-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGas chromatography – mass spectrometry studies of the component composition of carboxylic acids of the rhizomes of Iris medwedewii and Iris carthaliniae (Iridaceae)
Studie složení karboxylových kyselin rhizomů Iris medwedewii a Iris carthaliniae (Iridaceae) pomocí plynové chromatografie ve spojení s a hmotnostní spektrometrií
Složení karboxylových kyselin v rhizomech Iris carthaliniae a Iris medwedewii pomocí plynové chromatografie ve spojení s hmotnostní spektrometrií dosud nebylo studováno. Celkový obsah karboxylových kyselin u I. carthaliniae byl 1,34 %, včetně (%) – 0,65 mastných; 0,36 mono-, di - a tri-karboxylových; 0,33 fenolických karboxylových kyselin. Výtěžek karboxylových kyselin u I. medwedewii byl 1,58 %, včetně (%) – 0,73 mastných; 0,38 mono-, di - and tri-karboxylových; 0,47 fenolických karboxylových kyselin. Hlavní postavení v rhizomech I. carthaliniae měly kyselina myristová (25 %), kyselina palmitová (14,41 %), kyselina stearová (10,51 %) a kyselina linolová (6,05 %). Kromě toho kyselina levulová (15,84 %) a kyselina šťavelová (4,42 %) převládaly mezi organickými kyselinami, zatímco kyselina ferulová (2,43 %), kyselina citronová (1,88 %) a kyselina jablečná (3,62 %) převládaly mezi hydroxykyselinami. V rhizomech I. medwedewii byly zjištěny mastné kyseliny – kyselina palmitová (6,51 %), linolová (8,38 %), olejová (3,87 %) a kaprinová (3,21 %). Dále bylo zjištěno, že v rhizomech I. medwedewii mezi organickými kyselinami převládaly kyselina levulová (29,39 %), malonová (1,45 %) a jantarová (1,72 %); kyselina citronová (25,37 %), jablečná (3,30 %) a ferulová (2,42 %) pak převládaly mezi hydroxykyselinami.
Klíčová slova:
Iris carthaliniae • Iris medwedewii • rhizomy • karboxylové kyseliny • plynová chromatografie
Authors: Javanshir I. Isayev; Оlga O. Mykhailenko; Vladumir M. Kovalyov; Gamid M. Gurbanov; Murad Y. Suleymanov
Published in the journal: Čes. slov. Farm., 2017; 66, 9-14
Category: Původní práce
Summary
The composition of carboxylic acids of the rhizomes of Iris carthaliniae and Iris medwedewii by the gas chromatography – mass spectrometry method has been studied for the first time. The total content of carboxylic acids for I. carthaliniae was 1.34%, including (%) – 0.65 fatty; 0.36 mono-, di - and tri-carboxylic; 0.33 phenol carboxylic acids. The yield of carboxylic acids for I. medwedewii was 1.58%, including (%) – 0.73 fatty; 0.38 mono-, di - and tri-carboxylic; 0.47 phenol carboxylic acids. The dominant fatty acids in the rhizomes of I. carthaliniae were myristic acid (25%), palmitic acid (14.41%), stearic acid (10.51%) and linoleic acid (6.05%). Besides, levulinic acid (15.84%) and oxalic acid (4.42%) prevailed among organic acids, while ferulic acid (2.43%), citric acid (1.88%) and malic acid (3.62%) prevailed among the hydroxy acids. The fatty acids palmitic acid (6.51%), linoleic acid (8.38%), oleic acid (3.87%) and capric acid (3.21%) were found in the rhizomes of I. medwedewii, and also, levulinic (29.39%), malonic (1.45%), succinic (1.72%) acids prevailed among the organic acids and citric (25.37%), malic (3.30%) and ferulic (2.42%) acids in the rhizomes of I. medwedewii prevailed among the hydroxy acids.
Key words:
Iris carthaliniae • Iris medwedewii • rhizomes • carboxylic acids • GC-MS analysisIntroduction
The carboxylic acids are synthesized in a significant amount in the plant cell1, 2), they are products of anabolism (assimilation) and necessary for cell and organism activities. They are the products of transformation of the main nutrients: fats, proteins, and carbohydrates3, 4). Organic acids have antioxidant, anti-inflammatory and immunomodulatory properties, they are involved in metabolism, have a positive affect on digestion, and create favorable conditions for the life of beneficial intestinal microorganisms5, 6). Fatty acids are involved in the synthesis of prostaglandins and in the stabilization of cellular membranes1, 3, 7). Therefore, the search of the new plant sources rich in organic acids is a very important direction of research.
There are 40 species of iris in the flora of Azerbaijan but the most common species of iris are I. medwedewii Fomin and I. carthaliniae Fomin8, 9). They are perennial plants with thick horizontal creeping rhizomes8, 10). According to the classification of Rodionenko (1961)10), I. carthaliniae belongs to the subgenus Xyridion (Tausch) Spach em Rodion. (sect. Xyridion, ser. Spuria (Diels) Lawrence em Rodion.) and I. medwedewii belongs to the subgenus Iris (sect. Hexapogon (Bunge) Baker em. Rodion., subsect. Oncocyclus (Siemss) Benth.). The Iris species have been cultivated as decorative plants since the ancient times. These cultivated Irises have a wide variety of colours in their showy and usually perfumed blossoms11–13). The plants of Iris species are rich in xanthones14), flavonoids15–16), iridoidoids, essential oils17–18), saponins, tannins, etc. Various kinds of Iris species in different regions of the world are used as diuretic, hemostatic, astringent, antipyretic, tonic, anti-inflammatory, cardiac, immunomodulatory, antioxidant, antimicrobial19), antiviral and tonic agents17–18). The rhizomes of this plant have been extensively used against fever, kidney infections and as ingredients of tooth powders etc. The chemical composition of secondary metabolites of these Irises has not been studied18, 20).
The previous papers reported the qualitative and quantitative composition of fatty acids of the rhizomes of I. hungarica Waldst. et Kit21) and I. pseudacorus L.22). According to the classification of Rodionenko (1961)10) I. hungarica belongs to the subgenus Iris, section Iris, series Elatae Lawrence; I. pseudacorus – subgenus Limniris (Tausch) Spach em. Rodion, section Limniris, series Laevigatae (Diels) Lawrence). In the rhizomes of I. hungarica, 19 fatty acids have been identified (total amount – 0.5%). Myristic (C14 : 0; 41.0%), linoleic (C18 : 2ω6; 12.1%), palmitic (C16 : 0; 11.6%) fatty acids were prevalent. And in the rhizomes of I. pseudacorus, 12 fatty acids have been identified (total amount – 1.07%), the dominant components being palmitic (C16 : 0) 46.60%, linoleic (C18 : 2ω6) 28.91%, heptadecanoic (C17 : 0) 4.20% and linolenic (C18 : 3ω3) 4.73% fatty acids.
<em>I. carthaliniae</em> Fomin 
Fig. 1. General view of the living plants and herbariums of the studied species of Irises 
The aim of this study is to determine the qualitative and quantitative composition of carboxylic acids of the rhizomes of two Azerbaijan Iris species, namely I. medwedewii and I. carthaliniae by the gas chromatography-mass spectrometry (GC/MS) method.
Experimental part
Plant materials
The objects of the study were the rhizomes of I. medwedewii Fomin and I. carthaliniae Fomin, prepared in Gosmalyan and Pirasora villages of the Lerik region of Azerbaijan, in the blooming phase at the height of more than 2000 m above the sea level in May 2014. Voucher specimens have been deposited in the Herbarium of the Pharmacognosy Department and Botany Department, National University of Pharmacy, Kharkiv, Ukraine, and also at the Department of Pharmacognosy and Botany, Azerbaijan Medical University, Baku, Azerbaijan.
Preparation of the extracts
The analysis of the methyl ethers of carboxylic acids was carried out by the method of chromatography-mass-spectrometry25–27) on a 5973N/6890N MSD/DS Agilent Technologies (USA). The internal standard (solution of tridecane (50.0 µg) in hexane) and 1.0 ml of the methylating agent (14% BCl3 in methanol, Supelco 3-3033) were added to a weighed sample of the product (50.0 mg) in a vial of 2.0 ml. The mixture was held at 65 °С in a sealed vial for 8 hours. In this time organic acids were completely extracted from the plant material and hydrolysis and methylation of fatty acids occurs. Free organic and phenolcarboxylic acids were methylated simultaneously. The reaction mixture was elutriated from the plant material and diluted with 1.0ml of distilled water. Methyl ethers were extracted with 0.2 ml of methylene chloride, carefully shaken up several times within an hour and then the obtained extract was chromatographed23, 24).
Chromatographic conditions
Sample introduction (2.0 µl) was carried out into a chromatographic column in the splitless mode without the split ratio within 0.2 minute. A capillary column HP-INNOWAX (30 m × 250 µm × 0.50 µm) was used for separation. The mobile phase: helium, gas flow rate: 1.2 ml/min. Temperature of the sample injection heater: 250 °С. Temperature of furnace is programmable from 50 to 320 °С with the rate of 4 degree/min. For component identification, the data from the mass-spectra libraries NIST05 and WILEY 2007 with the total number of spectra of more than 470,000 were used combined with identification programs AMDIS and NIST25,26).
For quantitative calculations, the internal standard method was used27). Calculation of components content С (mg/kg) was carried out using the formula:
С= P1·50·1000/ P2 M,
where P1 – peak area of the tested substance, P2 – peak area of the standard, 50 – mass of the internal standard (μg) injected into the sample; m – sample mass (g).
The relative content of carboxylic acids was determined in % of their sum.
Results and discussion
The analysis of carboxylic acid composition of the rhizomes of I. carthaliniae and I. medwedewii showed the presence of 35 common acids and 2 acids specific only for certain species.
The analysis of the content in the rhizomes revealed the presence of the total content of the carboxylic acids for I. carthaliniae, 13447.54 mg/kg or 1.34%, including (%) – 0.65 fatty; 0.36 mono-, di - and tri-carboxylic; 0.33 phenol carboxylic acids. The yield of the carboxylic acids for I. medwedewii was 15829.61 mg/kg or 1.58%, including (%) – 0.73 fatty; 0.38 mono-, di - and tri-carboxylic; 0.47 phenol carboxylic acids. Qualitative composition of the carboxylic acids varied: there are lower and higher carboxylic acids; mono-, di - and tricarboxylic acids by the number of the carboxyl groups; aliphatic and aromatic acids by the nature of the hydrocarbonic radical connected with a COOH-group; saturated and unsaturated acids by the level of saturation. The lowest acids are presented by both free organic acids and hydroxyacids. The length of carbon chains in fatty acids is from 6 to 24 atoms. The results are given in Figs. 2–3 and Tables 1–2 below.
Fig. 2. GC-MS chromatogram of carboxylic acids of the rhizomes of I. carthaliniae 
Fig. 3. GC-MS chromatogram of carboxylic acids of the rhizomes of I. medwedewii 
Tab. 1. Lower carboxylic acids of the rhizomes of I. carthaliniae and I. medwedewii 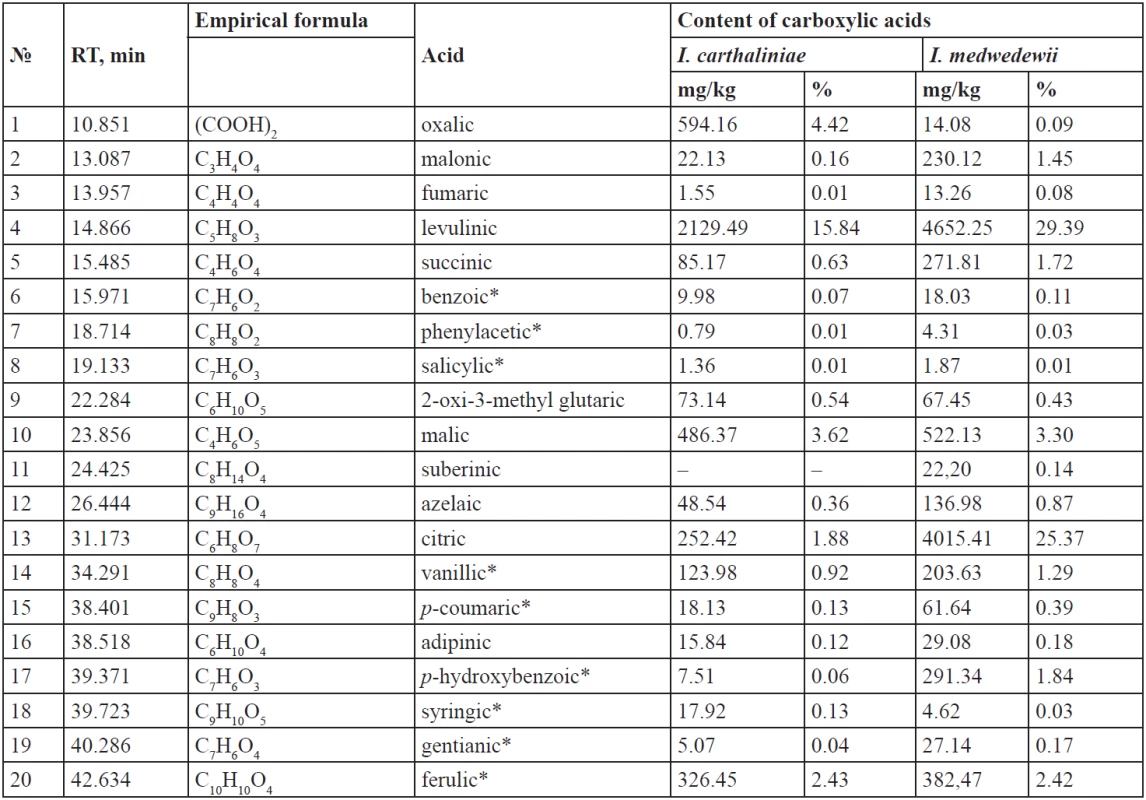
*phenol carboxylic acids; – means that the substance was not identified Tab. 2. Higher carboxylic acids of the rhizomes of I. carthaliniae and I. medwedewii 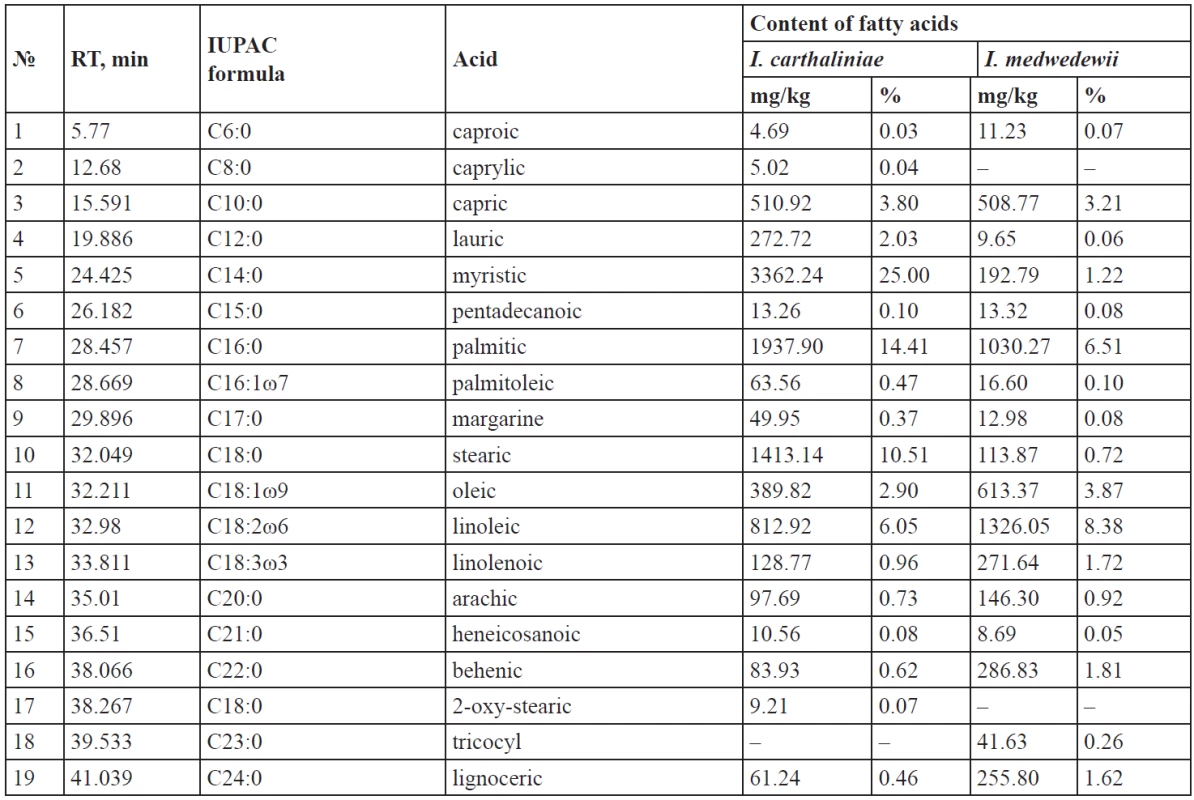
– means that the substance was not identified The content of the organic acids for I. carthaliniae is 31.38% of the total carboxylic acids content; 9.3% of them are hydroxy acids. Oxalic, malonic, fumaric, levulinic, succinic, 2-oxi-3-methylglutaric, and azelaic acids are common for the both species of irises. 10 aliphatic acids were identified in the rhizomes of I. carthaliniae, their total content as 27.58%, relative to the total amount of acids. Levulinic acid (15.84%), malic acid (3.62%), oxalic acid (4.42%), citric acid (1.88%), succinic (0,63%) prevail among the organic acids in the rhizomes of I. carthaliniae. Adipinic acid (0.12%) was found only in the rhizomes of I. carthaliniae. In addition, in the raw materials 9 aromatic acids were identified, their content being 3.8%. The content of ferulic acid (up 2%) significantly dominates in the both species of irises.
The yield of the organic acids in the rhizomes of I. medwedewii is 69.32% and the total of 20 acids were identified, the hydroxy acids content was 34.96%, which is almost 4 times higher than in the rhizomes of I. carthaliniae. Among the aliphatic acids dominated: levulinic (29.39%), citric (25.37%), malic (3.30%) malonic (1.45%), succinic (1.72%); their total content being 63.02% of the total acids. Additionally, in I. medwedewii the suberinic acid (0.14%) was identified. The content of aromatic acids was 6.29%. Benzoic, phenylacetic, salicylic, malic, citric, vanillic, р-coumaric, р-hydroxybenzoic, syringic, gentianic, ferulic acids were common hydroxy acids for both Irises.
The content of the saturated fatty acids in the rhizomes of I. carthaliniae was 58.25%, which far exceeds the amount of the unsaturated (10.38%) ones of the total of carboxylic acids. Among the saturated acids dominated myristic (25%), palmitic (14.41%), stearic (10.51%) acids; lower quantities of lauric (2.03%) and capric (3.80%) acids were found. Caprylic (0.04%) and 2-hydroxy-stearic (0.07%) acids were found only in I. carthaliniae.
ω-6 Dienoic linoleic acid (6.05%) prevailed among the unsaturated acids in the rhizomes of I. carthaliniae and monoenoic ω-9 oleic acid (2.90%) was second to it. The content of palmitoleic acid and linolenic acid were until 1%.
The composition of the fatty acids in the rhizomes of I. medwedewii was almost identical: caprylic and 2-oxy-stearic acid were absent, but tricocyl acid (0.26%) was found. The saturated fatty acids content was 16.61% and the unsaturated represented 14.07% of the total of carboxylic acids. Although, the qualitative composition of the Irises was similar, the content of the fatty acids in I. medwedewii did not exceed 0.1–2%, but the palmitic (6.51%), olein (3.87%) and linoleic (8.38%) acids made the exception.
Conclusion
- Qualitative and quantitative composition of the carboxylic acids in the rhizomes of I. carthaliniae and I. medwedewii was studied at the first time using the method of chromatо-mass spectrometry, 37 carboxylic acids being identified.
- Total content of the carboxylic acids for I. carthaliniae was 13447.54 mg/kg or 1.34%, including (%) – 0.65 fatty; 0.36 mono-, di - and tri-carboxylic; 0.33 phenol carboxylic acids. 10 aliphatic acids and 9 aromatic acids were identified in the rhizomes of I. carthaliniae, their total content as 27.58% and 3.8% respectively, relative to the total amount of acids. Myristic acid (25%), palmitic acid (14.41%), stearic acid (10.51%) and linoleic acid (6.05%) prevailed among the fatty acids; levulinic acid (15.84%) and oxalic acid (4.42%) prevailed among the organic acids; ferulic acid (2.43%), citric acid (1.88%) and malic acid (3.62%) prevailed among the hydroxy acids.
- Yield of the carboxylic acids for I. medwedewii was 15829.61 mg/kg or 1.58%, including (%) – 0.73 fatty; 0.38 mono-, di - and tri-carboxylic; 0.47 phenol carboxylic acids. Palmitic acid (6.51%), linoleic acid (8.38%), oleic acid (3.87%) and capric acid (3.21%) prevailed among the fatty acids; levulinic (29.39%), malonic (1.45%), succinic (1.72%) acids prevailed among the organic acids; citric (25.37%), malic (3.30%) and ferulic (2.42%) acids prevailed among the hydroxy acids.
- The results of this study are significant for the determination of beneficial compounds in the rhizomes of the Iris species, so making use of these plant raw materials as a source of new medicines in the future is possible.
Acknowledgement
The authors are grateful to Stanislav Uluantsev, acting as a chemical engineer of the Laboratory of Analytical Studies of the National Institute of Vine and Wine “Magarach” (Yalta, Russian Federation), for his help with the experimental carboxylic acids analysis.
Conflict of interest: The authors have declared no financial relationships with any organizations that might have an interest in the submitted work; no any other relationships or activities that could appear to have influenced the submitted work.
Received: November 21, 2016
Accepted: February 16, 2017
Javanshir I. Isayev • Gamid M. Gurbanov
Department of Pharmacognosy and Botany, Azerbaijan Medical University, Baku, Azerbaijan
Оlga O. Mykhailenko, PhD in Pharmacy, Assistant Professor of Botany department (∗)
National University of Pharmacy
str. Valentunivska 4, 61168 Kharkiv, Ukraine
e-mail: z_ola07@mail.ru
Vladumir M. Kovalyov
Department of Pharmacognosy, National University of Pharmacy,
Kharkiv, Ukraine
Murad Y. Suleymanov
Analytical Expertise Center, Azerbaijan Republic Ministry
of Health, Baku, Azerbaijan
Zdroje
1. Ohlrogge J., Browse J. Lipid biosynthesis. The plant cell 1995; 7, 957–970.
2. Goodwin T., Mercer E. Vvedenie v biokhimiiu rastenii [Introduction to biochemistry of plants] tom 1. Moscow: Mir 1986. (in Russian).
3. Bernardi G. New comprehensive biochemistry. Biochemistry of lipids, lipoproteins and Membranes. Amsterdam: Elsevier 1996; 31, 141–152.
4. Gunstone F. D. Fatty acids and lipid chemistry. London: Blackie Academic and Professional 1996.
5. Gusakova S. V., Sagdulayev Sh. Sh., Khushtakova Z. A. Lipophilic extracts in herbal medicine and bodycare, their preparation and biological properties. Khimiya prirodnykh soedinenii. 1998; 4, 437–447 (in russian).
6. Marri P., Grenner D., Meyes P., Rodyel V. Biochemistry of man: in 2 tom. Мoscow: Mir 1993 (in russian).
7. Plant lipids: biology, utilization and manipulation/eds. Denis J. Murphy. Wiley-Blackwell 2005.
8. Flora of Azerbaijan, in 8 vols. Baku, 1952. T. 2 (in Russian).
9. Isayev D. I., Gurbanov G. M. Summery phytochemical investigations of some plants species belonging to the genus İris from Azerbaijan flora. The modern achivements of Azerbaijan medicine. 2014; 2, 104–109.
10. Rodionenko G. I. An outline of a new and evolutionary botanical classification of Irises. The Iris Year Book. The British Iris Society 1962; 103–119.
11. Goldblatt P., Manning J. C. The Iris family: natural history and classification, Timber Press: Portland 2008.
12. Dykes W. R. The genus Iris. New York: Dover Publications 1974.
13. Austin C. Irises: a gardeners encyclopedia; Portland: Timber Press 2005.
14. Isayev D. I., Kovalev V. N., Gurbanov G. M., Mykhailenko O. A. Quantitative determination of mangiferin in some species of the genus Iris of flora Azerbaijan by HPLC. Plant resources. 2015; 51(3), 444–447.
15. Isayev D. I., Gurbanov G. M. Quantitative determination of flavonoids by spectrophotometry in the raw material of I. carthaliniae and I. medwedewii plants. Azerbaijan pharmaceutical and pharmacotherapy journal. 2014; 1, 27–30.
16. Iwashina T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000; 113(3), 287–299.
17. Kassak P. Secondary metabolites of the choosen genus Iris spesies. J. Acta universitatis agriculturae et silviculturae mendelianae brunensis 2012; 32(8), 269–280.
18. Kukula-Koch W., Sieniawska E., Widelski J., Urjin O. Major secondary metabolites of Iris spp. Phytochemistry reviews 2013; 12(4), 1–32.
19. Zatylnikova O. A., Osolodchenko T. P., Kovalev V. N. Antimicrobial activity of extracts of Iris pseudacorus L. Scientific J. Annals of Mechnikov’s Institute 2010; 4, 43–47 (in Ukrainian).
20. Williams Ch. A., Harborne J. B., Colasante M. Flavonoid and xanthone patterns in bearded Iris species and the pathway of chemical evolution in the genus. Biochemical Systematics and Ecology 1997; 25(4), 309–325.
21. Kovalyov V. N., Mykchailenko O. A., Krechun A. V. Investigation of lipid composition of rhizomes with roots of Iris hungarica. Rastitelnye resursu. 2015; 3, 406–415 (in Russian).
22. Zatylnikova O. O., Kovalyov S. V., Osolodchenco T. P. The chemical stady of lipophilic fraction from rhizomes of Yellow Iris. Visnuk farmacii 2008; 3(5)5, 9–12 (in Ukrainian).
23. Carrapiso A. I, García C. Development in lipid analysis: some new extraction techniques and in situ transesterification. Lipids 2000; 35(11), 1167–1177.
24. Bicchi C., Brunelli C., Cordero C., Rubiolo P. Direct resistively heated column gas chromatography (Ultrafast module-GC) for high-speed analysis of essential oils of differing complexities. J. Chromatogr. A. 2004; 1024(1–2), 195–207.
25. NIST Mass Spec Data Center SES. 2005a. Mass Spectra, 6th edn. National Institute of Standards and Technology: Gaithersburg MD.
26. NIST Mass Spec Data Center SES. 2005b. Retention.
27. State Pharmacopoeia of Ukraine. 1st ed. Appendix 1. Kharkiv: State Enterprise: Scientific and Expert Pharmacopoeial Centre 2004 (in Ukrainian).
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2017 Číslo 1- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Cytotoxic, anti-cancer, and anti-microbial effects of different extracts obtained from Artemisia rupestris
- Self-emulsifying drug delivery system (SEDDS) of Ibuprofen: formulation, in vitro and in vivo evaluation
- Medical and non-medical costs of Parkinson disease – comparison of Europe, USA, Asia an Australia
- The fate of Jewish pharmacists from the Czech Lands during the holocaust
- Sympozia Sekce dějin farmacie ČFS v roce 2016
- Gas chromatography – mass spectrometry studies of the component composition of carboxylic acids of the rhizomes of Iris medwedewii and Iris carthaliniae (Iridaceae)
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The fate of Jewish pharmacists from the Czech Lands during the holocaust
- Medical and non-medical costs of Parkinson disease – comparison of Europe, USA, Asia an Australia
- Self-emulsifying drug delivery system (SEDDS) of Ibuprofen: formulation, in vitro and in vivo evaluation
- Cytotoxic, anti-cancer, and anti-microbial effects of different extracts obtained from Artemisia rupestris
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





