-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDetermination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
Authors: Zdenka Bedlovičová; Lucia Ungvarská Maľučká; Aneta Salayová; Jarmila Harvanová; Peter Očenáš
Authors place of work: University of Veterinary Medicine and Pharmacy, Košice, Slovak Republic
Published in the journal: Čes. slov. Farm., 2015; 64, 202-205
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Cordyceps sinensis is the fungi parasiting larvae, pupae and imagoes of insect as well as fruiting bodies of truffles of the genus Elaphomyces1). The fungi is known in both traditional Chinese medicine and in modern medicinal methods. It is used as a dietary supplement (CORDYCEPS MRL.®, ACAI DETOX®). The fact of Cordyceps sinensis consequence is supported by many scientific studies, which have shown its positive effects, for example in anti-tumor therapy2), in the treatment of HIV/AIDS, asthma, liver diseases and it also has a positive effect on female fertility etc.3). Chemical compounds are responsible for these properties, which is currently characterized by the parasite. It was found that the fungi are rich in natural substances such as cordycepin, cordycepic acid, respectively, D-mannitol4–6), polysaccharides7, 8), nucleotides9), proteins and amino acids10, 11).
This paper is focused on the basic research of studied biologically active compounds (nucleosides, amino acids) identification. To date nucleosides are believed to be the active compounds in Cordyceps12). Several methods including thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC) and nuclear magnetic resonance spectroscopy (NMR) have been used for the identification of the compounds under study.
Experimental methods
The mushrooms for extraction were obtained dried and grinded from the Technical University of Zvolen, Faculty of Forestry, in co-operation with team of Ing. Martin Paulík, PhD.
For the identification of biologically active compounds occurrring in Cordyceps sinensis we used methanolic extracts of eight fungi samples (1–8). For the separation of the content compounds thin layer chromatography was used (silica gel plates by Kiesselgel 60 F254 from Merck) in chloroform. Detection of chromatographs was provided by UV light at 254 nm and by freshly prepared 2% ninhydrine in methanol. For HPLC detection on a UHPLC Ultimate 3000 (ThermoScientific) a DAD detector was used. The measurements were performed at the temperature of 25 °C on the column Polaris 5 C18-A 250 ⋅ 4,6 mm (Varian), the injection volumes were 20 μl, flow rate was 1 ml/min. The mobile phase consisted of 2% acetonitrile hypergrade for chromatography (Merck) in water for chromatography (Merck). Standards were purchased: cytidine and uridine (Sigma-Aldrich), guanosine and adenosine (Acros Organics), thymidine (ABCR) and inosine (Calbiochem). NMR spectra were measured on a Varian VNMRS 600 MHz in D2O (Merck).
Sample preparation
Samples of Cordyceps sinensis for analysis were prepared by extraction in methanol. 10 g of crude mushroom was mixed in boiling methanol for 8 h and then filtered. The solvent was evaporated and the extract was dried under vacuum. Extraction yields: 7.1% (1), 2.2% (2), 8.0% (3), 4.8% (4), 8.3% (5), 7.6% (6), 15.1% (7), 9.1% (8).
Samples were filtered through a 0.22 μm syringe filters before HPLC analysis. Concentrations of samples prepared as water solution were 10 mg/ml.
From the extract of sample 7 we obtained a fraction crystallized from methanol, which we used for NMR experiments (1H NMR, 13C NMR, DEPT, gCOSY, gNOESY, gHSQC and gHMBC) in amount of 20 mg dissolved in 0.6 ml of D2O.
Results and discussion
HPLC analysis
In all samples the presence of the nucleosides adenosine, cytidine, uridine, inosine, guanosine and thymidine was studied (Fig. 1).
Fig. 1. Structure of studied compounds 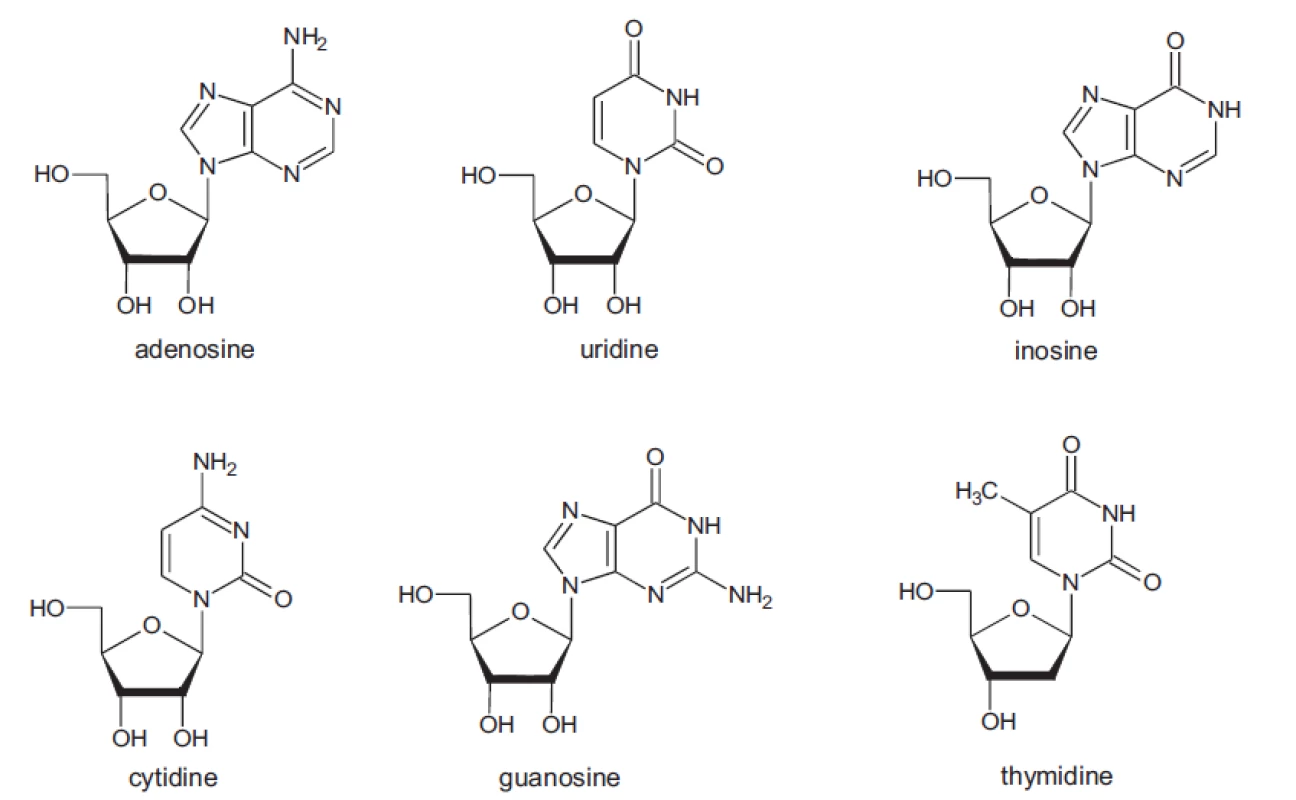
Figure 2 shows typical chromatograms of nucleoside standards and an example of a C. sinensis extract (6). The results showed that the components were obviously variant depending on different cultivation conditions of Cordyceps sinensis.
Fig. 2. HPLC-UV/VIS chromatograms of representative samples: above – standards, below – extract 6 (cultivated on millet) of C. sinensis 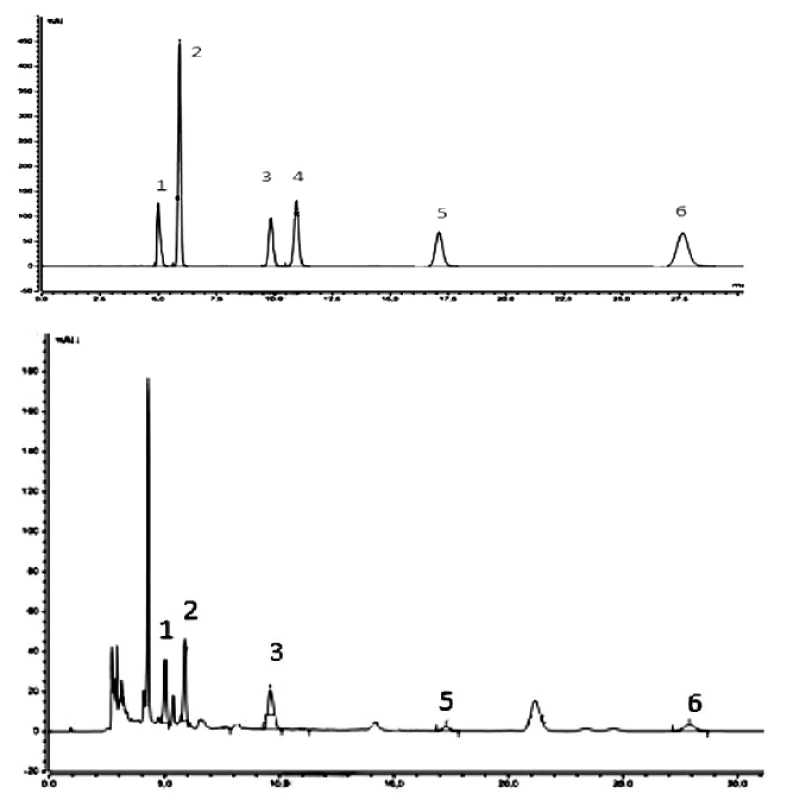
1 – cytidine, 2 – uridine, 3 – inosine, 4 – guanosine, 5 – thymidine, 6 – adenosine In all samples all nucleosides under study were not determined. The qualitative analysis showed that all nucleosides studied are represented only in samples 2, 3, 4, 5. In other samples cytidine (sample 8), inosine (samples 1, 6 and 7), and guanosine (sample 1) did not occur. These results demonstrate that qualitative parameters of the compounds under study are affected by cultivation conditions of fungi.
NMR analysis
With regard to the identification of chemical structures contained in Cordyceps sinensis, NMR analysis, which is an invaluable source of new information on the molecular structure, is only little employed.
A NMR study of the extract fraction achieved by crystallisation from methanol (sample 7) was provided. The TLC and HPLC analyses performed the presence of five compounds in an approximately equal amount. As we could not identify the compounds, we used NMR analysis. On the basis of 1D (1H NMR, 13C NMR, DEPT) and 2D (gCOSY, gNOESY, gHSQC and gHMBC) NMR experiments, we defined the structure of five amino acids. The chemical shifts (δδ, ppm) are described in Table 1. The chemical shifts correlated with the database13).
Tab. 1. Assignment 1H and 13C NMR signals for isolated compounds of Cordyceps sinensis methanolic extract 7 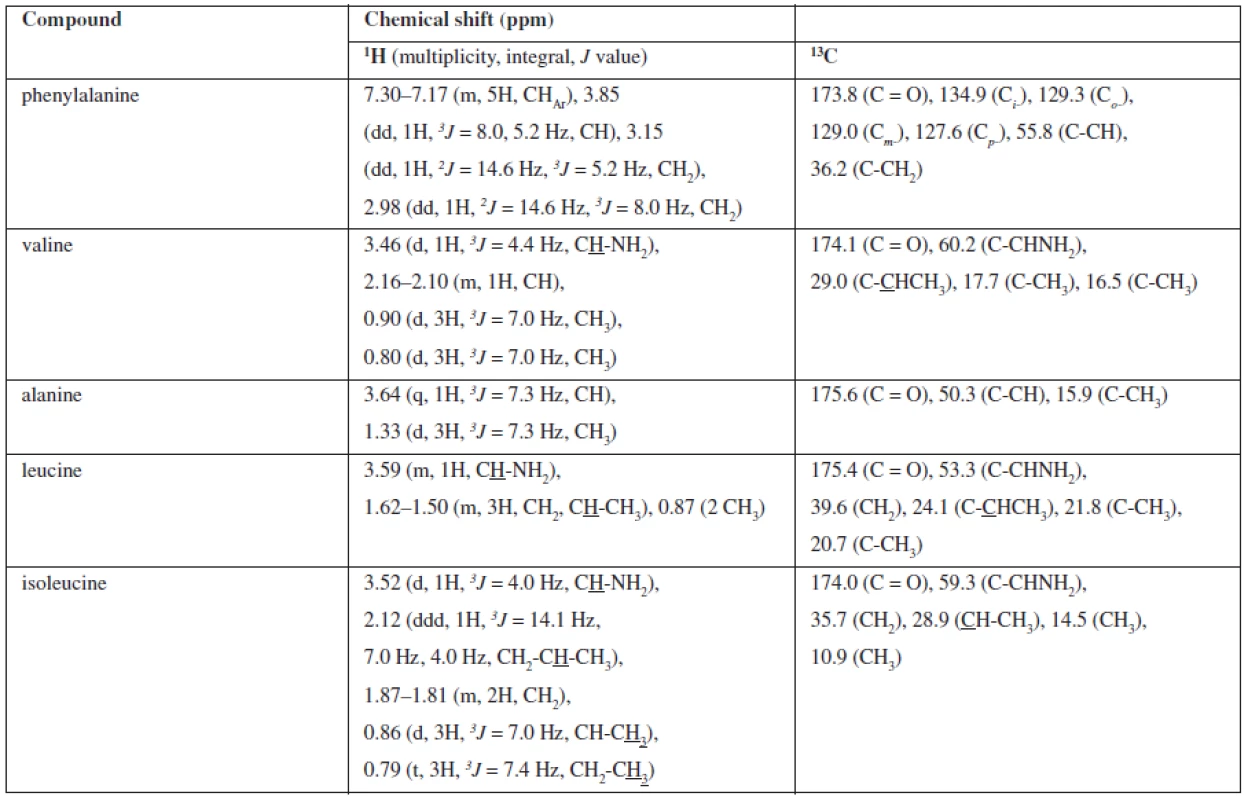
Conclusions
HPLC-UV/VIS and NMR analyses were performed for qualitative determination of nucleosides in Cordyceps sinensis fungi. Six nucleosides were determined by HPLC and five unknown compounds were identified by NMR as amino acids.
The authors would like to acknowledge the contribution of Assoc. Prof. RNDr. Ján Imrich, CSc., and RNDr. Mária Vilková, PhD., from the Laboratory of NMR, Faculty of Science, UPJŠ, Košice, to the measurements of NMR spectra.
Conflicts of interest: none
RNDr. Zdenka Bedlovičová, PhD.
University of Veterinary Medicine and Pharmacy
Komenského 73, 04181 Košice, Slovak Republic
e-mail: zdenka.bedlovicova@uvlf.sk
Zdroje
1. Hobbs Ch. Medicinal mushrooms: an exploration of tradition, healing, and culture. Santa Cruz (CA): Botanica Press 1995.
2. Wang B. J., Won S. J., Yu Z. R., Su Ch. L. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide, Food and Chemical Toxicology 2005; 43, 543–552.
3. Gong Y. X., Li S. P., Li P., Liu J. J., Wang Y. T. J. Simultaneous determination of six main nucleosides and bases in natural and cultured Cordyceps by capillary electrophoresis, Chromatogr. A 2004; 1055, 215–221.
4. Huang L. F., Liang Y. Z., Guo F. Q., Zhou Z. F., Cheng B. M. Simultaneous separation and determination of active components in Cordyceps sinensis and Cordyceps militarris by LC/ESI-MS, J. Pharm. Biomed. Anal. 2003; 33, 1155–1162.
5. Sprecher M., Sprinson D. B. J. A reinvestigation of the structure of “Cordyceps acid”. J. Org. Chem. 1963; 28, 2490–2491.
6. Cai L. I., et al. Obsarvation of the structure morphology of Cordyceps polysaccharide by atomic force microscope, J. Chin. Electron. Microsc. Soc. 1999; 18, 103–105.
7. Wu Y., et al. Isolation and characterization of a mannoglucan from edible Cordyceps sinensis mycelium, Carbohydr. Res. 2007; 342, 870–875.
8. Li S. P., et al. The nucleosides content and their variation in natural Cordyceps sinensis and cultured Cordyceps mycelia, J. Chin. Pharm. Sci. 2001; 10, 175–179.
9. Hsu T. H., et al. A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom, Food. Chem. 2002; 78, 463–469.
10. Ji S., et al. Introduction of the research status in chemical constituents of Cordyceps, J. Fujian Coll. Trad. Chin. Med. 1999; 9, 46–47.
11. Fan H., Li S. P., Xiang J. J., Lai C. M., Yang F. Q., Gao J. L., Wang Y. T. Qualitative and quantitative determination of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography-electrospray ionization tandem mass spectrometry (HPLC-ES-MS/MS), Analytica Chimica Acta 2006; 567, 218–228.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 5- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Metody používané ve farmaceutické technologii ke zvyšování biologické dostupnosti špatně rozpustných léčiv po perorálním podání
- Výdaje na léky a péči pro stárnoucí populaci
- Úroveň a faktory ovplyvňujúce pacientsku spokojnosť s lekárenskou starostlivosťou na Slovensku
-
Pracovní den sekce technologie léků České farmaceutické společnosti ČLS JEP
Pokroky v lékových formách - Drug bioavailability increasing by formulation of liquisolid systems
- Hodnocení sypných a konsolidačních vlastností prášků ve farmaceutické technologii
- Evaluation of compressibility of tableting mixtures using the compaction equation
- Branched polyesters as mucoadhesive carriers of drugs
- Formulační aspekty orodispergovatelných tablet
- Evaluation of water absorption rate of tablets by using an Enslin-Neff device
- Evaluation of the influence of lubricants on the viscoelastic properties of tablets using the stress relaxation test
-
44th Conference drug synthesis and analysis
(Brno, 2nd to 4th September 2015) – Part 1 - Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
- Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
- A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
- Determination of acid-base dissociation constants of newly synthesized arylethanolamine derivatives using capillary zone electrophoresis
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
- The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
- On-line hyphenated capillary electrophoresis and tandem mass spectrometry used for the analysis of selected biogenic amines in grape leaves
- Validation of spectrophotometric methods of assaying metronidazole in capsules
- Prof. RNDr. Jaroslav Květina, DrSc. dr.h.c. FCMA – 85letý
- Farmakochemie
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Metody používané ve farmaceutické technologii ke zvyšování biologické dostupnosti špatně rozpustných léčiv po perorálním podání
- Formulační aspekty orodispergovatelných tablet
- Prof. RNDr. Jaroslav Květina, DrSc. dr.h.c. FCMA – 85letý
- Hodnocení sypných a konsolidačních vlastností prášků ve farmaceutické technologii
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



