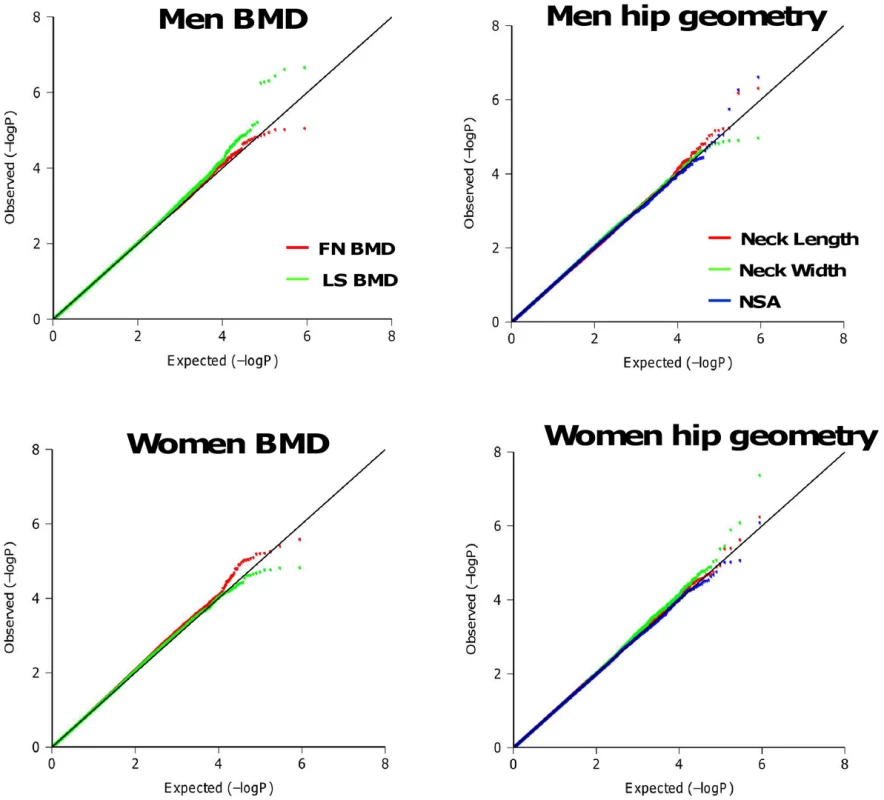
The distributions of observed p-values did not deviate from the null distribution, which rules out systematic bias due to bad genotyping or population substructure in our study samples. Stage II: Meta-Analysis
All genotyped SNPs (n = 431–593 for sex-specific phenotypes) with association test p-values <10−3 in Stage I were examined for replication in the Rotterdam Study (both men and women) and TwinsUK Study (women only). We performed meta-analyses by combining results from the Framingham Study and Rotterdam Study in men and all three cohorts in women. P-values <4.3×10−7 from meta-analyses are considered as genome-wide significant associations (See statistical methods section for details). We listed the most significant SNP on each chromosome locus with meta-analysis p-values <10−6 in Table 1. The most significant association for men was found with NSA for SNP rs2278729 located in the intron 4 of TBC1D8 on chromosome 2q11.2 (p = 1.48×10−7). SNP rs7227401 located in intron 4 of OSBPL1A (18q11.2) was found to be strongly associated with NW (p = 4.22×10−7) in men. The most significant association for women from meta-analysis was found with LS BMD for SNP rs2062375 located in the intergenic region of TNFRSF11B and COLEC10 genes on chromosome 8q24.12 (p = 2.68×10−11). SNP rs494453 located in the intron 2 of RAP1A on chromosome 1p13.2 was also strongly associated with NW (p = 2.80×10−7). The association became more significant for SNP rs494453 when combining women and men together (p = 3.6×10−8). None of the above associated SNPs are exonic coding SNPs. For SNPs listed in Table 1, no significant heterogeneity across studies was found and the p-values (as well as regression coefficients) were not changed with or without adjustment of body weight. The quality scores of imputed SNPs in Table 1 were >0.98 (IMPUTE confidence score) for the TwinsUK Study and >0.84 (MACH variance ratio) for the Rotterdam Study.
Tab. 1. The most significant SNP in each locus with joint-analysis p-value <10−6. 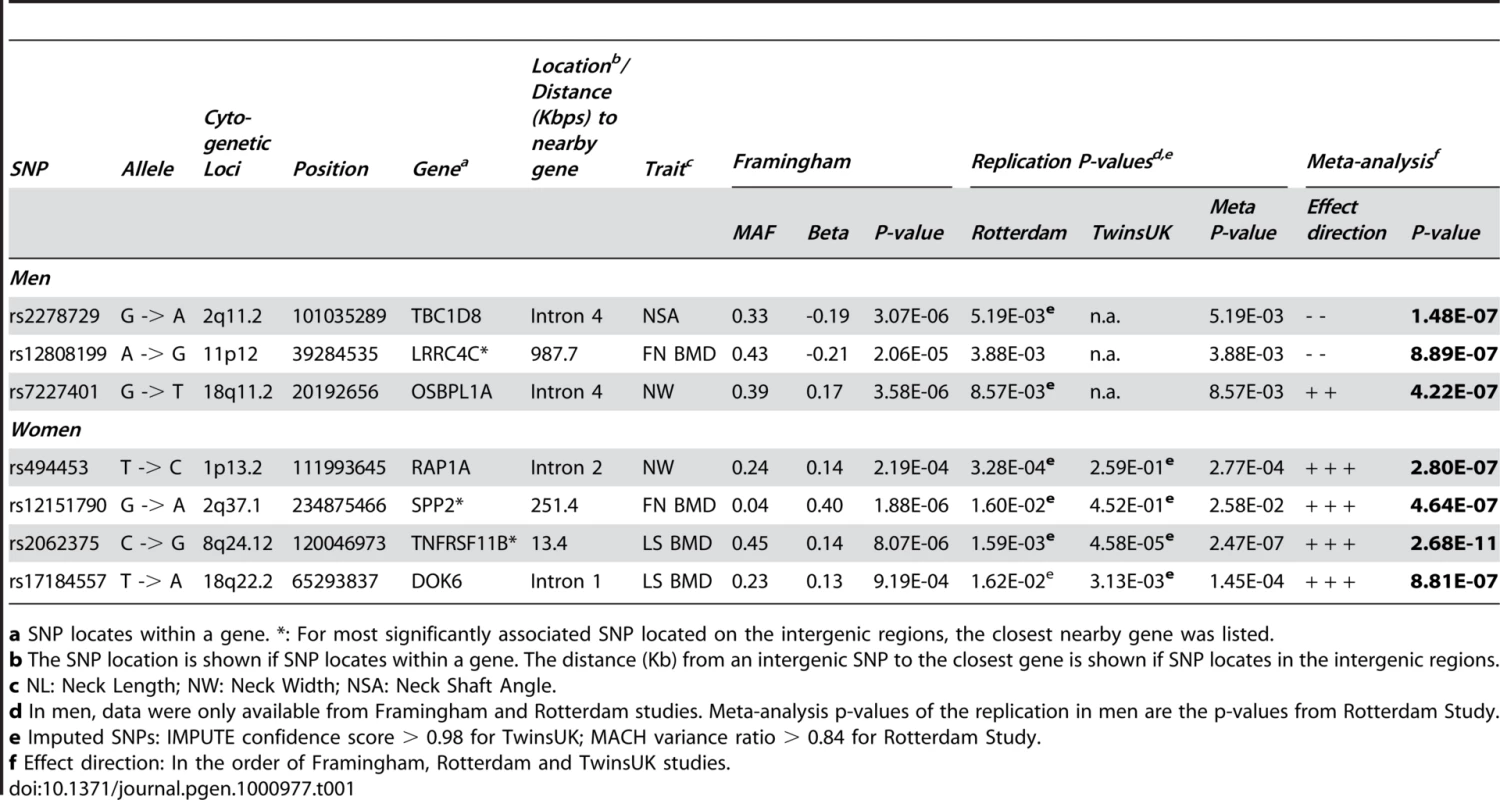
a SNP locates within a gene. eQTL in Multiple Human Tissues
Cis-eQTLs were analyzed for eight candidate genes located within 500 kb in four genome-wide significant loci (Table 2). All eight candidate genes were expressed in bone tissue estimated by either expressed sequence tag (EST) in the CGAP EST cDNA library (Figure S1) or human primary osteoblast samples (Table 2). However, since transcripts were not presented on expression arrays, expression of TBC1D8 was not available in human primary osteoblast samples. P-values <0.005 estimated by false discovery rate (FDR) were considered as significant. SNP rs494453 was found to be significantly associated with transcript levels of the RAP1A gene. Allele C of rs494453 is in LD with allele A of rs3767595 (haplotype). The haplotype CA was associated with lower expression of RAP1A, but higher NW (stronger bone structure) in women. We also performed eQTL analyses in human lymphocytes and liver tissue. Expression level of the RAP1A gene was not available for either lymphocytes or liver tissue. SNPs on chromosome 2q11.2 (TBC1D8 and RLP31) and 8q24.12 (TNFRSF11B) loci were associated with gene expressions in lymphocytes (Table 2). The most significant eSNP was found for SNP rs2278729 (chromosome 2q11.2) with TBC1D8 expression in lymphocytes (p = 2.58×10−10) and liver tissue (p<10−16, Figure S3). Allele A of rs2278729 was associated with smaller NSA in men and also with lower expression of TBC1D8 transcript. The same allele A was also associated with lower RPL31 expression in lymphocytes and was marginally significant in osteoblasts. Consistency between the direction of effect on transcript levels in lymphocytes and LS BMD was observed for TNFRSF11B at the chromosome 8q24.12 locus, which confirmed a previous report that increased TNFRSF11B expression levels have been shown to inhibit bone resorption [32]. A previous study also demonstrated that alleles associated with decreased BMD were associated with differential allelic expression of the TNFRSF11B in lymphocytes [22]. However, we did not observe associations of genome-wide significant SNPs in/near the TNFRSF11B gene region with TNFRSF11B expression levels in human primary osteoblasts, possible due to lack of power.
Tab. 2. Cis-expression quantitative trait locus analyses of genome-wide significant SNPs (p < 4.3 x 10-7) selected from Table 1 with transcript levels in human lymphocytes and primary osteoblasts. 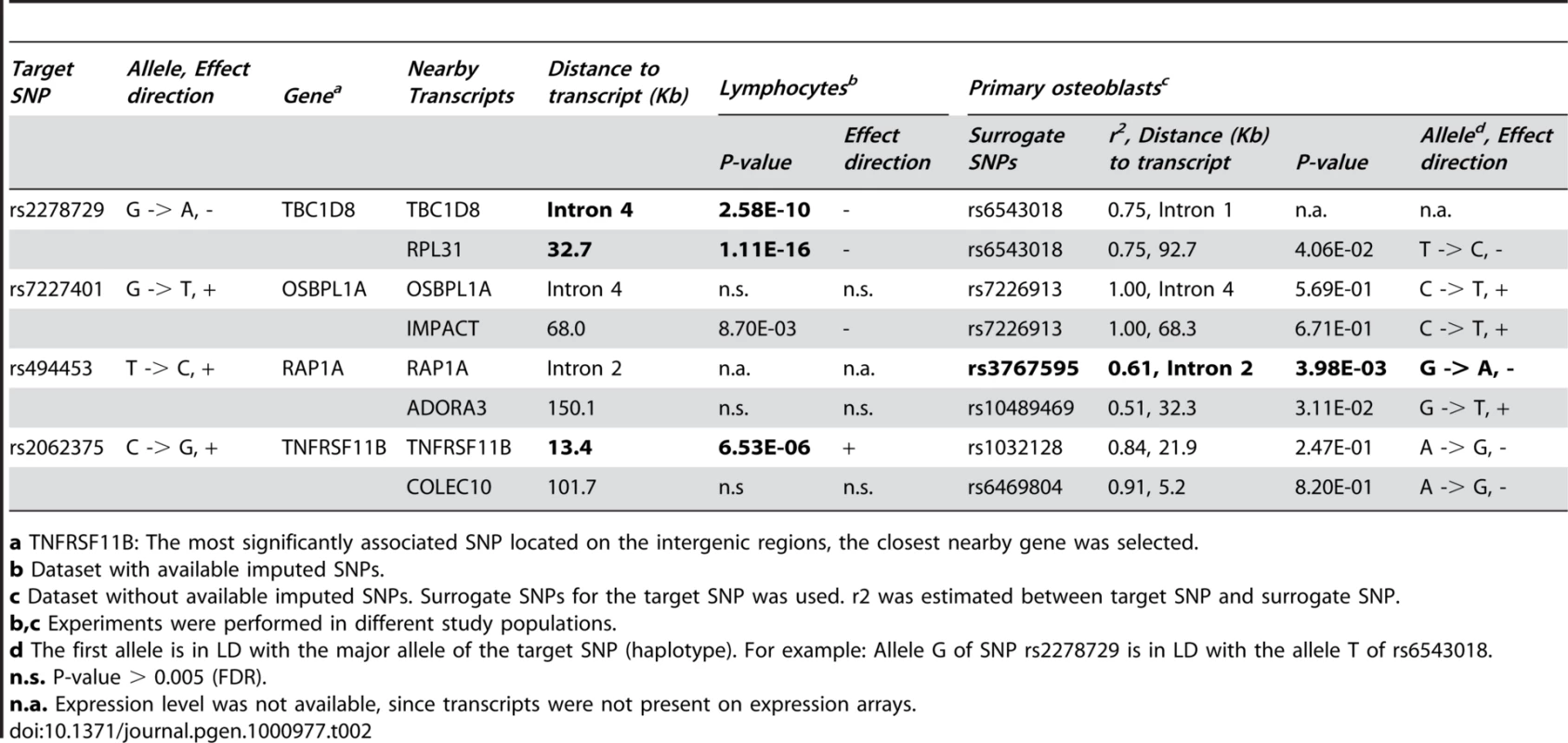
a TNFRSF11B: The most significantly associated SNP located on the intergenic regions, the closest nearby gene was selected. Mouse Expression Profiling Experiments
We investigated the candidate genes corresponding to the genome-wide significant SNPs in 4 chromosomal regions by looking at reported gene functions (including biological processes, canonical pathways and organism processes in human and mouse), microRNA targets and gene-related human diseases (Table S3). Except for the TNFRSF11B gene, there were few additional data regarding the potential biological significance of other candidate genes being involved in skeletal development and bone remodeling; therefore, we performed additional analyses on expression profiles in animal experiments (Table 3). In experiment 1, we found that PTH negatively regulated expressions of OSBPL1A and TNFRSF11B. RPL31, IMPACT and RAP1A genes were expressed in PTH stimulated osteoblasts, but not regulated by PTH. TBC1D8 were not expressed in PTH stimulated osteoblasts. In experiment 2, we analyzed the differential expression of candidates during osteoblast maturation. As a quality control measure, we looked at a number of known osteoblast markers, including runt-related transcription factor 2 (Runx2), collagen type 1, alpha 1 (Col1a1), collagen type 1, alpha 2 (Col1a2), osteocalcin, osteopontin and osteonectin. The expected expression patterns (differential expression during maturation) were observed in all cases. We observed that the expression of OSBPL1A, IMPACT and COLEC10 was significantly different across a time course (Day 4, 5, 6, 8, 16, 25 and 30 post-differentiation) of osteoblast development (p<0.0083). In the third experiment using the LCMS algorithm in the B6XC3H F2 intercross mice, we found that OSBPL1A, IMPACT, RAP1A and COLEC10 genes were predicted to be causally linked with bone phenotypes (detailed phenotypes listed in Table S4) based on the evidence of significantly pleiotropic effects on trait QTL and eQTL.
Tab. 3. Expression profiles for 4 genome-wide significant loci in mice osteoblast gene expression experiments and Likelihood-based Causality Model Selection (LCMS) regulatory network analysis in inbred mice. 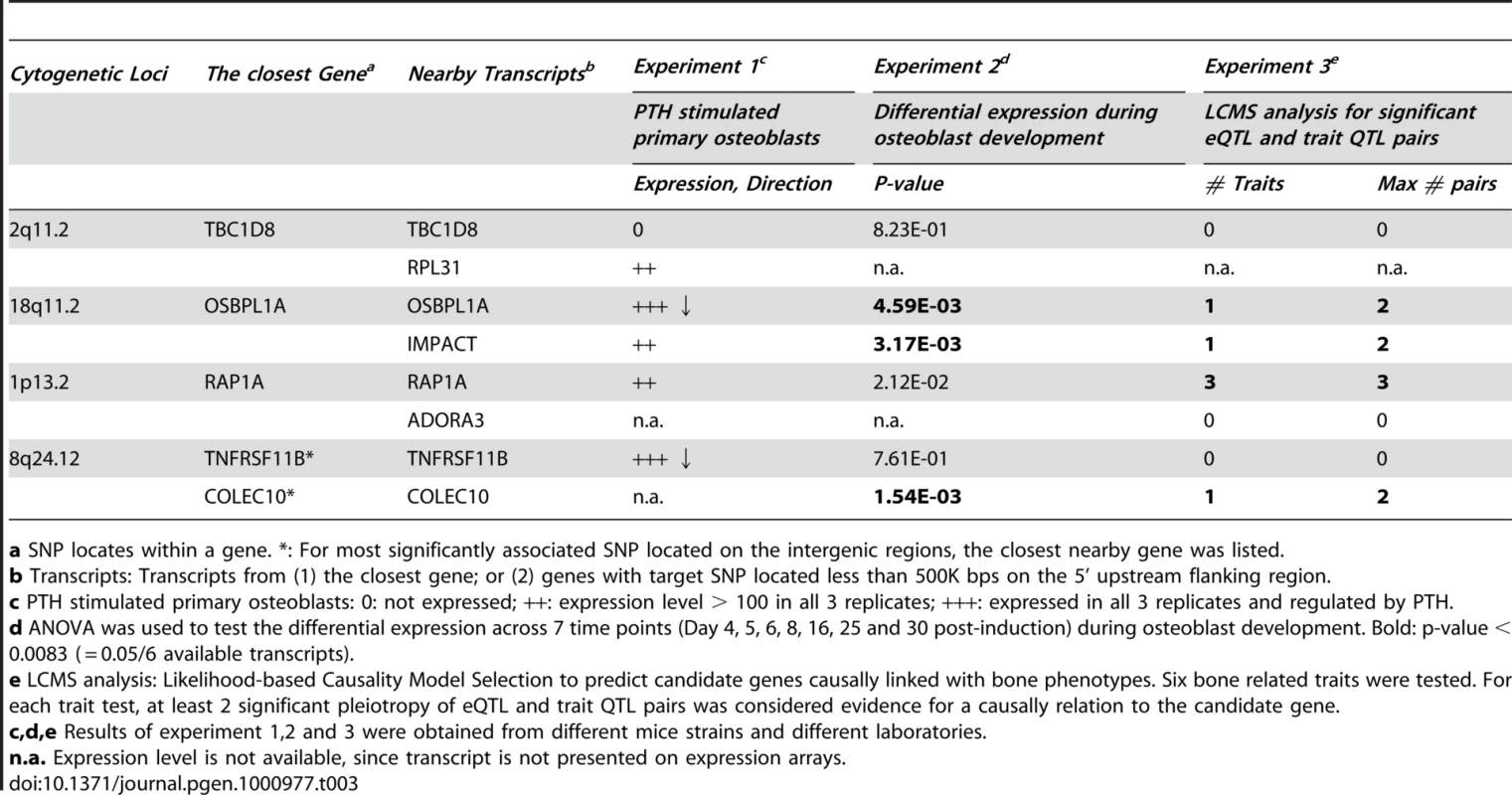
a SNP locates within a gene. Prioritization of the Genome-Wide Suggestive Candidate Genes
A total of 109 suggestive genome-wide associated regions/genes (most significant SNP with meta-analysis 4.3×10−7< p-value ≤5×10−5) were selected based on the criteria that p-values showed nominal association in the Framingham, Rotterdam and TwinsUK studies. Among them, 16 candidate genes were prioritized with results either involving the differential expression in osteoblasts or causally linked (LCMS algorithm) with bone phenotypes in mice (Table 4). Among 16 prioritized candidate genes/loci, PPAP2B, GPR177, TGFBI, DOCK1, SOX6 and PDGFD gene expressions were regulated by PTH in osteoblasts. Significant differential expression during osteoblast development was found for GPR177, TGFBI, SOX6 and CDH2 genes. IRX2, TGFBI and CDH2 genes showed strong expression in the skeleton compared to 24 other subsets of organ/tissue systems of the mouse embryo. Using the LCMS algorithm in inbred mice, 12 genes were predicted to be causally linked with bone phenotypes (detailed phenotypes listed in Table S4). All of the prioritized candidate genes are expressed in bone tissues. 10 genes were found to be expressed in human bone tissue from the CGAP EST cDNA library (Figure S1) and the remained genes (HECW2, CASR, MMRN1, IRX2, SOX6 and SALL1) were found to be expressed in human primary osteoblasts.
Tab. 4. Prioritized suggestive genome-wide loci based on mice experiments of Likelihood-based Causality Model Selection (LCMS) regulatory network analyses, gene expression signature profiles in osteoblasts, and transcriptome atlas of mouse embryos. 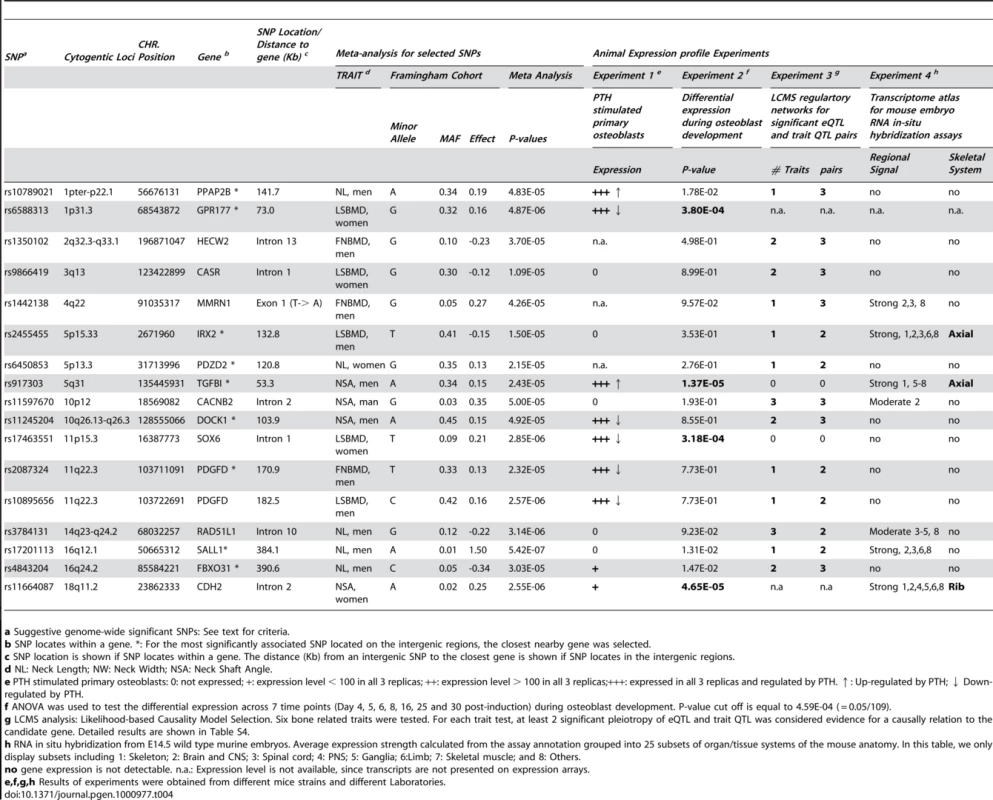
a Suggestive genome-wide significant SNPs: See text for criteria. Gene Set Enrichment Test
To test the probability of our candidate genes clustering into a particular biological pathway, we performed a gene set enrichment test on 24 candidate genes (20 loci) from Table 2 and Table 4. Due to lack of biological or functional annotation, IRX2 and FBXO31 genes were excluded from analyses. We found a significant clustering (Fisher exact test p = 1.65×10−4; Benjamini-Hochberg multiple testing corrected p-value = 0.03) of genes involved in adhesion of cells, including CASR, CDH2, PPAP2B, RAP1A, TGFBI and TNFRSF11B genes. We also estimated expression abundance by number of expressed sequence tag (EST) sequences per 200,000 tags in the CGAP EST cDNA library for these 24 candidate genes. Among 48 human tissues and organs, candidate genes were expressed in bone (17 candidate genes), liver (22 candidate genes), muscle (18) and adipose tissue (12) (Figure S1 and Figure S2). Expression levels of RAP1A (p = 2.51×10−4), RPL31 (p = 3.03×10−7) and TNFRSF11B (p = 1.69×10−3) genes showed over-representation in bone (Figure S1).
Discussion
In this study we performed sex-specific genome-wide association studies for BMD at the LS and FN skeletal sites as well as geometric indices of the hip in adults from the Framingham Osteoporosis Study and then replicated the top finding in two independent studies. As a result of meta-analyses on 7,633 women and 3,657 men, we discovered three novel genome-wide significant loci, including chromosome 1p13.2 RAP1A locus (p = 3.62×10−8; NW in men and women combined), 2q11.2 TBC1D8 locus (p = 1.48×10−7, NSA in men) and 18q11.2 OSBPL1A locus (p = 4.22×10−7, NW in men). We also confirmed TNFRSF11B gene on chromosome 8q24.12 to be associated with LS BMD in women only (p = 2.68×10−11).
The RAP1A gene (chromosome 1p13.2) was predicted to be causally linked with bone phenotypes in B6xC3H F2 intercross mice. Compared to other tissues, expression levels of RAP1A showed over-representation in human bone tissue. An eSNP (rs494453) located in intron 2 of RAP1A gene was also found to be significantly associated with RAP1A gene expression in human primary osteoblasts. A marginally significant differential expression during osteoblast maturation was also found in our study. RAP1A, a GTPase that mediates calcium signal transduction, has been found to mediate activities of JnK [33]. JnK has been reported to be involved in late stage osteoblast differentiation [34] and apoptosis of osteoblasts [35]. Therefore, variants in the RAP1A gene may change the activities of JnK and then impact osteoblast maturation. Further experiments are necessary to explore the role of the RAP1A gene. Both OSBPL1A and IMPACT genes located in chromosome 18q11.2 region were predicted to be causally linked with bone phenotypes in mice. Expressions of both genes were found to be significantly differential during osteoblast maturation. However, only expression of the OSBPL1A gene in osteoblasts was regulated by PTH. No significant eQTL was found in this region. Given the genome-wide significant SNPs were located in the OSBPL1A gene, we still cannot rule out the involvment of the nearby IMPACT gene. In addition, an in vitro study has shown that DDIT3 over-expression enhances osteoblastic differentiation in ST-2 stromal cells, a mechanism that may involve the formation of heterodimers with C/EBP-β and the sensitization of the BMP/Smad signaling pathway [36]. IMPACT protein has found to decrease expression of mouse DDIT3 protein [37]; therefore, IMPACT may negatively regulate bone formation.
We estimated the statistical power of our meta-analysis at an α-level of 10−7. In women, the power was 62–99% and >80% for effect size (h2) equal to 1% and 2%, respectively. In men, the statistical power was 35–75% and >70% for effect size equal to 1% and 2%, respectively. Inadequate statistical power seems to be one of the limitations in our study. Therefore, we prioritized 16 candidate genes/loci out of 109 suggestive genome-wide suggestive candidate genes (4.3×10−7<p≤5×10−5) based on the expression profiling and the LCMS modeling relevant to the skeletal system. Among 16 prioritized candidate genes/loci, PPAP2B, GPR177, SOX6 and CDH2 genes have been reported to be involved in Wnt-signaling. CASR, TGFBI and CACNB2 genes are involved in ossification, endochondrial bone formation in cartilage and calcium ion transportation, respectively (Table S5). CASR knockout mice have demonstrated decreased bone density and abnormal bone mineralization [38]. Variants in GPR177, SOX6 and CASR genes were associated with LSBMD in women. Variants in GPR177 and SOX6 (2 out of 3 above genes) have been successfully replicated in a large-scale meta-analysis of BMD on 19,195 Caucasian subjects (majority of whom were women) with association p-values <10−9 [27], but none of the non-prioritized candidates (associated with BMD) did. These results support the concept of our prioritization strategy. Candidate gene/SNP prioritization strategies by gene expression and bioinformatic databases leverage the complexity of the disease phenotypes, which offers some advantages over traditional association studies that rely on strictly p-value driven approaches. A recent study demonstrated that using functional information in published references to identify the key biological relationships between genes was able to predict the success of validation in replication genotyping [39], which also provides additional evidence for the soundness of using biological functional relevance to prioritize candidate genes from GWAS for future validation.
We exploited eSNP/eQTL in multiple human tissues. Given that (1) disease-related human tissues are often difficult to obtain for research purposes; (2) eQTL analysis requires a large sample size to reach the statistical power necessary to observe subtle changes in gene expression [40]; and (3) all of the selected candidate genes were expressed in bone tissues, we believe that performing eQTL in multiple tissues, although not replacing eQTL analysis in bone tissue, does provide complementary information. Genetic control of biological functions may be tissue-specific. Analysis of cis - eQTL in the tissue type directly relevant to the phenotype has been generally shown to be more informative than the same analysis in unrelated tissue types (such as blood). However, studies have found that cis-eQTLs are conserved across tissues, when genes are actively expressed in those tissues [10], [12], [13], [41]–[44]. eQTL analyses in liver, adipose, brain and muscle tissues from the same individual mice suggested that, for a gene exhibiting significant cis-eQTL associations in one tissue, 63–88% (dependent on tissue types) of them also exhibit cis-eQTL associations in another tissue [42]. Two recent studies, quantifying allele-specific gene expression in four human cell lines (lymphoblastoid cell, two primary fibroblasts and primary keratinocytes) from the same individuals, observed that only 2.3–10% of the mRNA-associated SNPs showed tissue-specific cis-expression across these cell lines [43], [44]. They also found that the variation of allelic ratios in gene expression among different cell lines was primarily explained by genetic variations, much more so than by specific tissue types or growth conditions [43]. Among the highly heritable transcripts (within the upper 25th percentile for heritability), 70% of expression transcripts that had a significant cis-eQTL in adipose tissue also had a significant cis-eQTL in blood cells [45]. Comparing eQTL in human primary fibroblasts, Epstein-Barr virus-immortalized B cells and T cells revealed that cell-type-shared eQTL tend to have larger effects, higher significance and to cluster tightly around the transcription start site [46]. As for bone tissue, comparing gene expression in 58 human primary osteoblast samples and 57 lymphoblastoid cell samples, despite tissues obtained from different individuals, indicated that overall, there is a large overlap in genes expressed in these two cell types, as well as the associated functional pathways [47]. 60% of the top 100 eSNP in human lymphoblastoid cells also showed associations in human primary osteoblasts, which indicated that both tissue-independent and dependent eSNP were observed in primary osteoblasts and lymphoblastoid cells [47]. Taken together this evidence suggests that if genes are expressed across tissues, their allele-specific expression can be preserved and highly correlated across tissues. Thus, the expression of a gene in liver or other non-bone tissues may not directly cause a change in bone; however, it is possible that its allele-specific expression in liver is highly correlated with allele-specific expression in bone. Because of these correlations it is possible that a gene's expression in adipose or liver can serve as surrogate markers to study the eQTL; however, the real causal relationship would be occurring in bone.
It is important to note that a lack of evidence from mining publicly available gene expression experiments does not necessarily exclude a gene's involvment in skeletal metabolism, given that (1) experimental models such as osteoblastogenesis or early skeletal development, do not represent all relevant processes related to osteoporosis; (2) variation in a gene leading to disease may affect protein function but not expression; and (3) absence of association between a transcript and disease-associated SNP may be due to limited statistical power or under different environmental conditions. An inherent limitation in most of the cell line in-vitro gene expression profiling experiments, such as our PTH treated osteoblasts or Epstein-Barr virus-immortalized lymphoblastoid cell lines used to perform eQTL analysis in most of the GWAS, is that the expression profiling of the cultured cells may be varying from actual expression within in-vivo cells [10]. An additonal challenge of using available experimental data is that most of the studies performed gene profing using commercialized “genome-wide” chips, which usually have a fixed number of genes and often do not include all set of genes of given interest. Therefore, prioritization of candidate genes will be biased towards well-studied genes.
Few published GWAS have addressed the potential sex-difference in genetic risks of diseases. BMD and hip geometry for men and women are known to differ, as does the prevalence of osteoporotic fractures [48]. Gender differences in the heritability of osteoporosis-related phenotypes have been reported (reviewed in [49]). In the current study, few overlapping associated SNPs between men and women were found, which may be expected based on epidemiological and clinical data and may also be due to lack of power. Sex-specific associations may be due to lifestyle and environmental variation between men and women. However, it also indicates that common genetic effects for both genders may be relatively rare and therefore, larger sample sizes of men and women is needed to detect their existence. Another limitation is that we are unable to distinguish the gender-specific differential expressions, since gene expression is measured in a pooled mixture of osteoblasts from males and females, although, differentiated expression between sexes is actually less likely to occur in-vitro.
In summary, our study identified three novel genome-wide significant loci and prioritized 16 genome-wide suggestive candidate genes for BMD and hip geometry traits. Beyond generating a list of top associated SNPs by statistical signals, we highlighted the efficiency of our approach to reasonably prioritize association findings by utilizing publicly available expression profiling relevant to the skeletal system in cellular or whole animal models; and to provide supportive biological information for future functional validation of their involvements in bone metabolism. Resequencing of these loci is needed to determine the causal variants and genes, along with experimental functional studies to establish their precise mechanism linked to bone health related phenotypes.
Materials and Methods
A four-stage approach was applied (Figure 1). We first performed genome-wide association analyses of the BMD and hip geometry traits in the Framingham Osteoporosis Study (discovery stage I) and replicated SNPs with association test p<10−3 using meta-analysis by combining results from the Rotterdam Study, TwinsUK Study and Framingham Study (Stage II), with a subsequent assessment of the functional relevance of the replicated findings (Stage III and IV). All study subjects were of self-reported Caucasian origin.
Discovery Stage (I)
Framingham Osteoporosis Study
The Framingham Osteoporosis Study (FOS) is an ancillary study of the Framingham Heart Study (FHS) [50]. The current study involved participants from the FHS Original Cohort [51] and Offspring Cohort [52]. The Original Cohort participants underwent bone densitometry by DXA with a Lunar DPX-L (Lunar Corp., Madison, WI, USA) during their examination 22 (1992–1993) and examination 24 (1996–1997). The Offspring Cohort was scanned with the same machine at or between their examination cycle 6 or 7 (between 1996 and 2001). Participants in current study were a subset from the Original and Offspring cohorts who provided blood samples for DNA and had DXA scans of the hip and spine. Other than being selected on the basis of having bone phenotypes and DNA, the participants were not selected on any trait. In total, 2,038 females and 1,531 males had both available genotyping and bone phenotypes (Table S1). Informed consent was obtained from participants before entry into the study. This study was approved by the Institutional Review Boards for Human Subjects Research at Boston University and the Hebrew Rehabilitation Center.
Quantitative bone phenotypes and covariates
Femoral neck (FN) and L2–L4 lumbar spine (LS) BMD (g/cm2) was measured by DXA with a Lunar DPX-L for all FOS participants. The coefficients of variation (CV) in normal subjects for the DPX-L have been previously reported to be 0.9% for the LS and 1.7% for the FN BMD [50]. A hip structure analysis computer program (HSA) [53] was used to derive a number of hip geometry variables from the femoral DXA scans. The regions assessed were the narrowest width of the femoral neck (NN), which overlaps or is proximal to the standard Lunar femoral neck region. Although the program derived a number of structural variables, in the current study we only performed analyses for femoral neck length (NL, cm), neck-shaft angle (NSA), as well as subperiosteal diameter (neck width, NW, cm), which are direct measurements independent of the DXA machines (Figure 3). The maximum coefficients of variation were previously reported to be 4.2%, 1.8% and 2.6%, respectively for NL, NSA and NW [54]. Covariates potentially influencing BMD and hip geometry were obtained at the time of DXA measurements along with an overall medical history. Details of these measurements have been reported previously [50]. These variables included age, sex, height, weight, and estrogen use/menopausal status (for women). Each woman was assigned to one of two estrogenic status groups: 1) premenopausal or postmenopausal on estrogen replacement therapy (estrogen-replete) or 2) postmenopausal not on estrogen (estrogen-deplete) where menopause was defined as having no menstrual period for at least one year.
Fig. 3. Hip geometry indices. 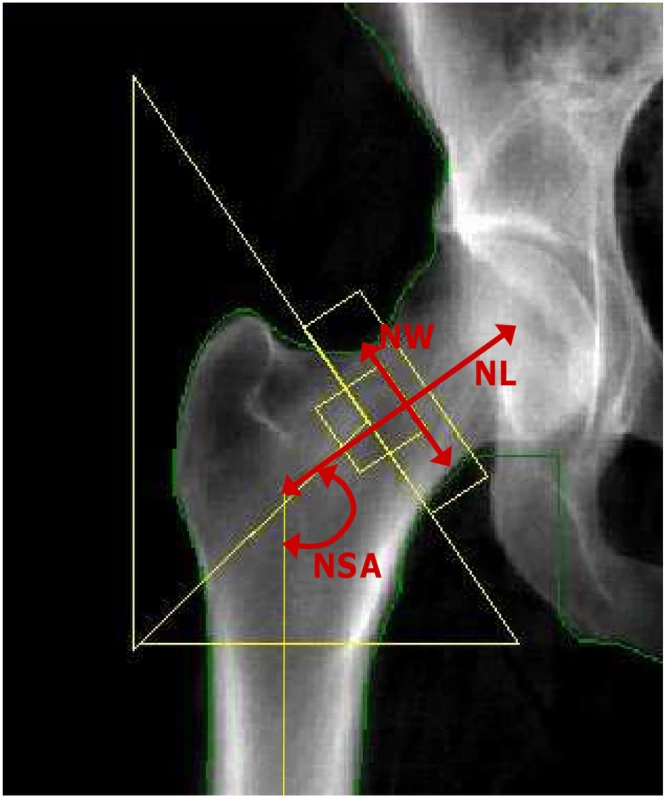
Red arrows indicate three hip geometry indices in a typical DXA image of the right hip. NL: Femoral neck length (cm); NW: Narrow neck width (cm); and NSA: Neck-shaft angle. Genotyping and exclusion of SNPs
Genotyping was conducted by the FHS SHARe (SNP Health Association Resource) project, for which 549,827 SNPs (Affymetrix 500K mapping array plus Affymetrix 50K gene center array) were genotyped in over 9,274 FHS subjects from over 900 families [55]. By estimation, we expected 80% genomic coverage (pair-wise genotype correlation r2>0.8) of the HapMap Phase I+II common SNPs (minor allele frequency, MAF ≥0.05) for the Caucasian population [56]. We excluded 793 individuals with an average SNP call rate <0.97. We also excluded SNPs with call rate <0.95 (34,868 SNPs; 6.3%); Hardy-Weinberg equilibrium (HWE) test p-value <10−6 (8,531 SNPs; 1.6%); MAF <0.01 (66,829 SNPs; 12.2%); or unknown genomic annotation (6,089 SNPs; 1.1%). Ultimately, 433,510 SNPs were used in the genome-wide analyses.
Population substructure
Principal components analysis (PCA) was used to estimate population substructure in Framingham Study. We first applied PCA by EIGENSTRAT [57] to all available genotypic data to infer continuous axes of genetic variation (principal components, PCs) describing ancestral heterogeneity (top eigenvectors of a covariance matrix). Since the Framingham Study is family-based, the top 10 PCs were first built using a subset of 200 biologically unrelated subjects and projected to all study samples. The first two PCs showed gradients similar to those previously reported in individuals of European ancestry, such as northwest, southeast European and Ashkenazi Jewish [58]. Next, we assessed the association between top 10 PCs and each of the 5 phenotypes using regression models to examine if PCs were significantly associated with each phenotype with adjustment of age, sex, cohort, height and BMI. The top 4 PCs, PC1 to PC4 were all associated with FNBMD, LSBMD and NL at nominal p-values less than 0.05. However, the top 4 PCs together only accounted for 0.1∼0.7% of the variation in phenotypes. PCs were not significantly associated with NW and NSA. To account for potential population substructure in the SNP-phenotype association tests in Framingham Study, we adjusted PC1 to PC4 along with other covariates in the mixed effect regression models.
Statistical analysis
Sex - and cohort (Original and Offspring)-specific standardized residuals (mean = 0; SD = 1) of phenotypes were calculated using multivariate regression. For BMD phenotypes, the covariates adjusted in the regression models included PC1–PC4, age, age2 and estrogenic status (in women only). For hip geometry, the covariates included height, BMI, PC1–PC4, age, age2 and estrogenic status (in women only). Age2 was considered in the models to account for potential non-linear age effects. These residuals were used in association analyses described below. We performed both sex-specific and combined-sexes GWAS using linear mixed effects regression models (LME), with fixed SNP genotype effects, and random individual effects that correlate within pedigree according to kinship relationship [59]. The R package KINSHIP was used in the analyses. Although LME accounts for the within family correlation, like any population-based test for association, LME is sensitive to population admixture; therefore, PC adjusted residuals were used. Single SNP association tests were performed, using an additive genetic effect model that estimated the effect of one copy increment of the minor allele. To estimate how well the distribution was calibrated, for each phenotype, we estimated the genomic inflation factor (λGC) based on the median chi-squared test of all study participants [60].
Replication Stage (II)
Joint analysis for results from both discovery and replication stages almost always results in greater power than analyzing discovery and replication stages separately [61]. We selected SNPs with association test p-values less than 10−3 from Stage I discovery GWAS, and replicated them using meta-analysis by combining results from the Framingham Study and two independent population-based cohorts including the Rotterdam Study and the TwinsUK Study. Since both the Rotterdam and TwinsUK studies performed whole-genome genotyping using different platforms (Illumina platforms), SNP imputation was performed. Fixed effect meta-analyses were then used to estimate combined p-values.
Rotterdam Study
The Rotterdam Study is a prospective population-based cohort study of chronic disabling conditions in Dutch elderly individuals aged 55 years and over [62]. Microarray genotyping was performed in the whole original Rotterdam Study cohort using the Infinium II HumanHap550K Genotyping BeadChip version 3 (Illumina). The detail of genotyping procedures and quality control was reported elsewhere [27]. For population substructure, 102 individuals (>3 standard deviations) and 129 individuals (>97% probabilities) deviating from population mean of the IBS clustering [63] were excluded from association analysis. MACH [64], [65] was used to impute all autosomal SNPs from the HapMap I+II project. To account for the uncertainty of imputation, instead of using the “best guess” genotype for each individual, the additive dosage of the allele from 0 to 2, which is a weighted sum of the genotypes multiplied by their estimated probability, was used to perform association tests (MACH2QTL package). The ratio of the empirically observed dosage variance (from the imputed genotypes) to the expected (under binomial distribution) dosage variance (computed from the estimated minor allele frequency) was estimated for every SNP as a quality score for imputation. SNPs with the variance ratio <0.3 were excluded.
Age, gender and the distributions of phenotypes are shown in Table S1. Hip structural analysis measurements were done as described previously [66]. Sex-specific standardized residuals of phenotypes were calculated using general linear regression models adjusted for age, age2, height (for hip geometry only), and BMI (for hip geometry only). A linear regression model with additive genetic effect was used to estimate p-values for single SNP GWAS. The λGC for each trait ranged from 0.98 to 1.06, suggesting that there was no major residual confounding by population stratification, systematic genotyping error, or little evidence of cryptic relatedness between individuals.
TwinsUK study
The TwinsUK cohort consists of approximately 10,000 monozygotic (MZ) and dizygotic (DZ) adult Caucasian twins aged 16 to 85 years recruited from the general population all over the United Kingdom [67]. This study was approved by the Research Ethics Committee of St. Thomas' Hospital, and written informed consent was obtained from each participant. BMD measurements (g/cm2) of the lumbar spine (L1–L4) and femoral neck were performed by DXA using a Hologic QDR 2000W densitometer (Hologic, Bedford, MA, USA). HSA software developed by Beck et al. [53] was used to measure hip geometry from the DXA scans as described in Framingham Study. The genotyping methods and quality control have been described previously [22]. In brief, 2,820 participants were genotyped by the Hap300Duo, Hap300 or Hap550 SNP Infinium assay (Illumina, San Diego, CA, USA). For potential population substructures, the STRUCTURE program was used to assess participants' ancestry genetic background [68]. After excluding 14 outliers (individuals) that lay outside the CEPH cluster from STRUCTURE analysis, the λGC for the distribution of test statistic of BMD and hip geometry ranged from 0.99 to 1.02, suggesting that there was no residual confounding by population stratification, nor any apparent systematic genotyping error, and little evidence of cryptic relatedness. IMPUTE [69] was used to impute all autosomal SNPs in the HapMap I+II project based on Map (release 22, build 26, CEU population) reference panel. The “best-guess” imputed genotypes were used in analyses. For each SNP, a confidence score was calculated as the average of the maximum posterior probabilities of the imputed genotypes. Individual genotypes with confidence score less than 0.9 were excluded.
2,734 women with both BMD and genotypes were in the final analyses (Table S1); however the sample size was smaller for HSA as these measurements have not been completed in all cohort members. Standardized residuals of phenotypes were calculated using general linear regression models adjusted for age, age2, height (for hip geometry only), and BMI (for hip geometry only). A score test implemented in MERLIN [70] was used to estimate p-values for single SNP analyses. An additive genetic effect model was tested.
Joint analysis using fixed effect meta-analysis model
We combined results from Framingham, Rotterdam and TwinsUK studies using inverse-variance fixed effect meta-analysis approaches to estimate combined p-values. The METAL program (http://www.sph.umich.edu/csg/abecasis/Metal/) was used. All association results were expressed relative to the forward strand of the reference genome based on HapMap (dbSNP126). The Cochran's Q heterogeneity test across studies was also estimated. Cochran's Q p-values less than 0.05 indicate large heterogeneity beyond chance. However, since only 2 or 3 cohorts were meta-analyzed, there was insufficient number of studies for the Q -statistics to be accurate calculated.
Multiple-testing
Recent GWAS have used different genome-wide significant thresholds in between 5×10−7 and 5×10−8 [71]–[76]. Several GWAS on multiple correlated traits estimated the genome-wide significant thresholds by FDR [73]–[76]. We performed gender-specific GWAS on 5 correlated traits (LS BMD, FN BMD, NSA, NL and NW). Since the pair-wise genetic correlation is 0.7 between LSBMD and FNBMD, Bonferroni correction for multiple testing is considered too conservative for correlated association tests. Therefore, we estimated genome-wide significant threshold by false discovery rate (FDR) [77]. A total of 4,336,025 association tests were performed in the discovery stage and in the meta-analysis replication stage. We then estimated the q-value (positive false-discovery rate) of each association test [78]. Based on the q-value and the number of significant tests (defined as an association test with q-value less than a particular q-value cutoff), we estimated the maximum number of false associations at each q-value cutoff. We set up the threshold for genome-wide significance of p-values as 4.3×10−7, and this threshold resulted in ≤1 expected false discovery of genome-wide significant association tests in our GWAS. The corresponding q-value is 0.011.
Expression Profiling (Stage III): Human Tissues
We conducted expression quantitative trait locus (eQTL) analysis to evaluate whether the genome-wide significant SNPs for each locus also influence transcript levels of nearby genes as a cis-effect regulator (eSNP) in human primary osteoblasts, lymphocytes and liver tissue. In each locus, we selected nearby genes in which the genome-wide significant SNP was located within 500 Kb in the 5′ upstream of candidate genes with the assumption that SNPs are located in (or in LD with the variants located in) regulation elements of candidate genes. Expression experiments in primary osteoblasts, lymphocytes and liver tissues were conducted in three different study samples. For un-genotyped SNPs, imputed SNPs (MACH variance ratio >0.3) were used in the lymphocyte expression dataset and surrogate SNPs with LD r2≥0.5 were used in primary osteoblasts and liver tissue datasets.
Primary osteoblasts
A gene expression profile with 18,144 known genes (Illumina Human Ref8v2 BeadChips) and genome-wide genotyping of 561,303 SNPs (Illumina 550k Duo chips) were available in 95 human Caucasian primary osteoblast samples. Human trabecular bone from the shaft of proximal femora obtained from donors undergoing total hip replacement. Primary osteoblasts were derived from bone tissue. Tissue collection, RNA and DNA isolation, expression profiling, and DNA genotyping have been described in detail [79]. All gene expression levels were adjusted for sex and year of birth. We studied the cis-expression quantitative trait loci (cis-eQTL) of genome-wide significant SNPs or their proxy (eSNP) with selected transcripts within 500 kb of the SNP position. The linear regression model implemented in PLINK [63] was used to determine association between adjusted expression levels and genotypes.
Lymphocytes
Expression experiments in two different samples were performed. A gene expression profile with 20,599 genes (Affymetrix U133 Plus 2.0) and genome-wide genotyping of 408,273 SNPs (Illumina HumanHap300 Genotyping Beadchip) were available on 400 children from families recruited through a proband with asthma. The detailed study design was described elsewhere [9]. We also profiled expression levels using the Illumina Human 6 BeadChips on additional 550 children from the UK (recruited from families with atopic dermatitis probands). These individuals were genotyped using Illumina HumanHap300 Genotyping Beadchip. Inverse normal transformation was used to normalize the skewed distribution in both samples. MACH [64] was used to impute un-genotyped SNPs based on Phase II HapMap CEU panel. Association analysis was applied with FASTASSOC option implemented in MERLIN [80]. Only cis-effects within 500 Kb of the transcript were tested.
Liver tissue
A gene expression profile with 34,266 known genes (Agilent custom array) and genome-wide genotyping of 782,476 SNPs (Affymetrix 500K and Illumina 650Y SNP genotyping arrays) were available on 957 human Caucasian liver samples. Liver samples were either postmortem or surgical resections from organ donors. Tissue collection, RNA and DNA isolation, expression profiling, and DNA genotyping have been described previously [12]. All gene expression levels were adjusted for age, sex, race, and center. We studied the cis-eQTL of genome-wide significant SNP or its proxy (eSNP) with selected transcripts within 500 kb of the SNP position. The Kruskal-Wallis test was used to determine association between adjusted expression levels and genotypes.
Expression Profiling (Stage III): Animal Models
Experiment 1: PTH stimulated gene expression profiling of mouse primary osteoblasts
Primary osteoblastic cultures were obtained from 2–3 day-old wild type C57BL/6J neonatal mice calvariae, half samples from males and half from females. Osteoblastic cell culture and PTH treatments were described elsewhere [81]. The 48-hour treatment cycle (incubation in medium with PTH for 6 hours, and incubation for the next 42 hours in medium without PTH) was repeated for 2 weeks. Cells were harvested and total RNA was isolated at day 14, after the last 6 hours of PTH exposure. Triplicate arrays were run for each condition/treatment with Affymetrix Mouse Genome 430A 2.0 arrays (approximately 14,000 genes per chip). Differences in gene expression levels between PTH and vehicle samples were evaluated. PTH-regulated genes were defined as follows: i) gene expression was detectable in all 3 PTH - and/or 3 vehicle-treated samples, ii) the average level of gene expression in PTH-treated samples was at least 1.5-fold higher or lower than in vehicle-treated samples, iii) gene expression levels differed by ≥1.5 fold between PTH and vehicle-treated samples in at least 7 out of 9 comparisons (each PTH-treated sample compared to vehicle-treated sample).
Experiment 2: Differential expression during osteoblast maturation
To determine whether the top associated genes were differentially expressed in maturing osteoblasts, we analyzed gene expression profiles of D3 murine embryonic stem cells that were undergoing directed differentiation toward the osteoblast lineage by treatment with vitamin D3, β-glycerophosphate and ascorbic acid. The gene expression dataset is publicly accessible via Gene Expression Omnibus, NCBI (GEO accession GSE3792). Gene expression patterns were generated using Affymetrix Mouse Genome 430A arrays at seven time points (Day 4, 5, 6, 8, 16, 25 and 30 post-induction). Triplicate arrays were run for each time point. The arrays were processed using the R AFFY package [82]. The robust multi-array average (RMA) algorithm was used for normalization [83]. ANOVA was used to identify genes whose expression differed across time points.
Experiment 3: Likelihood-based causality model selection
To identify causal relationships for the top associated genes discovered in our GWAS, we used the likelihood-based causality model selection (LCMS) algorithm by incorporating information of genotype, expression, and clinical traits together to construct regulatory networks. The description of the cross, genotyping and gene expression analysis have been described previously [12], [84]. The expression data is available via NCBI's Gene Expression Omnibus (GEO) database for adipose (GSE11065), liver (GSE11338) and muscle (GSE12795) tissues. The trabecular density measurement of the L5 vertebra from each F2 mouse was determined using a desktop μCT imaging system (μCT 40; Scanco Medical, Bassersdorf, Switzerland). The trabecular region was defined by contouring the inner section of the vertebral body with exclusion of the growth plate. Quantitative measurements of bone volume fraction (BV/TV), trabecular number (TbN), trabecular thickness (TbTh), trabecular separation (TbSp), mineral density of the bone volume fraction (DBV) and total femoral areal BMD (BMD) from mice were calculated using the Scanco software. μCT-derived trabecular bone data were evaluated against a hydroxyapatite standard in the same setting. LCMS procedure has been previously described [13], [31]. LCMS has been shown capable of recovering known causal relationships and we recently validated this approach by characterizing transgenic or knockout mouse models for 10 genes predicted causal for obesity by LCMS, seven of which significantly affected fat mass [85]. LCMS requires evidence of significant pleiotropy of eQTL/clinical trait QTL pairs using a likelihood modeling to test the fit of pleiotropy versus close linkage models. Three potential models were tested including: 1) Causal model: DNA variation affects a gene's expression which affects a clinical trait; 2) Reactive model: DNA variation affects a clinical trait which affects a gene's expression; and 3) Independent model: DNA variation independently affects both a gene's expression and clinical trait. The model with the lowest Bayesian Information Criteria (BIC) deemed the best fit. Reliability of each model call was determined by repeating LCMS on 1000 bootstrap samples. Candidate genes with at least 2 significant pleiotropy for eQTL and trait QTL pairs were considered to be causally related to differences in bone-related traits.
Experiment 4: Embryonic mouse in vivo gene expression atlas database
To determine where and when genes in the genome are expressed in the developing embryo in vivo [86], we ascertained the anatomic locations that genes were expressed during embryonic development in E10.5 and E14.5 wild type murine embryos from EURExpress database. Gene expression profiling on whole mounts and tissue sections of murine embryos were carried out by RNA in-situ hybridization with non-radioactive probes. Level and pattern of expression within each single organ or region are scored according to a standard scheme. Three different levels of expression were defined as (A) weak expression, (B) medium expression, and (C) strong expression. If no colored precipitate is seen, the gene expression is not detectable [87].
Prioritization of genome-wide suggestive candidate genes
To prioritize candidate genes from the list of suggestive genome-wide associated SNPs for further functional validation, we selected 109 suggestive candidate genes in which SNPs located within those regions were required to have all of the following criteria: (1) meta-analysis p-values of association test in the range of 4.3×10−7<p≤5×10−5, (2) p-values from the discovery stage <10−3, (3) p-values ≤0.05 from the replication stage and (4) the same direction of effect from both discovery and replication stages. We then prioritized these candidate genes based on results from either (1) two significant results supported by three expression signature profiles including PTH regulated genes (+++) from experiment 1, differentiated expression during osteoblast maturation (p<4.59×10−4, Bonferroni correction for 109 tests) from experiment 2 and at least moderate expression in skeletal sites of mouse embryos from experiment 4; or (2) candidate genes with at least 2 pairs of significantly pleiotropic QTL/eQTL effects from LCMS modeling (experiment 3).
Bioinformatic Approaches (Stage IV)
Gene-set enrichment tests on functional similarity
To explore functional similarity of our prioritized associated genes, we performed a gene-set enrichment test to examine the probability of our candidate genes clustering in particular biological/functional pathways as defined by the Gene Ontology (GO) project [88]. The GO Consortium provides controlled vocabularies, which model “Biological Process”, “Molecular Function” and “Cellular Component” that are structured into directed acyclic graphs based on published literature and databases. Gene products may be annotated to one or more GO nodes. To determine whether any GO terms annotate a specified list of genes at a frequency greater than that would be expected by chance, a p-value was calculated using the hyper-geometric distribution [89]. To correct for multiple testing, false discovery rate (FDR) was estimated [77].
Gene-set enrichment tests on expression abundance in human tissues
It has been well accepted that the content of the expressed sequence tag (EST) pool for a given tissue type reflects the composition of original mRNA samples used for creation of the complementary DNA library [90]. We estimated gene expression abundance for our top associated genes in an EST cDNA library (48 types of human normal tissues and organs) from the Cancer Genome Anatomy Project, National Cancer Institute (http://cgap.nci.nih.gov/Tissues). We estimated the expected expression levels and performed hyper-geometric tests to evaluate over - or under-representation of individual genes in selected tissues, including bone, liver, muscle and adipose tissue.
Supporting Information
Zdroje
1. ManolioTA
CollinsFS
CoxNJ
GoldsteinDB
HindorffLA
2009 Finding the missing heritability of complex diseases. Nature 461(7265) 747 53
2. MoonesingheR
KhouryMJ
LiuT
IoannidisJP
2008 Required sample size and nonreplicability thresholds for heterogeneous genetic associations. Proc Natl Acad Sci U S A 105(2) 617 22
3. SteinGS
LianJB
OwenTA
1990 Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J 4 13 3111 3123
4. WuXB
LiY
SchneiderA
YuW
RajendrenG
2003 Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest 112(6) 924 34
5. LeeS-K
LorenzoJA
1999 Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: Correlation with osteoclast-like cell formation. Endocrinology 140 35523561
6. PearsonTA
ManolioTA
2008 How to interpret a genome-wide association study. JAMA 299(11) 1335 44
7. CheslerEJ
LuL
ShouS
QuY
GuJ
2005 Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet 37 233 242
8. StrangerBE
NicaAC
ForrestMS
DimasA
BirdCP
2007 Population genomics of human gene expression. Nat Genet 39 1217 24
9. DixonAL
LiangL
MoffattMF
ChenW
HeathS
2007 A genome-wide association study of global gene expression. Nat Genet 39(10) 1202 7
10. CooksonW
LiangL
AbecasisG
MoffattM
LathropM
2009 Mapping complex disease traits with global gene expression. Nat Rev Genet 10 184 194
11. SchadtEE
LambJ
YangX
ZhuJ
EdwardsS
2005 An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet 37(7) 710 7
12. SchadtEE
MolonyC
ChudinE
HaoK
YangX
2008 Mapping the genetic architecture of gene expression in human liver. PLoS Biol 6 e107 doi:10.1371/journal.pbio.0060107
13. ChenY
ZhuJ
LumPY
YangX
PintoS
2008 Variations in DNA elucidate molecular networks that cause disease. Nature 452(7186) 429 35
14. KielDP
DemissieS
DupuisJ
LunettaKL
MurabitoJM
2007 Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet 8 Suppl 1 S14
15. CummingsSR
BlackDM
1995 Bone mass measurements and risk of fractures in Caucasian women: A review of findings from prospective studies. Am J Med 98 24 28
16. FaulknerKG
WackerWK
BardenHS
SimonelliC
BurkePK
2006 Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporos Int 17(4) 593 9
17. PulkkinenP
JämsäT
LochmüllerEM
KuhnV
NieminenMT
2008 Experimental hip fracture load can be predicted from plain radiography by combined analysis of trabecular bone structure and bone geometry. Osteoporos Int 19(4) 547 58
18. RalstonSH
de CrombruggheB
2006 Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev 20(18) 2492 506
19. HsuYH
XuX
TerwedowHA
NiuT
HongX
2007 Large-scale genome-wide linkage analysis for loci linked to BMD at different skeletal sites in extreme selected sibships. J Bone Miner Res 22(2) 184 94
20. IoannidisJP
NgMY
ShamPC
ZintzarasE
LewisCM
2007 Meta-analysis of genome-wide scans provides evidence for sex - and site-specific regulation of bone mass. J Bone Miner Res 22 173 183
21. RichardsJB
KavvouraFK
RivadeneiraF
StyrkársdóttirU
EstradaK
2009 Genetic Factors for Osteoporosis Consortium. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 151 528 37
22. RichardsJB
RivadeneiraF
InouyeM
PastinenTM
SoranzoN
2008 Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371(9623) 1505 12
23. StyrkarsdottirU
HalldorssonBV
GretarsdottirS
GudbjartssonDF
WaltersGB
2008 Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358(22) 2355 65
24. StyrkarsdottirU
HalldorssonBV
GretarsdottirS
GudbjartssonDF
WaltersGB
2009 New sequence variants associated with bone mineral density. Nat Genet 41(1) 15 7
25. TimpsonNJ
TobiasJH
RichardsJB
SoranzoN
DuncanEL
2009 Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum Mol Genet 18(8) 1510 7
26. XiongDH
LiuXG
GuoYF
TanLJ
WangL
2009 Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am J Hum Genet 84(3) 388 98
27. RivadeneiraF
StyrkársdottirU
EstradaK
HalldórssonBV
HsuYH
2009 Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41(11) 1199 206
28. HsuYH
NiuT
TerwedowHA
XuX
FengY
2006 Variation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in men. Hum Genet 118(5) 568 77
29. van MeursJB
TrikalinosTA
RalstonSH
BalcellsS
BrandiML
2008 Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 299(11) 1277 90
30. IoannidisJP
RalstonSH
BennettST
BrandiML
GrinbergD
2004 Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA 292(17) 2105 14
31. UitterlindenAG
RalstonSH
BrandiML
CareyAH
GrinbergD
2006 The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med 145 255 64
32. YasudaH
ShimaN
NakagawaN
YamaguchiK
KinosakiM
1998 Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95(7) 3597 602
33. LingL
ZhuT
LobiePE
2003 Src-CrkII-C3G-dependent activation of Rap1 switches growth hormone-stimulated p44/42 MAP kinase and JNK/SAPK activities. J Biol Chem 278(29) 27301 11
34. MatsuguchiT
ChibaN
BandowK
KakimotoK
MasudaA
2009 Jnk Activity is Essential for Atf4 Expression and Late-Stage Osteoblast Differentiation. J Bone Miner Res 24 398 410
35. KousteniS
HanL
ChenJR
AlmeidaM
PlotkinLI
2003 Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest 111(11) 1651 64
36. PereiraRC
DelanyAM
CanalisE
2004 CCAAT/enhancer binding protein homologous protein (DDIT3) induces osteoblastic cell differentiation. Endocrinology 145(4) 1952 60
37. PereiraCM
SattleggerE
JiangHY
LongoBM
JaquetaCB
2005 IMPACT, a Protein Preferentially Expressed in the Mouse Brain, Binds GCN1 and Inhibits GCN2 Activation. J Biol Chem 280(31) 28316 23
38. HoC
ConnerDA
PollakMR
LaddDJ
KiforO
1995 A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet 11 389 94
39. RaychaudhuriS
PlengeRM
RossinEJ
NgACY
International Schizophrenia Consortium 2009 Identifying Relationships Among Genomic Disease Regions: Predicting Genes at Pathogenic SNP Associations and Rare Deletions. PLoS Genet 5 e1000534 doi:10.1371/journal.pgen.1000534
40. GiladY
RifkinSA
PritchardJK
2008 Revealing the architecture of gene regulation: the promise of eQTL studies. Trends Genet August 24(8) 408 415
41. PetrettoE
MangionJ
DickensNJ
CookSA
KumaranMK
2006 Heritability and tissue specificity of expression quantitative trait loci. PLoS Genet 2 e172 doi:10.1371/journal.pgen.0020172
42. MengH
VeraI
CheN
WangX
WangSS
2007 Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proc Natl Acad Sci U S A 104(11) 4530 5
43. ZhangK
LiJB
GaoY
EgliD
XieB
2009 Digital RNA allelotyping reveals tissue-specific and allele-specific gene expression in human. Nat Methods 6(8) 613 8
44. LeeJH
ParkIH
GaoY
LiJB
LiZ
2009 A robust approach to identifying tissue-specific gene expression regulatory variants using personalized human induced pluripotent stem cells. PLoS Genet 5 e1000718 doi:10.1371/journal.pgen.1000718
45. EmilssonV
ThorleifssonG
ZhangB
LeonardsonAS
ZinkF
2008 Genetics of gene expression and its effect on disease. Nature 452 423 428
46. DimasAS
DeutschS
StrangerBE
MontgomerySB
BorelC
2009 Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325(5945) 1246 50
47. KwanT
GrundbergE
KokaV
GeB
LamKC
2009 Tissue effect on genetic control of transcript isoform variation. PLoS Genet 5 e1000608 doi:10.1371/journal.pgen.1000608
48. SeemanE
2001 Clinical review 137: Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab 86(10) 4576 84
49. KarasikD
FerrariSL
2008 Contribution of gender-specific genetic factors to osteoporosis risk. Ann Hum Genet 72(Pt 5) 696 714
50. HannanMT
FelsonDT
Dawson-HughesB
TuckerKL
CupplesLA
2000 Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 5 710 720
51. DawberTR
MeadorsGF
MooreFEJ
1951 Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health 41 279 86
52. KannelWB
FeinleibM
McNamaraPM
GarrisonRJ
CastelliWP
1979 An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110(3) 281 90
53. BeckTJ
RuffCB
ScottWWJr
PlatoCC
TobinJD
1992 Sex differences in geometry of the femoral neck with aging: a structural analysis of bone mineral data. Calcif Tissue Int 50 24 9
54. KhooBC
BeckTJ
QiaoQH
ParakhP
SemanickL
2005 In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone 37(1) 112 21
55. MailmanMD
FeoloM
JinY
KimuraM
TrykaK
2007 The NCBI dbGaP database of genotypes and phenotypes. Nat Genet 39(10) 1181 6
56. de BakkerPI
MallerJ
YelenskyR
AltshulerD
DalyMJ
2006 Evaluating and improving power in whole-genome association studies using fixed marker sets. Nat Genet 38(6) 663 7
57. PriceAL
PattersonNJ
PlengeRM
WeinblattME
ShadickNA
2006 ‘Principal Components Analysis Corrects for Statification in Genome-Wide Asssociation Studies’. Nature Genetics 38 904 909
58. KathiresanS
WillerCJ
PelosoGM
DemissieS
MusunuruK
2009 Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 41(1) 56 65
59. AbecasisGR
CardonLR
CooksonWO
ShamPC
ChernySS
2001 Association analysis in a variance components framework. Genet Epidemiol 21 Suppl 1 S341 S346
60. DevlinB
RoederK
1999 Genomic Control for Association Studies. Biometrics 55;4 997 1004
61. SkolAD
ScottLJ
AbecasisGR
BoehnkeM
2006 Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet 38 209 13
62. HofmanA
BretelerMM
van DuijnCM
JanssenHL
KrestinGP
2009 The Rotterdam Study: 2010 objectives and design update. Eur J Epidemiol 24 553 72
63. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 7
64. LiY
AbecasisGR
2006 Mach 1.0: Rapid Haplotype Reconstruction and Missing Genotype Inference. Am J Hum Genet S79 2290
65. BurdickJT
ChenWM
AbecasisGR
CheungVG
2006 In silico method for inferring genotypes in pedigrees. Nature Genet 38 1002 1004
66. RivadeneiraF
ZillikensMC
De LaetCE
HofmanA
UitterlindenAG
2007 Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res 22 1781 90
67. SpectorTD
WilliamsFMK
2006 The UK adult twin registry (TwinsUK). Twin Res and Hum Genetics 9 899 906
68. PritchardJK
StephensM
DonnellyP
2000 Inference of population structure using multilocus genotype data. Genetics 155 945 59
69. MarchiniJ
HowieB
MyersS
McVeanG
2007 A new multipoint method for genome-wide association studies via imputation of genotypes. Nature Genetics 39 906 913
70. LiM
BoehnkeM
AbecasisGR
2006 Efficient study designs for test of genetic association using sibship data and unrelated cases and controls. Am J Hum Genet 78 778 92
71. The International HapMap Consortium 2005 A haplotype map of the human genome. Nature 437 1299 1320
72. The Wellcome Trust Case Control Consortium 2007 Genomewide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 661 678
73. DudbridgeF
GusnantoA
2008 Estimation of significance thresholds for genomewide association scans. Genet Epidemiol 32(3) 227 34
74. BishopDT
DemenaisF
IlesMM
HarlandM
TaylorJC
2009 Genome-wide association study identifies three loci associated with melanoma risk. Nat Genet 41(8) 920 5
75. SabattiC
ServiceSK
HartikainenAL
PoutaA
RipattiS
2009 Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet 41(1) 35 46
76. VasanRS
GlazerNL
FelixJF
LiebW
WildPS
2009 Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA 302(2) 168 78
77. BenjaminiY
HochbergY
1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57 289 300
78. StoreyJD
2003 “The positive false discovery rate: A Bayesian interpretation and the q-value”. Annals of Statistics 31 (6) 2013 2035
79. GrundbergE
KwanT
GeB
LamKC
KokaV
2009 Population genomics in a disease targeted primary cell model. Genome Res 9(11) 1942 52
80. AbecasisGR
ChernySS
CooksonWO
CardonLR
2002 Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nature Genet 30 97 101
81. BianchiEN
FerrariSL
2009 β-arrestin2 regulates parathyroid hormone effects on a p38 MAPK and NFκB gene expression network in osteoblasts. Bone 45 716 725
82. GautierL
CopeL
BolstadBM
IrizarryRA
2004 affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004 20(3) 307 15
83. IrizarryRA
HobbsB
CollinF
Beazer-BarclayYD
AntonellisKJ
2003 Exploration, Normalization, and Summaries of High Density Oligonucleotide Array Probe Level Data. Biostatistics. Vol. 4, Number 2 249 264
84. FarberCR
van NasA
GhazalpourA
AtenJE
DossS
2009 An integrative genetics approach to identify candidate genes regulating BMD: combining linkage, gene expression, and association. J Bone Miner Res 24(1) 105 16
85. YangX
DeignanJL
QiH
ZhuJ
QianS
2009 Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat Genet. Nat Genet 2009 Apr;41(4) 415 23
86. DavidsonD
BaldockR
2001 Bioinformatics beyond sequence: mapping gene function in the embryo. Nature Rev Genet 2 409
87. ViselA
ThallerC
EicheleG
2004 GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Research 32 D552 D556
88. AshburnerM
BallCA
BlakeJA
BotsteinD
ButlerH
2000 Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25 25 29
89. BoyleEI
WengS
GollubJ
JinH
BotsteinD
2004 GO::TermFinder–open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 20 3710 5
90. BoonK
OsórioEC
GreenhutSF
SchaeferCF
ShoemakerJ
2002 Proc Natl Acad Sci USA 99 11287 11292
Štítky
Genetika Reprodukční medicína
Článek The Met 470 Allele Is Associated with Lower Birth Rates in Fertile Men from a Population IsolateČlánek Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 6
-
Všechny články tohoto čísla
- Translational Selection Is Ubiquitous in Prokaryotes
- Whole-Genome Sequencing of a Single Proband Together with Linkage Analysis Identifies a Mendelian Disease Gene
- Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits
- The Met 470 Allele Is Associated with Lower Birth Rates in Fertile Men from a Population Isolate
- Contributions of Status and Allele Expression, But Not Copy Number Variation, to the Control of SIVmac251 Replication in Indian-Origin Rhesus Monkeys
- A Genome-Wide Association Study of Optic Disc Parameters
- Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
- The Transcription Factor REST Is Lost in Aggressive Breast Cancer
- 14-3-3 Mediates Histone Cross-Talk during Transcription Elongation in
- Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
- Use of Genome-Wide Expression Data to Mine the “Gray Zone” of GWA Studies Leads to Novel Candidate Obesity Genes
- Genome-Wide RNAi Screen Identifies Multiple Regulators of HIF–Dependent Transcription in Hypoxia
- The CYCLIN-A CYCA1;2/TAM Is Required for the Meiosis I to Meiosis II Transition and Cooperates with OSD1 for the Prophase to First Meiotic Division Transition
- Inactivation of hnRNP K by Expanded Intronic AUUCU Repeat Induces Apoptosis Via Translocation of PKCδ to Mitochondria in Spinocerebellar Ataxia 10
- Mice with Alopecia, Osteoporosis, and Systemic Amyloidosis Due to Mutation in , a Gene Coding for Palmitoyl Acyltransferase
- siRNA–Mediated Methylation of Telomeres
- Chromosome 4 Replicates in Two Phases That Correlate with Chromatin State
- Dynamic Switch of Negative Feedback Regulation in Akt–TOR Signaling
- On the Use of Variance per Genotype as a Tool to Identify Quantitative Trait Interaction Effects: A Report from the Women's Genome Health Study
- and Deficiency Cooperate in the Progression of Mouse Prostate Tumourigenesis
- An Integration of Genome-Wide Association Study and Gene Expression Profiling to Prioritize the Discovery of Novel Susceptibility Loci for Osteoporosis-Related Traits
- Consent and Internet-Enabled Human Genomics
- Understanding Adaptation in Large Populations
- Identification of a Functional Genetic Variant at 16q12.1 for Breast Cancer Risk: Results from the Asia Breast Cancer Consortium
- Evidence that Adaptation in Is Not Limited by Mutation at Single Sites
- The IG-DMR and the -DMR at Human Chromosome 14q32.2: Hierarchical Interaction and Distinct Functional Properties as Imprinting Control Centers
- Cushing's Syndrome and Fetal Features Resurgence in Adrenal Cortex–Specific Knockout Mice
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits
- The IG-DMR and the -DMR at Human Chromosome 14q32.2: Hierarchical Interaction and Distinct Functional Properties as Imprinting Control Centers
- Amplification of a Cytochrome P450 Gene Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid
- Copy Number Variation and Transposable Elements Feature in Recent, Ongoing Adaptation at the Locus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



