-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEstimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
Authors: Ľubica Havranová Sichrovská 1; Lukáš Stanzel 1; Ivan Malík 1; Matej Maruniak 1; Iva Kapustíková 1; Eva Sedlárová 1; Jozef Csӧllei 2
Authors place of work: Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Slovak Republic 1; Department of Chemical Drugs, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic 2
Published in the journal: Čes. slov. Farm., 2015; 64, 276-278
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Lipophilicity is considered to be a very important molecular descriptor. It plays a crucial role in determining pharmacokinetic ADMET-properties (absorption, distribution, metabolism, excretion, and toxicity) and the pharmacodynamic profile, which correlates well with their bioactivity in the end1). Successful drug development requires efficient delivery to target sites as the drug must be able to reach a specific biophase by crossing several biomembranes by passive and/or active transport. A predominant factor influencing the pharmacokinetic behaviour is lipophilicity. For example, commonly used β-blockers, which are structurally similar to β3-adreneregic receptor agonists, may be divided into lipophilic and hydrophilic drugs, or are in an intermediate position2): sotalol (log Poct = –0.79), atenolol (log Poct = 0.23), nadolol (log Poct = 0.71), practolol (log Poct = 0.76), pindolol (log Poct = 1.75), acebutolol (log Poct = 1.87), timolol (log Poct = 2.10), metoprolol (log Poct = 2.15), alprenolol (log Poct = 2.61) and propranolol (log Poct = 3.65). Lipophilicity of compounds can influence oral absorption, diffusion through biolog ical barriers (e.g. placenta or blood/brain), degree of metabolism/renal elimination, plasma half-life, receptor selectivity, or tissue concentration3). Highly lipophilic drugs are insoluble in aqueous media and bind strongly to plasma proteins, which results in a low free blood concentration, and are distributed only into lipid bilayers. On the other hand, highly polar compounds cannot be absorbed through the gut wall because of lower membrane solubility. Keeping the optimal lipophilicity range can lead to an improvement of therapeutic efficacy and side-effect profiles of new drugs4).
Experimental methods
To describe the transfer of a substance from the aquatic environment into an organism and its bioaccumulation potential, the partition coefficient of a substance between water and a lipophilic solvent (octan-1-ol, cyclohexane, heptane) was determined.
Partition coefficient (P) is defined as the ratio of the equilibrium concentrations (Ci) of a dissolved substance in a two-phase system consisting of two immiscible solvents5):
P = CLS/CW, [1]
where CLS is the concentration of a compound in the lipophilic phase and CW is the concentration of a compound in the aqueous phase. Partition coefficient is usually given in the form of its log arithm to the base ten (log P).
Studied compounds
Chemical structure and the basic physicochemical parameters of the studied substances BL-14S2-BL-44S2 (chemically 3-{4-[(alkoxycarbonyl)amino]phenoxy}-N-{2-[4-(aminosulfonyl)phenyl]ethyl}-2-hydroxypropan-1-ammonium chlorides) are shown in Table 1.
Tab. 1. General characterization of investigated molecules BL-14S2-BL-A4S2 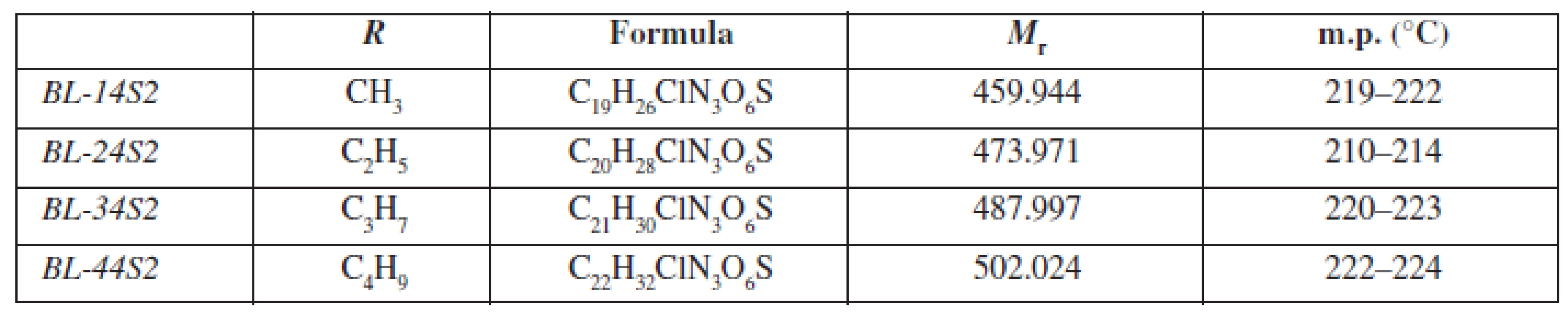
Devices
An analytical balance Chyo JL-180 (Chyo Balance Corporation, Japan), a mechanical shaker, a UV spectrophotometer (Shimadzu, UV-1800, Japan), a pH-meter (Hanna Instruments, Slovak Republic).
Chemicals
The aqueous phase was represented in all cases by phosphate buffer prepared from a water solution of disodium hydrogen phosphate, p.a. (CentralChem, Slovak Republic) with the concentration c = 0.2 mol · l–1 and a water solution of citric acid, p.a. with the concentration c = 0.1 mol · l–1. Measurements were performed under equilibrium conditions at pH = 7.4. The lipophilic phase was represented by high purity analytical grade octan-1-ol (Merck, Germany), cyclohexane (CentralChem, Slovak Republic) and heptane (CentralChem, Slovak Republic).
Estimation of partition coefficient log P2
In the present study, the generally accepted and well-known shake-flask method for obtaining the log P values in three mediums was used. The first one was octan-1-ol/phosphate buffer (log PO), second cyclohexane/phosphate buffer (log PC) and the third was heptane/phosphate buffer (log PH). The authors prepared 50 ml of basic solutions of the studied compounds BL-14S2-BL-44S2 in phosphate buffer at pH = 7.4 with the concentration c = 5.10–5 mol · l–1 and measured their absorbance A1. To 10 ml of the solution, 0.5 ml of the lipophilic medium represented by octan-1-ol, cyclohexane and heptane, respectively, was added. This system was shaken for 1h, the phases of the solvent system were mutually saturated and after that settled for 1 h, then the lipophilic and aqueous phases were separated. The absorbance A2 of the aqueous phase was measured. The values of absorbance were in both cases measured at the wavelength λ = 227 nm. The data of log PO, log PC, log PH for each compound were calculated from the equations 2–46):
Pexp = (1000 × g) − (a × cH2O × Mr) / b × cH2O × Mr [2]
cH2O = A2 [3]
ε = A1/c, [4]
where g is the weight of the studied compound in grams in 10 ml of measured solution, a is the number of millilitres of the aqueous phase, b is the number of millilitres of the lipophilic phase, Mr is the relative molecular weight of the compound under study, cH2O is the amount of the inspected compound in the aqueous phase after shaking, c is the concentration of the measured solution expressed in mol · l−1.
Tab. 2. Values of absorbances for calculation log PO, log PC, log PH for studied compounds BL-14S2-BL-44S2 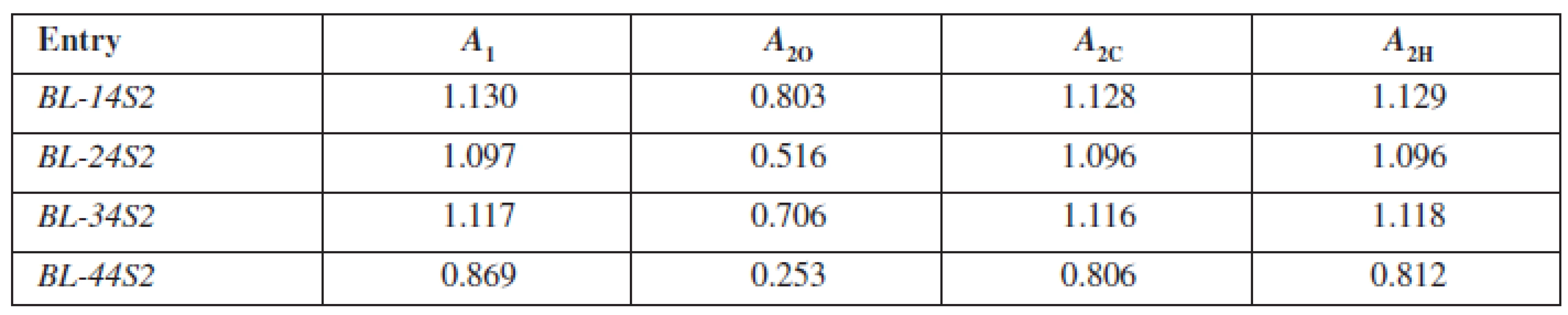
A1 – absorbance measured before shaking, A2O – absorbance measured after shaking for the system octan-1-ol/phosphate buffer, A2C – absorbance measured after shaking for the system cyclohexane/phosphate buffer, A2H – absorbance measured after shaking for the system heptane/phosphate buffer Results and discussion
This short research paper was focused on the estimation of lipophilicity of four newly synthesized substances potentially active as β3-adrenergic receptor agonists. Solubility in lipids refers to the ability of a compound to dissolve in fats, oils, lipids and non-polar solvents7). The experimentally estimated partition coefficient (log P) is routinely used as an assessment of lipid solubility in vivo and it is a key event of molecular desolvation in the transfer from aqueous phases to the cell membrane and protein bindings. The values of log P were experimentally determined in three systems, whereby the aqueous phase was always composed of phosphate buffer with pH = 7,4 and the lipophilic phase was formed of octan-1-ol, cyclohexane and heptane. Octan-1-ol represents a simple model of the cell phospholipidic membrane. The log P data acquired from the systems with cyclohexane and heptane signify the margin of penetration through the stratum corneum and blood-brain barrier. Octan-1-ol has eight atoms of carbon which are responsible for its lipophilic properties and on the other hand, it has also hydroxyl functionality which is accountable for its hydrophilic attribute. Cyclohexane is a lipophilic solvent composed of a planar six-membered cycle; therefore the molecules with benzene rings should easily incorporate into this system. Heptane is also a lipophilic solvent but it is composed of linear aliphatic chains. Compounds with aromatic scaffold cannot incorporate into heptane molecules so easily8).
The evaluated compounds BL-14S2-BL-44S2 are structurally based on the aryloxyaminopropanol pharmacophore bearing a benzene sulfonamide fragment in the basic part of the molecule differing from each other in the alkoxylcarbonylamino moiety. The presence of two aromatic rings is responsible for their lipophilic character. Furthermore, with elongation of the alkoxycarbonylamino fragment attached to the aromate, lipophilicity enhances. On the other hand, the presence of quaternary ammonium, hydroxyl and sulfonamide groups is responsible for their hydrophilicity. Whereas, cyclohexane and heptane are highly lipophilic solvents, only the substance BL-44S2 with a butoxylcarbonylamino moiety was able to penetrate into them. The partition coefficient in the medium cyclohexane/phosphate buffer (log PC) system was of the value of 0.22 and was higher than the partition coefficient estimated in heptane/phosphate buffer, which was of the value of 0.17, in consequence of a different structural character of the molecules of the solvents mentioned above. Into the non-polar environment of octan-1-ol all of the substances were able to penetrate and the values of log PO ranged from 0.90 to 1.70 (Table 3), whereby with an elongation of the alkoxylcarbonylamino fragment, the log PO values increased constantly.
Tab. 3. Experimentally estimated values of partition coefficients for inspected compounds BL-14S2-BL-A4S2 in octan-1-ol/phospate buffer (log P<sub>O</sub>), cyclohexane/phosphate buffer (log P<sub>C</sub>) and heptane/phosphate buffer (log P<sub>H</sub>) 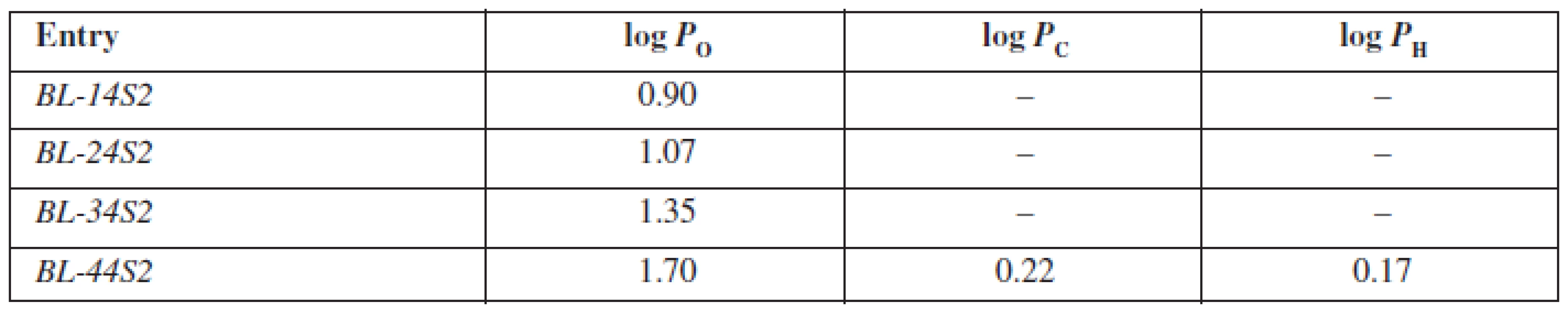
Conclusions
In conclusion, following the obtained results it can be assumed that the currently investigated compounds BL-14S2-BL-44S2 which could be classified as potential β3-adrenergic agonists, are not highly lipophilic and according to that, they are unlikely to cross the blood-brain barrier and cause some serious undesirable side effects on the CNS. It could be hypothesized that they would not be extensively metabolized, have not poor absorption, solubility, low bioavailability and a shorter half-life compared to their lipophilic analogues.
This work was supported by the following Grant projects: FaF UK/29/2015, UK/346/2015, FaF UK/28/2015, FaF UK/44/2015, FaF UK/63/2015; Comenius University in Bratislava Science Park supported by the Research and Development Operational Programme funded by the ERDF – Grant number: ITMS 26240220086.
Conflicts of interest: none.
PharmDr. Ľubica Havranová Sichrovská
Katedra farmaceutickej chémie
Odbojárov 10, 832 32 Bratislava, Slovak Republic
e-mail: l.sichrovska@gmail.com
Zdroje
1. Arnott J. A., Planey S. L. The influence of lipophilicity in drug discovery and design. Expert. Opin. Drug Discov. 2012; 10, 863–875.
2. Testa B., Crivori P., Reist M., Carrupt P. A. The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Perspect. Drug. Discov. 2000; 19, 179–211.
3. Brochard U. Pharmacolog ical properties of β-adrenoreceptor blocking drugs. J. Clin. Cardiol. 1998; 1, 5–9.
4. Lechat P. Clinical pharmacolog y of beta-blockers in cardiolog y: trial results and clinical applications. Hot Topics Cardiol. 2008; 10, 7–44.
5. Liu X., Testa B., Fahr A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011; 10, 1401–1408.
6. Sedlárová E., Čižmárik J. Štúdium lokálnych anestetík: časť 153. Vzťah medzi chemickou štruktúrou, fyzikálno-chemickými vlastnosťami a biolog ickou aktivitou v sérii piperidinopropylesterov alkoxysubstituovaných kyselín fenylkarbámových (In Slovak). Čes. slov. Farm. 2000; 49, 306–312.
7. Arnott J. A., Kumar R., Lobo Planey S. Lipophilicity indices for drug developement. J. Appl. Biopharm. Pharmacokinet. 2013; 1, 31–36.
8. Sedlárová E., Malík I., Andriamainty F., Kečkešová S., Csöӧllei J. Štúdium lipofility derivátov kyseliny fenylkarbámovej s bázickou časťou tvorenou substituovaným N-fenylpiperazínom(In Slovak). Farm. Obzor 2007; 4, 86–89.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Antibakteriální účinky přírodních látek – silice
- Organická syntéza, Laboratórny manuál
- Cholinergický systém srdca
- Proběhl 42. mezinárodní kongres k dějinám farmacie
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Sympozia Sekce dějin farmacie ČFS v roce 2015
- Autorský rejstřík
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Antibakteriální účinky přírodních látek – silice
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Cholinergický systém srdca
- Organická syntéza, Laboratórny manuál
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



