-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSelf-emulsifying drug delivery system (SEDDS) of Ibuprofen: formulation, in vitro and in vivo evaluation
Samoemulgující systém (SEDDS) pro podání léčiva ibuprofen: hodnocení vzorků v podmínkách in vitro a in vivo
Cílem této studie bylo vyvinout samoemulgující systém (SEDDS) pro perorální podání těžce rozpustného léčiva ibuprofenu a zhodnotit jeho biologickou dostupnost. K určení fázového chování mikroemulzí a srovnání účinnosti různých směsí povrchově aktivní látky a oleje byly sestaveny fázové diagramy. Samoemulgující systémy ibuprofenu byly připraveny ze směsi Labrafilu M2125, Cremophoru RH40 a Plurol oleique. U připravených emulzí bylo hodnoceno jejich chování v podmínkách in vitro a in vivo. FTIR hodnocení nepotvrdilo žádnou interakci mezi léčivem a pomocnými látkami. DSC studie prokázaly, že léčivo je v systému solubilizováno. Vzorky byly dále hodnoceny na termodynamickou stabilitu, dispersibilitu, index lomu, viskozitu a bod zákalu. Připravené vzorky ukázaly negativní náboj částic vnitřní fáze, což ukazuje na jejich stabilitu. Systém optimalizovaného složení představoval mikroemulzi s velikostí vnitřní fáze 177,5 nm. Množství uvolněného léčiva v podmínkách in vitro bylo významně vyšší než u přípravku dostupného na trhu a čistého léčiva. Farmakodynamické studie optimalizované složení ukázala vyšší protizánětlivý účinek ve srovnání s přípravkem dostupným na trhu a čistým léčivem. Hodnoty AUC a Cmax po perorálním podání byly vyšší pro SEDDS s obsahem ibuprofenu ve srovnání s přípravkem dostupným na trhu. Dosažené výsledky naznačují, že SEDDS by mohl být užitečnou alternativou pro zvýšení biologické dostupnosti špatně rozpustných léčiv.
Klíčová slova:
ibuprofen • samoemulgující systém • fázový diagram • zeta potenciál • protizánětlivá aktivita
Authors: Subhash Chandra Bose Penjuri; Saritha Damineni; Nagaraju Ravouru; Srikanth Reddy Poreddy
Published in the journal: Čes. slov. Farm., 2017; 66, 23-34
Category: Původní práce
Summary
The goal of the present study was to develop a self-emulsifying drug delivery system for the oral poorly water-soluble drug ibuprofen and to evaluate its oral bioavailability. Phase diagrams were constructed to determine the phase behaviour of the microemulsions and to compare the efficiency of various surfactant-oil mixtures. The SEDDS formulations of ibuprofen were prepared from a mixture of Labrafil M2125, Cremophor RH40, and Plurol oleique. The prepared emulsions were characterized for in vitro and in vivo behaviour. A FTIR study confirmed there is no interaction between the drug and excipients. DSC studies showed that the drug is in a solubilised form in the self-emulsifying formulations. The formulations were evaluated for thermodynamic stability, dispersibility, refractive index, viscosity and cloud point. Formulations showed a negative charge on globules which indicates their stability. The optimized formulation produced a microemulsion with a globule size of 177.5 nm. The in vitro release profile of the optimized formulation was significantly higher than that of the marketed formulation and pure drug. The anti-inflammatory activity of the optimized formulation was significantly higher than that of the marketed formulation and pure drug. The AUC and Cmax values after oral administration were higher for the ibuprofen SEDDS in comparison with the marketed product. These results suggest that SEDDS of Ibuprofen can be a useful tool to increase the bioavailability and an alternative to enhance the bioavailability of poorly soluble drugs.
Key words:
ibuprofen • self-emulsifying system • phase diagram • zeta potential, anti-inflammatory activityIntroduction
Oral delivery of poorly water soluble drugs is frequently associated with oral bioavailability of drugs1). One of the most popular approaches to enhance oral bioavailability is lipid-based drug delivery systems (LBDDS). LBDDS offer an excellent platform to improve the bioavailability of BCS class II (low solubility & high permeability) and class IV (low solubility & low permeability) drugs. LBDDS includes oil solutions, emulsions, microemulsions, self-emulsifying/microemulsifying/nanoemulsifying drug delivery system (SEDDS/SMEDDS/SNEDDS) and micellar systems2, 3). SEDDS are proportionate newer LBDDS with huge promise in the enhancement of oral bioavailability of drugs. These formulations avoid a slow and incomplete dissolution of a drug, increase the extent of transportation and bypass the P-gp efflux, thereby strengthening drug absorption from the GI tract4).
Self-emulsifying formulations are isotropic mixtures of oil, surfactant and a cosurfactant (or solubilizer), and a drug, which under gentle agitation have the ability to form fine oil-in-water (o/w) emulsions followed by aqueous phase dilution5).In recent years, medium chain triglyceride oils and nonionic surfactants which are less toxic are being used for the formulation of self-emulsifying drug delivery systems6). Potential advantages of these systems include enhanced oral bioavailability, reduction in dose, consistent temporal profiles of drug absorption, selective targeting of drug(s) towards specific absorption window in the GIT, and protection of drug(s) from the hostile environment in the gut7, 8).
Ibuprofen (2-(4-(2-methylpropyl) phenyl) propanoic acid) is a nonsteroidal anti-inflammatory drug (NSAID) used for relief of symptoms of arthritis and fever. Ibuprofen is a poorly water-soluble drug, and absorption was shown to occur mainly from the intestine and to a lesser, though significant, extent from the stomach9). The dissolution rate of poorly water-soluble drugs often becomes a rate-limiting step in their absorption from the GI tract10, 11). In the present study the objective was to develop self-emulsifying formulations of Ibuprofen and evaluate their physico-chemical properties, in vitro and in vivo release.
Experimental part
Materials
Ibuprofen (IBN) was obtained from Granules India Limited (Hyderabad). Commercial Ibuprofen soft gel capsules were purchased from the United States of America. Mefenamic acid was obtained from Dr. Reddy’s Laboratories Ltd. (Hyderabad). Acetonitrile was purchased from Qualigens fine Chemicals (Mumbai). Castor oil, Propylene glycol and PEG 400 were purchased from Merck (Mumbai). Labrafil M 2125, labrasol, Plurol oleique CC 497, Transcutol P and Labrafac WL 1349 were obtained from Gattefosse India Pvt Ltd. (Mumbai). Oleic acid was purchased from Merck (Mumbai). Soya bean oil and sunflower oil were purchased from Genuine Chemicals Co. (Mumbai). Isopropyl myristate was purchased from Lobachemie Pvt Ltd (Mumbai). Cremophor RH 40 was obtained from BASF (Mumbai). Tween 80 was purchased from Finar Chemicals (Ahmadabad). Water (HPLC grade) was purchased from Qualigens fine Chemicals (Mumbai).
Methods
Solubility study12–14)
Solubility of IBN in oils, surfactants and cosurfactants is an important criterion for the selection of oils/surfactants/cosurfactants in SEDDS. The solubility of IBN in various oils/surfactants/cosurfactants was determined by adding an excess amount of drug in 2 ml of various oils/surfactants/cosurfactants placed in vials and mixed using a vortex mixer. The vials were then sonicated (Vibra Cell, Sonics & Materials, USA) for 2 h to reach equilibrium and the equilibrated samples were centrifuged (Remi equipments, Mumbai, India) at 3000 rpm for 20 min. The samples were then filtered and diluted and IBN concentration was analysed by the UV spectrophotometric method (Shimadzu, Japan).
Emulsification efficiency15)
Equal ratios of oil and surfactant mixtures were taken in a beaker and uniformly homogenized with a gradual rise in temperature up to 37 °C. This mixture was diluted with equal amounts of distilled water in a volumetric flask to yield emulsion. The number of volumetric flask inversions required to yield a homogenous emulsion was recorded. The emulsion was then allowed to stand for equilibrium and its percentage transmittance was determined by a UV-Visible spectrophotometer (Shimadzu, Japan).
Compatibility studies
Fourier transform infrared spectroscopy (FTIR)
Compatibility studies between IBN, oil and Smix (surfactant and cosurfactant) were studied by FTIR (a Perkin Elmer Model 1600, USA). The pure drug and physical mixture were dissolved in dichloromethane separately. By using a capillary tube, a smear/thin film of the samples was placed on a NaCl crystal cell (hexagonal clear, about 2 mm thickness) and sandwiched with another NaCl crystal cell. The NaCl cells with the sandwiched sample were placed in a sample holder. The samples were scanned from 4000 to 400 cm–1 using a FTIR spectrophotometer.
Differential scanning calorimetry (DSC)
Compatibility studies between IBN, oil and Smix were studied by Differential Scanning Calorimetry (a DSC-60, Shimadzu Corporation, Japan). Liquid samples are placed in a dome-shaped sample holder and sealed. Then the sample is placed in the right side pocket, with an empty cell on the left side as a blank. Dry nitrogen was used as the effluent gas. All samples were scanned at a temperature ramp speed of 5 °C/min and the heat flow from 0° to 160 °C.
Construction of pseudoternary phase diagram16)
Pseudoternary phase diagrams were constructed with oil and surfactant/cosurfactant by the water titration method to determine the formation of a microemulsion region, from which a large number of microemulsions could be identified. The trails were conducted using oil and surfactant : cosurfactant in the ratios of 1 : 1 to 1 : 9. The surfactant to cosurfactant weight ratios used were 1 : 1 to 1 : 4 and 4 : 1 to 2 : 1 in the construction of pseudoternary phase diagrams. After each increment of water, the solution was observed visually for transparency and easy flowing microemulsion. Phase diagrams were constructed using Tri-Plot V1-4-2.
Thermodynamic stability studies17)
Heating cooling cycle
Formulations were exposed to 4 °C and 40 °C for about 6 cycles. The formulations were kept at each temperature for not less than 48 h.
Centrifugation test
Formulations were centrifuged at 3500 rpm for 30 min.
Freeze thaw test
Formulations were exposed to –21 °C and 25 °C in a cyclic way for about three times. Formulations were observed physically for turbidity or phase separation at the end of the test period.
Dispersibility test18)
The efficiency of self-emulsification of the formulations was assessed by their dispersibility. One millilitre of each formulation was added to 500 ml of water at 37 ± 0.5 °C. A standard stainless steel dissolution paddle is used with a rotating speed of 50 rpm to provide gentle agitation. The in vitro performance of the formulations is assessed visually as per the grading system as given in Table 1.
Tab. 1. Visual assessment of efficiency of self-emulsification 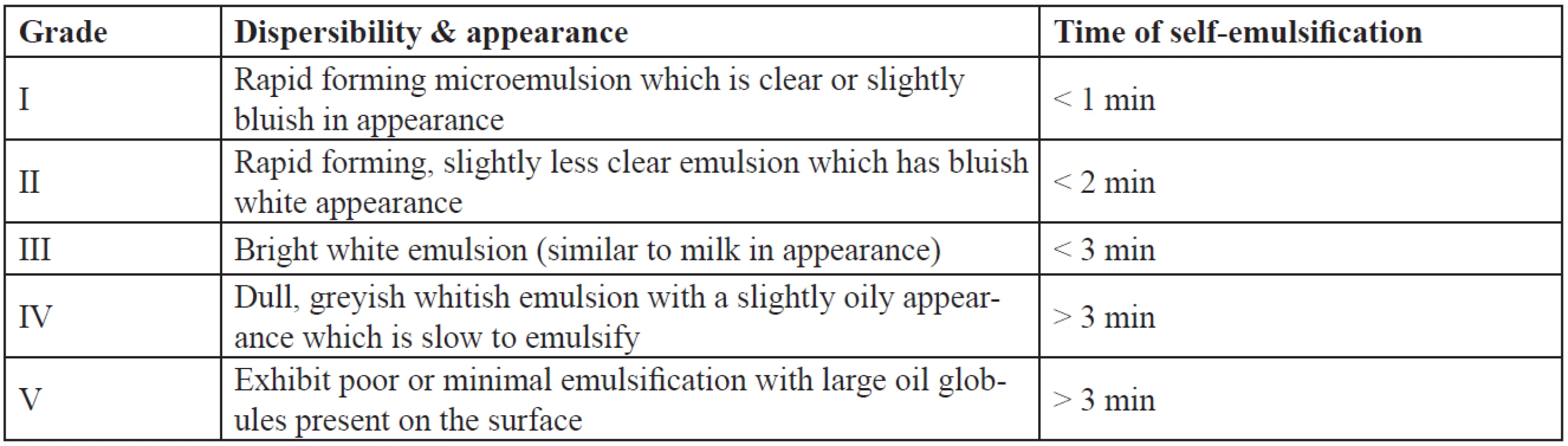
Drug precipitation study19)
IBN SEDDS were taken into 250 ml water and mixed under continuous stirring (100 rpm) on a magnetic stirrer. The formed emulsion was then observed visually after a period of 24 h for the precipitation of the drug. The self-emulsifying systems were then considered as stable (without precipitation) or unstable (with precipitation).
Preparation of SEDDS
Based on the solubility, emulsification ability and pseudo-ternary phase diagram studies excipients were selected. Formulation was limited to only the SEDDS that passed the thermodynamic stability studies, dispersion ability and precipitation study. An accurately weighed amount of IBN was dissolved initially in Labrafil M2125 (Oil). A Cremophor RH 40 (Surfactant) and Plurol oleique CC (Cosurfactant) mixture was accurately weighed and added slowly to the drug-oil mixture. The mixture was heated at 40 ºC on a magnetic stirrer, until IBN was perfectly dissolved. Then the mixture was sealed in a glass capped vial and stored at room temperature until used.
Evaluation of SEDDS
Drug content
The drug from pre-weighed SEDDS is extracted by dissolving in ethanol : water (1 : 1) the drug content was analyzed by a UV visible spectrophotometer at 221 nm (Shimadzu, Japan).
Refractive index and percent transmittance20)
One ml of the formulation diluted to 100 ml with water and a drop of it was placed on an Abbe’s refractometer prism, RI value was determined in the presence of a visible light source (Tungsten lamp). The percent transmittance of formulations was measured at 650 nm using a UV visible spectrophotometer (Shimadzu, Japan). Distilled water was used as blank.
Viscosity19)
0.5 gm of SEDDS was diluted to 10 times with distilled water with constant stirring on a magnetic stirrer and viscosity of the resultant emulsion was measured using a Brookfield LVDL 111 + CP viscometer (Brookfield Engineering Laboratories, Inc., Middleboro, MA, spindle # CPE40) at 5 rpm at 25 ± 1.0 °C.
Effect of pH and robustness to dilution21)
SEDDS were subjected to 100-fold dilution with distilled water, 0.1 N HCl and phosphate buffer pH 6.8. The resultant diluted emulsions were checked for coalescence of globules and phase separation after 24 h storage.
Turbidimetric evaluation22, 23)
Equal quantity of SEDDS and 0.1 N HCl was mixed under continuous stirring (50 rpm) on a magnetic hot plate at 37 ± 0.5 °C temperature. The turbidity was measured by using a turbidimeter (Digital nephelo-turbidity meter 132, Systronics, India) until equilibrium was reached. The increase in turbidity was measured until there was no further change in turbidity.
Cloud point measurement15)
The cloud point value of optimized formulations was determined and compared. Each formulation was diluted with water in the ratio of 1 : 100 and placed in a water bath with a gradual increase (2 °C/min) in temperature (from 25 to 80 °C). Cloud point was measured as the temperature at which there was a sudden appearance of cloudiness as seen visually.
Particle size analysis and homogeneity of SEDDS24)
The mean globule size, zeta potential and polydispersity index of SEDDS were measured by a photon correlation spectroscopy instrument (Zetasizer 3000HS, Malvern Instruments Corp., UK) at 25 °C. For measurement, SEDDS was pre-diluted by an addition of 0.6 ml of SEDDS to 90 ml of distilled water under slow agitation at room temperature (25 °C). The system was analyzed by dispersing it in 100 ml distilled water as the dispersant. Analysis was done in triplicate and the mean results are presented. The sizing and zeta potential of the SEDDS was determined in a small volume module. Samples were directly placed into the module and the data were collected for 10 minutes. All studies were repeated in triplicates, with good agreement being found between the measurements.
In vitro release study25)
The in vitro release of the optimized formulations (capsules filled with SEDDS) and the marketed product, each containing 200 mg of IBN, were carried using a USP type II dissolution apparatus (Model No TDT-08L, Electrolab, Mumbai) in pH 7.2 phosphate buffer (dissolution medium) maintained at 37 ± 0.5 °C with the paddle rotating at 50 rpm. Samples (1 ml) were withdrawn at regular intervals and the same volume of fresh dissolution medium was replaced to maintain sink condition. The samples were filtered, diluted and analyzed UV spectrophotometrically at 221 nm (Shimadzu, Japan). Dissolution studies were performed and the mean cumulative percentage of IBN was calculated and plotted against time. In vitro dissolution data were statistically analysed by a one-way ANOVA followed by a turkey post hoc test for multiple comparison using Graph Pad Prism. Differences were considered to be significant at a level of p < 0.05. The dissolution profiles of the optimized formulations and the marketed product were compared on the basis of their similarity factor (f2) and difference factor (f1).
The equations used are
f1 (Difference factor) = {[St =1n (Rt–Tt)]/ [St =1n Rt]} ×100
f2 (Similarity factor) =
50 × log {[1+ (1/n) St =1n (Rt–Tt)2]-0.5 ×100
Rt = Cumulative percentage dissolved of reference (marketed) product at time t
Tt = Cumulative percentage dissolved of test (prepared) formulation at time t
n = number of time points
Pharmacodynamic studies
Anti-inflammatory studies26, 27)
The anti-inflammatory activity of the optimized formulation in comparison to the marketed formulation was evaluated by the carrageenan-induced rat hind paw edema method by using a Digital plethysmometer (PLM-01 plus, Orchid Scientifics, India). The study protocol was approved by IAEC (Reg. No. IAEC/SUCP/08/2013). The animals were divided into three groups (six animals in each group) for anti-inflammatory studies.
Group I: Inflammation-induced and vehicle-treated control.
Group II: Inflammation-induced and formulated SEDDS (IBN) administered animals – 20 mg/kg body weight.
Group III: Inflammation-induced and marketed product (IBN) administered animals – 20 mg/kg body weight.
Wistar strain male albino rats weighing between (150–200 g) were used for the study. The animals were in a light-controlled 12 hours cycle with free access to food and water. Animals were fasted overnight before the experiment with free access to water. After one hour, paw edema was induced by injecting 50 µl of 1% w/v carrageenan into the subplanar region of the left hind paw. Paw volume was determined after five hours in all groups. The difference in the paw volume, determined before and after injection of the edema-provoking agent indicated the severity of edema. The volumes of the right hind paw of the controls and the treated animals were measured with a plethysmometer and the percentage inhibition of inflammatory reaction was determined for each animal by comparison with the control and calculated by the following formula.
where, Vcontrol = mean edema of rats in control group, Vtest = mean edema volume of rats in tested group
In vivo evaluation
Pharmacokinetic study28–32)
Pharmacokinetic study protocol was approved by the IAEC (Reg. No. IAEC/MNRCOP/08/2014). The oral pharmacokinetics of IBN was assessed in Wistar rats. Animals were fasted overnight with free access to water for at least 12 h before dosing. A single oral dose was given after collecting the zero-hour blood sample, and the animals were anesthetized and blood samples were withdrawn from the tail vein and placed in heparinised centrifuge tubes. The plasma was then separated and assayed for the concentration of IBN by the HPLC method.
Table 2 shows the specifications of animals used for the in vivo study. The pharmacokinetic parameters were calculated using the WinNonlin software. The animals were divided into three groups:
Group I: IBN suspension (pure IBN in 1% CMC) administered animals.
Group II: IBN-C1 administered animals.
Group III: Marketed product administered animals.
Tab. 2. Animal specifications for in vivo study 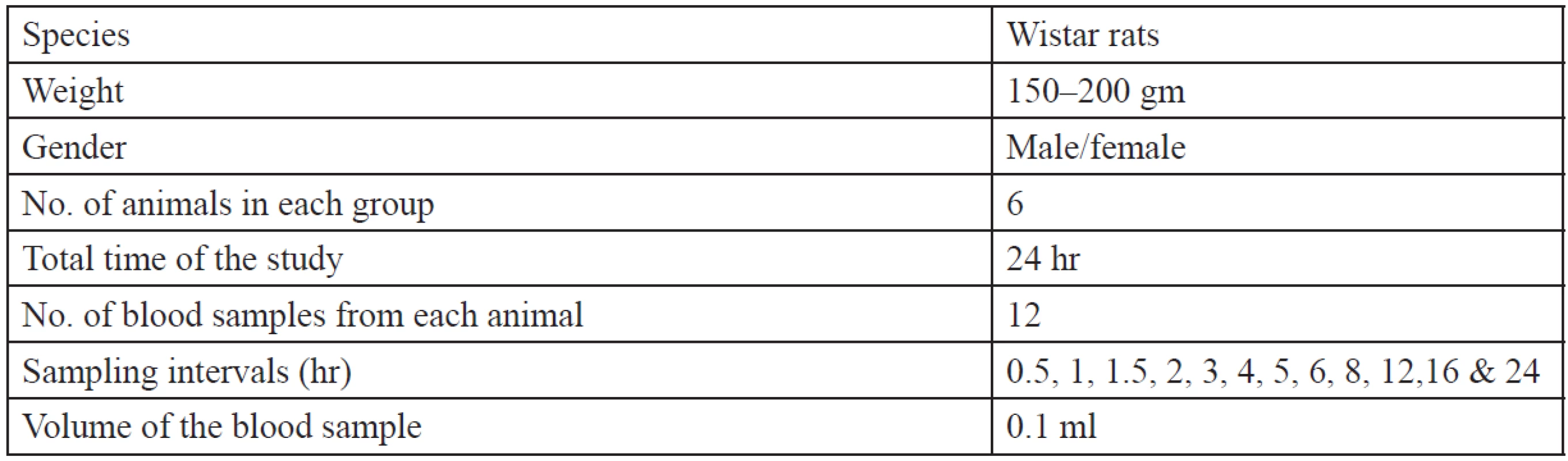
Stability study33, 34)
Stability studies were conducted according to the ICH Q1A (R2) guidelines. Formulations were packed in a screw capped bottle and were kept in a stability chamber at a temperature of 40 ± 2 ºC and 75 ± 5% RH for 6 months. Samples were withdrawn at the end of months 1, 2, 3 and 6 and analyzed for the drug content, homogeneity, clarity, globule size and release. Zero-time samples were used as the control for the study.
Results and discussion
Solubility of IBN in oils and surfactants
Solubility of IBN in various vehicles is an important parameter as the SEDDS should be clear and monophasic. The solubility of IBN in various oils, surfactants and cosurfactants is shown in Table 3. Among the oils, labrafil M2125 showed the maximum solubility which can be due to its lipophilicity (HLB 3–4), and was selected as the oil phase. The solubility of IBN in the surfactant and oil is important as the maximum solubilisation of the drug should occur in these phases. Cremophor RH 40 and labrasol, which showed good solubility, were selected as surfactants for further study. Cosurfactants on dilution with water may separate out resulting in micellar dispersion, thus reducing the solvent capacity for IBN35), so all the cosurfactants were further tested.
Tab. 3. Solubility of IBN in various oils/surfactants 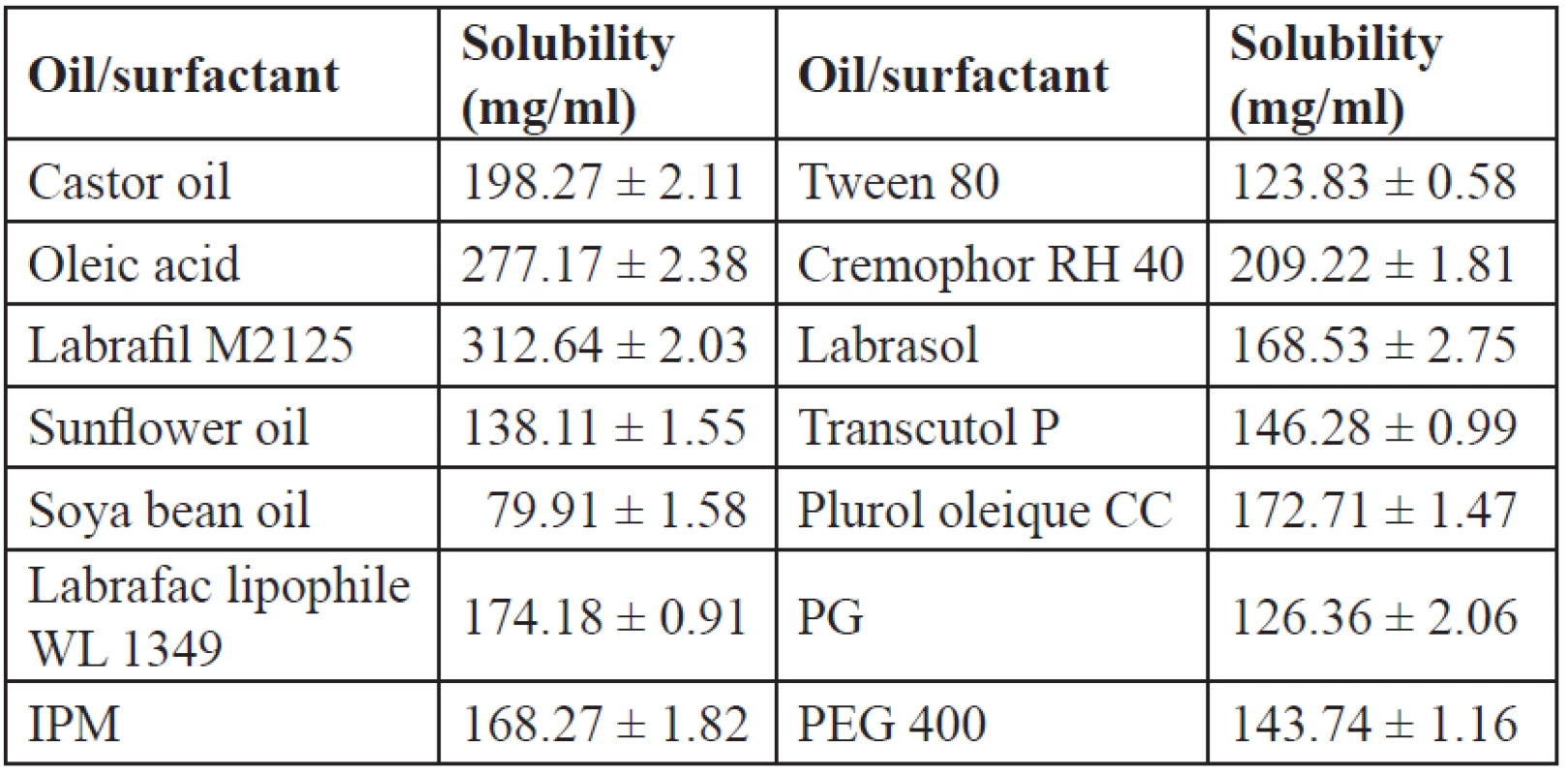
Mean ± SD, n = 3 Emulsification ability
The oil, surfactant and cosurfactants which showed good solubility were tested for their emulsification ability. Labrafil M2125 showed good solubility of IBN which helps to prevent precipitation in the intestinal fluids, so the emulsification efficiency of this oil is studied with the surfactants cremophor RH 40, labrasol and cosurfactants plurol oleique CC, PEG 400, transcutol P, PG. The emulsification efficiency results as indicated by inversions and percentage transmittance are given in Table 4. Better emulsification efficiency is given by a lesser number of inversions. The number of inversions required for cremophor RH 40, labrasol, plurol oleique CC, PEG 400, transcutol P and PG was found to be 7, 10, 8, 10, 13 and 15, respectively. From the emulsification efficiency and percentage transmittance studies, cremophor RH 40 showed a lesser number of inversions with 98.3% transmittance than labrasol. Among cosurfactants, plurol oleique CC showed 96.9% transmittance, while transcutol P has even good solubility of IBN and showed only 86.2% transmittance and required more number of inversions to attain homogeneity. So for the formulations, cremophor RH 40 was selected as the surfactant with plurol oleique as the cosurfactant.
Tab. 4. Emulsification efficiency of surfactants and cosurfactants with labrafil M2125 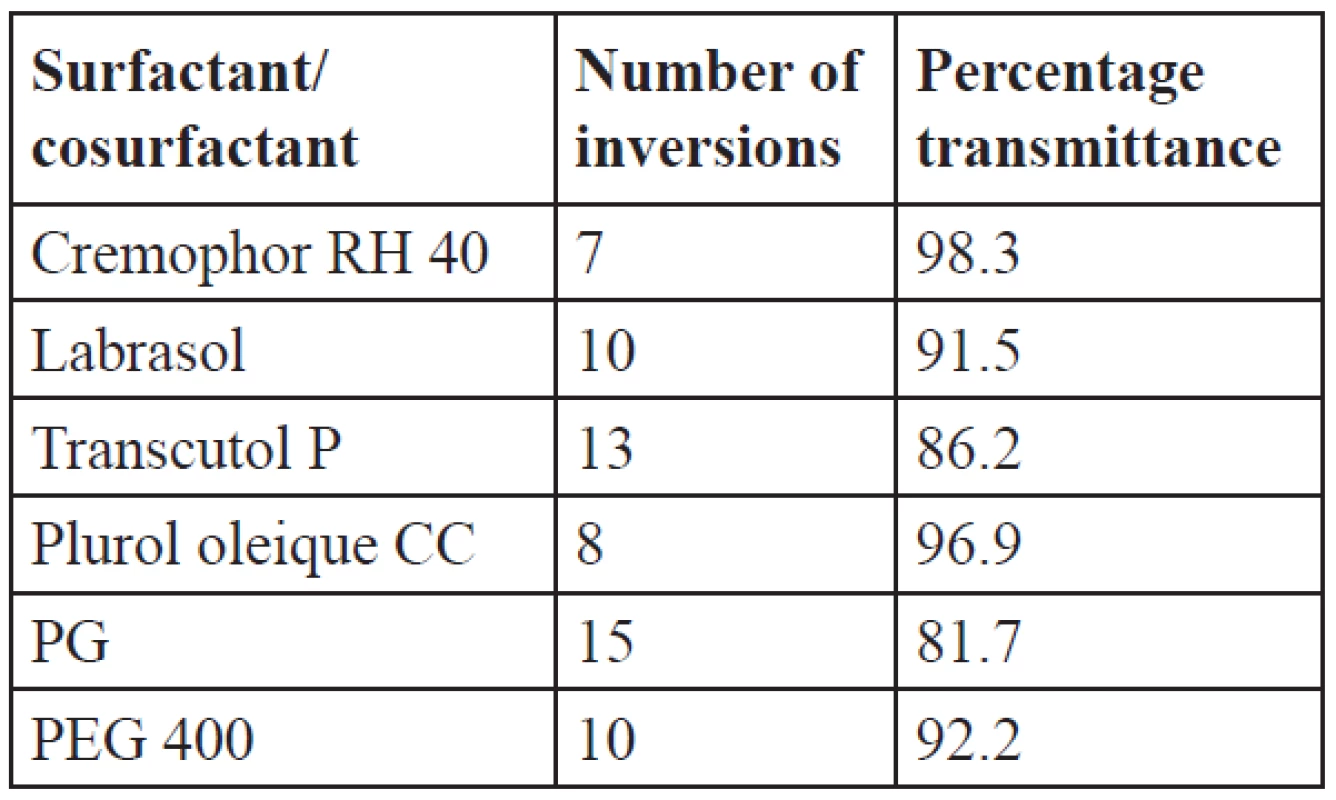
Compatibility studies
The FTIR spectra of IBN alone and the physical mixture are shown in Figure 1. The characteristic peaks of IBN are unchanged and prominently observed in the FTIR spectra of the physical mixture suggesting that there was no interaction between IBN and the selected oils and surfactants.
Fig. 1. FTIR spectra of IBN (A) and physical mixture (B) 
DSC thermograms of pure IBN and the physical mixture (Fig. 2) showed a sharp melting peak for IBN, at 77 °C. A small endothermic peak with low intensity was observed at 77 °C in the physical mixture, which may be due to reduction in the drug crystallinity and molecular dispersion of IBN in the lipid excipients.
Fig. 2. DSC thermograms of IBN (A) and physical mixture (B) 
Pseudo ternary phase diagrams were constructed with the selected excipients to know the self-emulsification region. Further from the study, formulations with Smix 1 : 1 to 3 : 1 and 2 : 1 ratios were developed for further characterization.
Thermodynamic stability studies
The stability of the formulations affects the performance and the instability may result in precipitation or phase separation. The effect of metastable forms, which are difficult to find, can be eliminated by thermodynamic studies. The formulations were subjected to centrifugation, heating cooling cycles, a freeze-thaw test. The results are shown in Table 5. The concentration of the surfactant and the cosurfactant has varied effects and is important for a stable microemulsion formation. Formulations which have passed the thermodynamic stability study, dispersion test and drug precipitation study were optimized and further evaluated. IBN-A1, IBN-B1, IBN-B2, IBN-C1, IBN-C2 were selected as optimized formulations as they passed the stability study.
Tab. 5. Results of thermodynamic stability studies & visual dispersion grading of IBN SEDDS 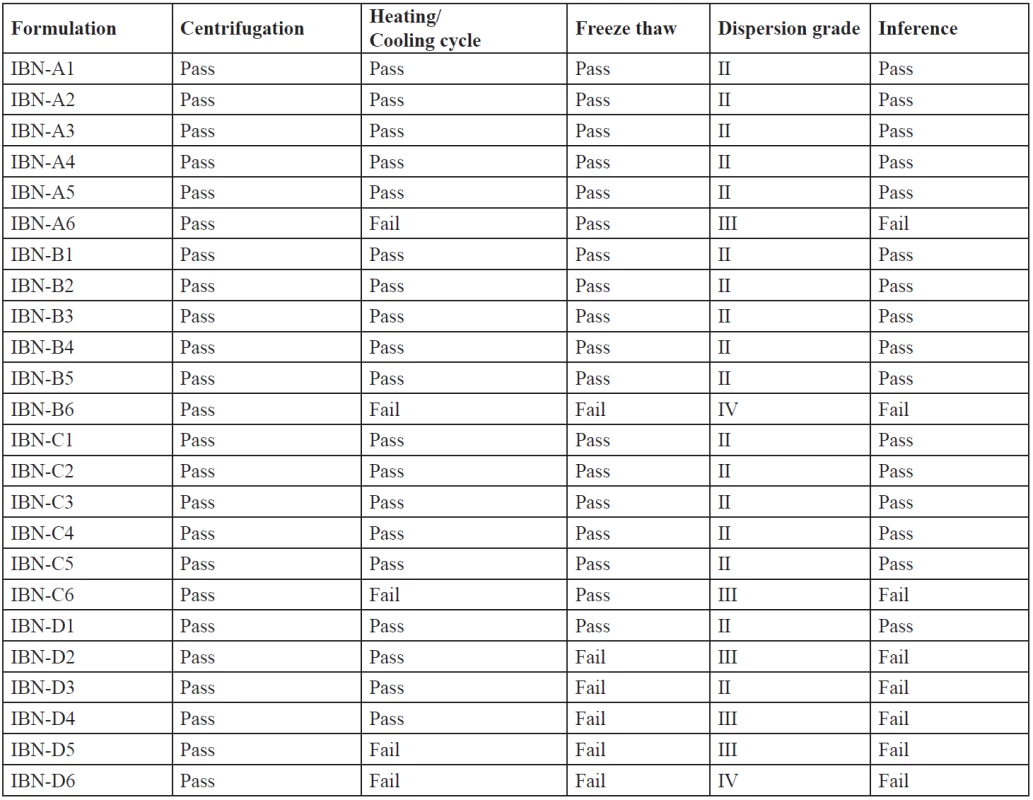
An efficient SEDDS formulation disperses very fast in seconds provided the condition of gentle stirring36). The dispersion rate depends on the interfacial barrier at the oil and dilution medium interface. Cremophor RH 40 and plurol oleique CC at ratios 1 : 1, 2 : 1, 3 : 1 has good dispersion which can be due to the formation of a stable interfacial film of surfactant as a monolayer on the emulsion droplets. While 1 : 2 showed poor dispersion ability which may be due to high cosurfactant concentration, which disperses in the water phase and may cause loss of solvent capacity37). From the thermodynamic stability study and drug precipitation analysis results five formulations were prepared (Table 6) and further evaluated.
Tab. 6. Composition of optimized IBN formulations 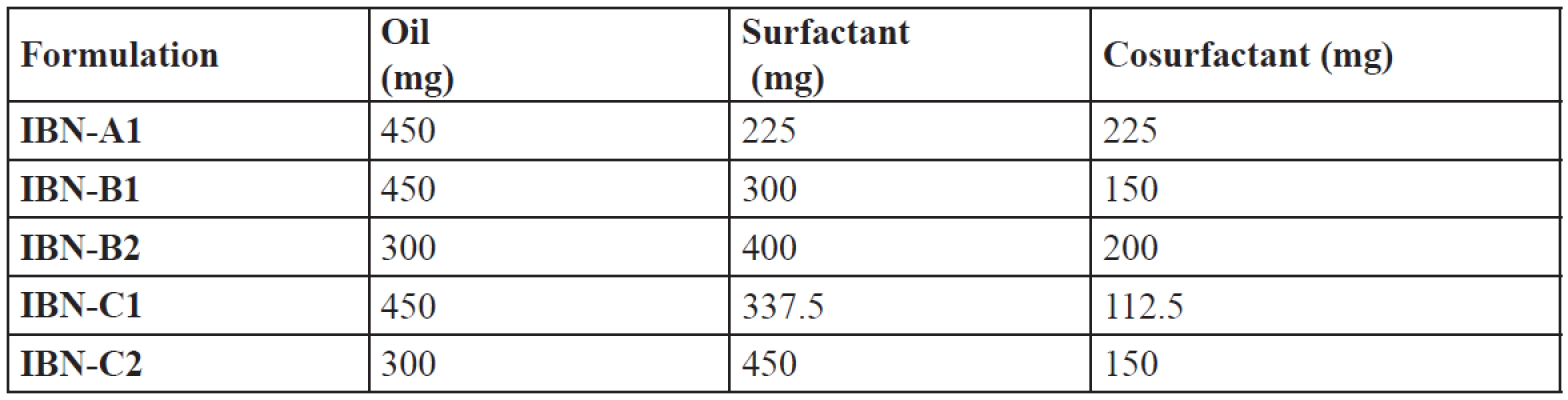
Evaluation of SEDDS
All the formulations have their drug content in the range of 98.94 ± 1.73 – 100.65 ± 0.93% (Table 7), which showed uniformity of drug in the formulations. The refractive index of the formulations was in the range of 1.433 ± 0.02 – 1.437 ± 0.03, nearly the same as that of water. The percentage transmission was found to be in the range of 95 to 97 (Table 7). So the prepared formulations were transparent and clear dispersions with maximum transmission. Viscosity of the system plays an important role in drug release. The lower viscosity values (Table 7) showed that the systems resulted in o/w emulsions. All the formulations were stable when diluted to 100 times with water, 0.1N HCl and pH 6.8 phosphate buffer. There was no phase separation or precipitation which indicates that the non-ionic surfactants used were stable to change in pH and the concentration of the electrolyte38).
Tab. 7. Drug content, refractive index, percentage transmission and viscosity of IBN SEDDS 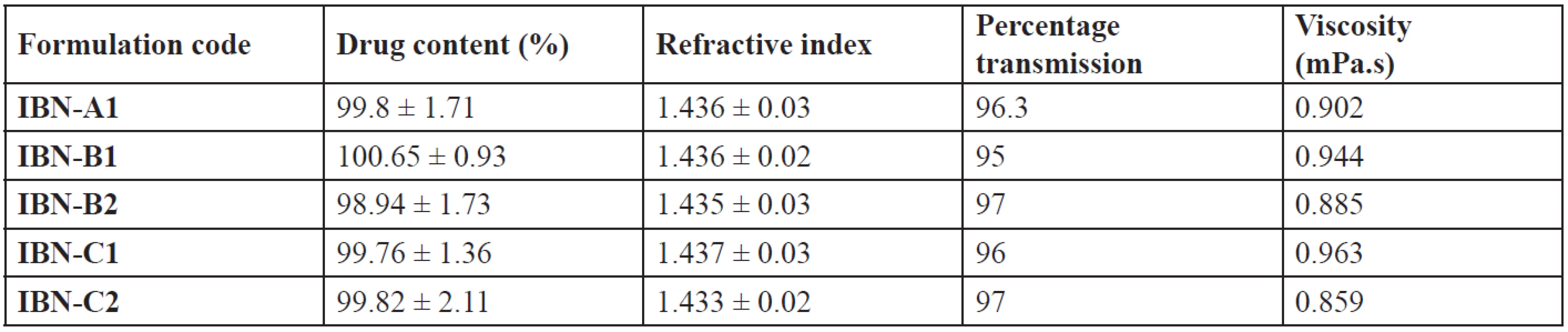
Mean ± SD, n = 3 The turbidity attributes to the clarity and uniformity of emulsification and hence stability of the formulations. A measure of turbidity also gives the knowledge of globule size39). The lower values (Table 8) indicate that the prepared systems were clear and may have a lower globule size40). At a temperature higher than the cloud point, formulation may undergo phase separation which is due to dehydration of polyethylene oxide moiety of the non-ionic surfactant. So, the cloud point of the formulation should be more than 37 °C. IBN formulations showed the cloud point at high temperature > 37 °C (Table 8), indicating stability at physiological temperature15).
Tab. 8. Globule size, PDI, zeta potential, turbidity and cloud point of IBN SEDDS 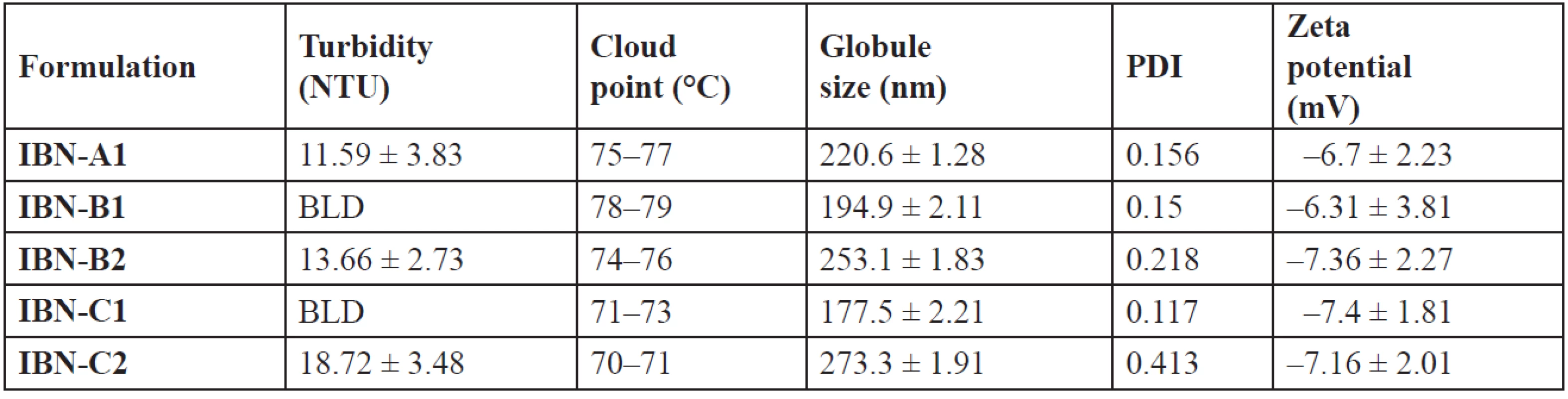
Mean ± SD, n = 3, BLD – Below limit of detection Emulsion globule size
The globule size of emulsions is an important factor which determines the drug release and hence absorption. Diffusion of a drug can be faster from smaller emulsion globules, which increases dissolution of the drug into aqueous medium5). The globule size was in the range of 177.5 nm to 273.3 nm (Table 8). The ratio of the oil, surfactant and cosurfactant mixture has varied effects on the size of globule. With an increase in the cremophor/plurol oleique ratio the globule size decreased41). The decrease in size may be due to the stabilisation of globules of oil, which can be due to the formation of a monolayer of surfactant molecules at the interface of oil-water3).
Fig. 3. Pseudoternary phase diagram showing the o/w emulsion region of labrafil, cremophor & plurol oleique at various Smix 
IBN-B2 and IBN-C2 showed a further increase in globule size than IBN-A1, which may be due to a higher surfactant concentration. The globule size of IBN-C1 is shown in Fig. 4. An increase in the amount of the surfactant results in more penetration of water into the emulsion globules resulting in larger sized globules8). The polydispersibility index of the formulations was less than 1 indicating the uniformity of size distribution within the formulation42). The globules were negatively charged (Fig. 5) and the zeta potential values were in the range of –6.31 to –7.4 mV (Table 8).
Fig. 4. Globule size data of IBN-C1 
The negative charge on the globules of all formulations attributes their stability, by causing repulsion between them and thus preventing their coalescence43). The negative charge may be due to the presence of surfactant/cosurfactant.
Fig. 5. Zeta potential data of IBN-C1 
In vitro release
The in vitro dissolution profile of pure IBN, IBN-A1, IBN-B1, IBN-B2, IBN-C1 and IBN-C2 was evaluated in pH 7.2 phosphate buffer and was shown in Figure 6. The faster release of IBN from SEDDS may be due to the dissolved state of the drug, the spontaneous formation of microemulsion and a smaller globule size as compared to plain IBN. The percentage release decreased with the increase in the size of the emulsion droplet. The formulation IBN-C1 showed the maximum release of 99.87 ± 1.98%, which may be due to its oil and surfactant mixture concentration ratios, which lead to rapid emulsification of oil for finer globules.
Fig. 6. In vitro dissolution profiles of IBN SEDDS 
A nano-sized globule provides a larger effective surface area for diffusion of solubilised IBN from SEDDS to dissolution media44). The SEDDS formulations were significantly different from pure IBN. Further in vitro release of IBN-C1 was carried out in 0.1N HCl in comparison to that of phosphate buffer pH 7.2. The release of IBN was 99.87 ± 1.98% and 96.97 ± 2.08% in phosphate buffer pH 7.2 and 0.1N HCl, respectively, at the end of 60 min.
The dissolution profiles were comparable (Fig. 7) showing no effect on the release of IBN with a change in pH in the gastrointestinal tract. The data were analyzed by t-test and a significant difference (p < 0.05) was observed between the means at 20, 30, 40 and 60 minutes.
Fig. 7. In vitro dissolution profile of IBN-C1 in pH 7.2 phosphate buffer & 0.1 N HCl 
Comparative dissolution profiles of IBN-C1 and the marketed product were carried out. The study showed that IBN-C1 and the marketed product has 99.87 ± 1.98 and 98.61 ± 1.73 release at the end of 60 min (Fig. 8).
Fig. 8. In vitro dissolution profile of IBN-C1 & marketed product 
The data were analyzed by the t-test and a significant difference was observed. The similarity factor f2 for IBN C1 and the marketed soft gelatin capsule was found to be 4% and the difference factor f1 was 71%. This indicates the dissolution profile of the optimized IBN SEDDS is different from the marketed product, which signifies that the optimized SEDDS shows an effective performance than the marketed formulation.
Anti-inflammatory activity
Anti-inflammatory activity was carried out by carrageenan-induced paw edema in rats. The anti-inflammatory activity of IBN-C1 was compared with the marketed product. The percentage inhibition and paw edema in rats showed a significant inhibition (p < 0.001) compared to control. Percentage inhibition of IBN-C1 and marketed product was found to be 86.45 ± 5.58 and 74.27 ± 5.6, respectively. IBN-C1 and the marketed product showed the maximum activity at the end of the 5th hour of single oral dose administration due to an increased absorption of IBN from lipids.
Pharmacokinetic studies
After single oral dose administration, the mean plasma concentration–time data of pure IBN, IBN-C1 and the marketed product were compared and are shown in Figure 9. The pharmacokinetic parameters of IBN are summarized in Table 9. The plasma level profiles were significantly increased for IBN-C1 and the marketed product compared to pure IBN. The mean plasma concentration–time profiles of IBN-C1 and the marketed product were found to be super imposable. The Cmax of IBN-C1 and the marketed product was 2.55 and 2.3 times higher than that of pure IBN, respectively. The Cmax was found to be 18.36 ± 8.88, 46.78 ± 12.94 and 42.26 ± 13.46 µg/ml for pure IBN, IBN-C1 and the marketed product, respectively. The AUC of IBN-C1 and the marketed product was 3.61 and 3.18 times higher than that of pure IBN, respectively. The AUC was found to be 64.30 ± 23.58, 232.17 ± 67.51 and 205.73 ± 57.61 µg/ml for pure IBN, IBN-C1 and the marketed product, respectively.
Fig. 9. Mean plasma concentration – time profiles of IBN 
Tab. 9. Pharmacokinetic parameters of IBN in rats after single oral dose of 20 mg/kg 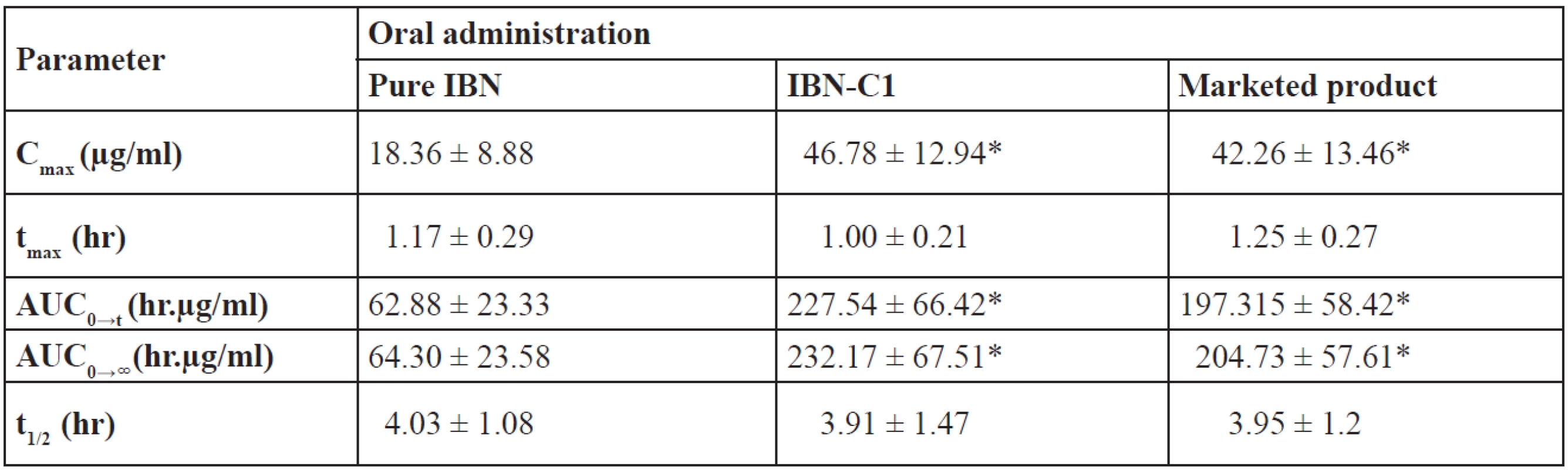
Mean ± SD, n = 6, *p < 0.05 vs. pure IBN The low AUC value of pure IBN may be due to less absorption into the body. On the contrary, IBN-C1 and the marketed product showed high AUC values indicating an increased/better absorption of the drug. The AUC of IBN-C1 and the marketed product was found to be significantly higher than pure IBN indicating an improvement in the bioavailability of IBN from SEDDS. The pharmacokinetic parameters of IBN C1 were better as compared with the marketed product and so it was in accordance with the in vitro dissolution profiles.
Stability study
Stability studies conducted showed there was no change in the physical parameters such as homogeneity and clarity. A slight increase in the globule size of 197.1 nm has been observed which may be due to aggregation of globules. The PDI was less than 1, which indicates uniformity of globules. The drug content and dissolution behaviour of the optimised formulation was well within the limits during storage and a significant difference (p < 0.05) was observed between the means of drug content at the 3rd and 6th months.
Conclusion
Ibuprofen is a non-steroidal anti-inflammatory drug which is poorly soluble in water. Most of the oral formulations show less absorption which may be due to solubility problem. In the present study prepared self-emulsifying formulations showed an enhanced release when compared to the marketed product. SEDDS formed microemulsion dispersion of the size 177.5 nm to 273.3 nm rapidly, showing good emulsification. The anti-inflammatory activity and pharmacokinetic study showed an improved activity as compared to pure IBN and the marketed product, due to solubilization of the drug by the self-emulsification process. Further results show a possible chance of improvement in the bioavailability of Ibuprofen with the formulated SEDDS. Thus this technique helps in improving solubility and can be applied to similar drugs used in the treatment of various diseases.
Received December 13, 2016
Accepted February 10, 2017
Conflict of interest: none.
Subhash Chandra Bose Penjuri • Srikanth Reddy Poreddy
Department of Pharmaceutics, MNR College of Pharmacy
Sangareddy 502 294, Telangana State, India
e-mail: penjurisubhash@gmail.com
Saritha Damineni
Department of Pharmaceutics, Sultan ul Uloom College of Pharmacy, Telangana State, India
Nagaraju Ravouru
Department of Pharmaceutics, Sri Padmavati Mahila Visvavidyalayam, Andhra Pradesh, India
Zdroje
1. Quan D. Q., Xu G. X., Wu X. G. Studies on preparation and absolute bioavailability of a self-emulsifying system containing puerarin. Chem. Pharm. Bull. 2007; 55, 800–803.
2. Humberstone A. J., Charman W. N. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv. Drug. Deliv. Rev. 1997; 25, 103–128.
3. Gursoy R. N., Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004; 58, 173–182.
4. Porter C. J., Charman W. N. Intestinal lymphatic drug transport: An update. Adv. Drug. Deliv. Rev. 2001; 50, 61–80.
5. Shah N. H., Carvajal M. T., Patel C. I., Infeld M. H., Malick A. W. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int. J. Pharm. 1994; 106, 15–23.
6. Pradeep Patil., Prasad Joshi., Anant Paradkar. Effect of formulation variables on preparation and evaluation of gelled self-emulsifying drug delivery system (SEDDS) of ketoprofen. AAPS. PharmSciTech. 2004; 5, 1–8.
7. Pouton C. W., Charman W. N. The potential of oily formulations for drug delivery to the gastro-intestinal tract. Adv. Drug. Deliv. Rev. 1997; 25, 1–2.
8. Pouton C. W. Formulation of self-emulsifying drug delivery systems. Adv. Drug. Deliv. Rev. 1997; 25, 47–58.
9. Kapsi S. G., Ayres J. W. Processing factors in development of solid solution formulation of itraconazole for enhancement of drug dissolution and bioavailability. Int. J. of Pharm. 2001; 229, 193–203.
10. Maeda T., Takenaka H., Yamahira Y., Noguchi T. Use of rabbits for GI drug absorption studies: Relationship between dissolution rate and bioavailability of griseofulvin tablets. J. Pharm. Sci. 1979; 68, 1286–1289.
11. Chiba Y., Kohri N., Iseki K., Miyazaki K. Improvement of dissolution and bioavailability for mebendazole, an agent for human echinococcosis, by preparing solid dispersion with polyethylene glycol. Chem. Pharm. Bull. 1991; 39, 2158–2160.
12. Prabagar Balakrishnan, Beom-Jin Lee, Dong Hoon Oh, Jong Oh Kim, Myung Ja Hong, Jun-Pil Jee, Jung Ae Kim, Bong Kyu Yoo, Jong Soo Wooa, Chul Soon Yong, Han-Gon Choia. Enhanced oral bioavailability of dexibuprofen by a novel solid self-emulsifying drug delivery system (SEDDS). Eur. J. Pharm. Biopharm. 2009; 72, 539–545.
13. Indian Pharmacopoeia, Ministry of Health and Family welfare, The Indian Pharmacopoeia commission, Ghaziabad, 6th edition., Vol I, 2010; 559–560.
14. Hong J. Y., Kim J. K., Song Y. K., Park J. S., Kim C. K. A new self-emulsifying formulation of itraconazole with improved dissolution and oral absorption. J. Control. Release. 2006; 110, 332–338.
15. Avachat A. M., Patel V. G. Self nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi. Pharm. J. 2015 : 23, 276–289.
16. Shafiq-un-Nabi S., Shakeel F., Talegaonkar S., Ali J., Baboota S., Ahuja A., Khar R. K., Mushir A. Formulation development and optimization using nanoemulsion technique: A technical note. AAPS. PharmSciTech. 2007; 8, E1–E6.
17. Sheikh S., Shakeel F., Talegaonkar S., Ahmad F. J., Khar R.K., Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007; 66, 227–243.
18. Khoo S. M., Humberstone A. J., Porter C. J., Edwards G. A., Charman W. N. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int. J. Pharm. 1998; 167, 155–164.
19. Patel D., Sawant K. K. Oral Bioavailability Enhancement of Acyclovir by Self Microemulsifying Drug Delivery Systems (SMEDDS). Drug. Dev. Ind. Pharm. 2007; 33, 1318–1326.
20. Ramadan E., Borg T. H., Abdelghani G. M., Saleh N. M. Formulation and evaluation of acyclovir microemulsions. Bull. Pharm. Sci. 2013; 36, 31–47.
21. Kallakunta V. R., Bandari S., Jukanti R., Veerareddy P. R. Oral self-emulsifying powder of lercanidipine hydrochloride: formulation and evaluation. Powder. Technol. 2012; 221, 375–382.
22. Taha E. I., Al-Saidan S., Samy A. M., Khan M. A. Preparation and in vitro characterization of self-nanoemulsified drug delivery system (SNEDDS) of all-trans-retinol acetate, Int. J. Pharm. 2004; 285, 109–119.
23. Nazzal S., Nutan M., Palamakula A., Shah R., Zaghloul A. A., Khan M. A. Optimization of a self-nanoemulsified tablet dosage form of Ubiquinone using response surface methodology: effect of formulation ingredients. Int. J. Pharm. 2002; 240, 103–114.
24. Cho H. J., Ku W. S., Termsarasab U., Yoon I., Chung C. W., Moon H. T., Kim D. D. Development of udenafil-loaded microemulsions for intranasal delivery: In vitro and in vivo evaluations. Int. J. Pharm. 2012; 423, 153–160.
25. Damineni Saritha., Penjuri Subhash Chandra Bose., Ravouru Nagaraju. Formulation and evaluation of self-emulsifying drug delivery system (SEDDS) of ibuprofen. Int. J. Pharm. Sci. Res. 2014; 5, 3511–3519.
26. Lichtenberger L. M., Romero J. J., Dial E. J., Moore J. E. Naproxen-PC: A GI safe and highly effective anti-inflammatory. Inflammopharmacology 2009; 17, 1–5.
27. Liles J. H., Flecknell P. A. The use of non-steroidal anti-inflammatory drugs for the relief of pain in laboratory rodents and rabbits. Laboratory. Animals. 1992; 26, 241–255.
28. Gola Shefali., Malhotra Anand S., Gupta Asheesh. RP-HPLC assay of ibuprofen in plasma using different extraction procedures. Int. J. of. Bio. Res. 2011; 2, 353–358.
29. Wang X. L., Han J., Zhang D., Liu H. C. Pharmacokinetics of ibuprofen enantiomers in rats after intravenous and oral administration of ibuprofen arginate. Yao. Xue. Xue. Bao. 2012; 47, 88−93.
30. Canapro R., Muntoni E., Zara G. P., Della Pepa C., Berno E., Costa M., Eandi M. Determination of Ibuprofen in human plasma by high-performance liquid chromatography: validation and application in pharmacokinetic study. Biomed. Chromatogr. 2000; 14, 219–226.
31. Minkler P. E., Hoppel C. L. Determination of ibuprofen in human plasma by high-performance liquid chromatography. J. Chromatogr. 1988; 428, 388–394.
32. Ting Li, Liang Fang, Changshun Ren, Manli Wang, Ligang Zhao. Determination of transdermally and orally applied indomethacin in rat plasma and excised skin and muscle samples. AJPS. 2008; 3, 269–275.
33. Bachhav Y. G., Patravale V. B. SMEDDS of glyburide: formulation, in vitro evaluation, and stability studies. AAPS. PharmSciTech. 2009; 10, 482–487.
34. Patel A. R., Vavia P. R. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007; 9, E344–E352.
35. Pouton C. W. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and self-microemulsifying drug delivery systems. Eur. J. Pharm. Sci. 2000; 11, S93–108.
36. Pouton C. W., Porter C. J. H. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv. Drug. Deliv. Rev. 2008; 60, 625–637.
37. Pouton C. W. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006; 29, 278–287.
38. Osborne D. W., Middleton C. A., Rogers R. L. Alcohol-Free Microemulsions. J. Dispers. Sci. Technol. 1988, 415–423.
39. Groves M. J., Mustafa R. M. Measurement of the spontaneity of self emulsifiable oils. J. Pharm. Pharmcol. 1974; 26, 671–681.
40. Pouton C. W. Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int. J. Pharm. 1985; 27, 335–348.
41. Zhong-gao, Han-Gon Choi, Hee-Jong Shin, Kyung-Mi Park, Soo-Jeong Lim, Ki-Jun Hwang, Chong-Kook Kim. Physicochemical characterization and evaluation of a microemulsion system for oral delivery of cyclosporin A. Int. J. Pharm. 1998; 161, 75–86.
42. Zhang P., Liu Y., Feng N., Xu J. Preparation and evaluation of self-emulsifying drug delivery system of oridonin. Int. J. Pharm. 2008; 355, 269–276.
43. Roland I., Piel G., Delattre L., Evrard B. Systematic characterization of oil - in - water emulsions for formulation design. Int. J. Pharm. 2003; 263, 85–94.
44. Ashish Deshmukh., Shirishkumar Kulkarni. Novel self micro-emulsifying drug delivery systems (SMEDDS) of efavirenz. J. Chem. Pharma. Res. 2012; 4, 3914–3919.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2017 Číslo 1- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Cytotoxické, protirakovinné a antimikrobiální účinky extraktů z Artemisia rupestris
- Samoemulgující systém (SEDDS) pro podání léčiva ibuprofen: hodnocení vzorků v podmínkách in vitro a in vivo
- Zdravotnické a nezdravotnické náklady na léčbu a péči Parkinsonovy choroby – srovnání Evropy, USA, Asie a Austrálie
- Osudy židovských farmaceutů z českých zemí během holocaustu
- Sympozia Sekce dějin farmacie ČFS v roce 2016
- Studie složení karboxylových kyselin rhizomů Iris medwedewii a Iris carthaliniae (Iridaceae) pomocí plynové chromatografie ve spojení s a hmotnostní spektrometrií
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Osudy židovských farmaceutů z českých zemí během holocaustu
- Zdravotnické a nezdravotnické náklady na léčbu a péči Parkinsonovy choroby – srovnání Evropy, USA, Asie a Austrálie
- Samoemulgující systém (SEDDS) pro podání léčiva ibuprofen: hodnocení vzorků v podmínkách in vitro a in vivo
- Cytotoxické, protirakovinné a antimikrobiální účinky extraktů z Artemisia rupestris
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




