-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
Reklama50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
article has not abstract
Published in the journal: . PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005198
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005198Summary
article has not abstract
Introduction
New species of Emmonsia-like fungi, with phylogenetic and clinical similarities to Blastomyces and Histoplasma, have emerged as causes of systemic human mycoses worldwide. They differ from classical Emmonsia species by producing a thermally-dependent, yeast-like phase rather than adiaspores, and by causing disseminated infections, predominantly in immunocompromised patients and often with high case-fatality rates. Such differences will be important for clinicians to consider in diagnosis and patient management, and for microbiologists who may encounter these fungi with increasing frequency.
Adiaspiromycosis Is a Rare and Limited Disease in Humans
Until recently, the clinical relevance of the genus Emmonsia was limited to a very rare and unusual pulmonary disease named adiaspiromycosis, caused by two species, Emmonsia crescens and Emmonsia parva. The disease follows inhalation of aerosolized conidia, released from mycelia found in soil. In the lungs, the conidia undergo a dramatic enlargement, from ~2–4 μm to 40–500 μm in diameter—a volume increase of up to a million-fold [1]. Emmons and Jellison called these swollen cells adiaspores, from the Greek α – (not, without), –δια – (by, through), and –σπορα (seed, sowing), in reference to the fact that they neither replicate nor disseminate [1]. However, their presence in the host may provoke a foreign body reaction, resulting in granulomatous lung disease [2,3]. Disease severity is dependent on inoculum size and host response, with a spectrum ranging from subclinical pneumonia to diffuse pulmonary disease causing hypoxic respiratory failure and, occasionally, death [2–4].
Adiaspiromycosis is common in rodents and other small terrestrial mammals. For instance, nearly a third of wild British mammals sampled had signs of the disease [5]. E. crescens has been reported to cause adiaspiromycosis in over 118 mammalian species with a global distribution [2]. Sequencing data from some E. parva-like isolates from animals has implicated different, as-yet-undescribed species (e.g., Emmonsia sp. from weasels in the United States [6] and Emmonsia sp. from mustelid in the Czech Republic), which are included in the phylogenetic tree of Fig 1.
Fig. 1. Maximum likelihood phylogeny inferred using RAxML v. 8.0.0 employing GTRCAT model and 1,000 bootstrap replicates. 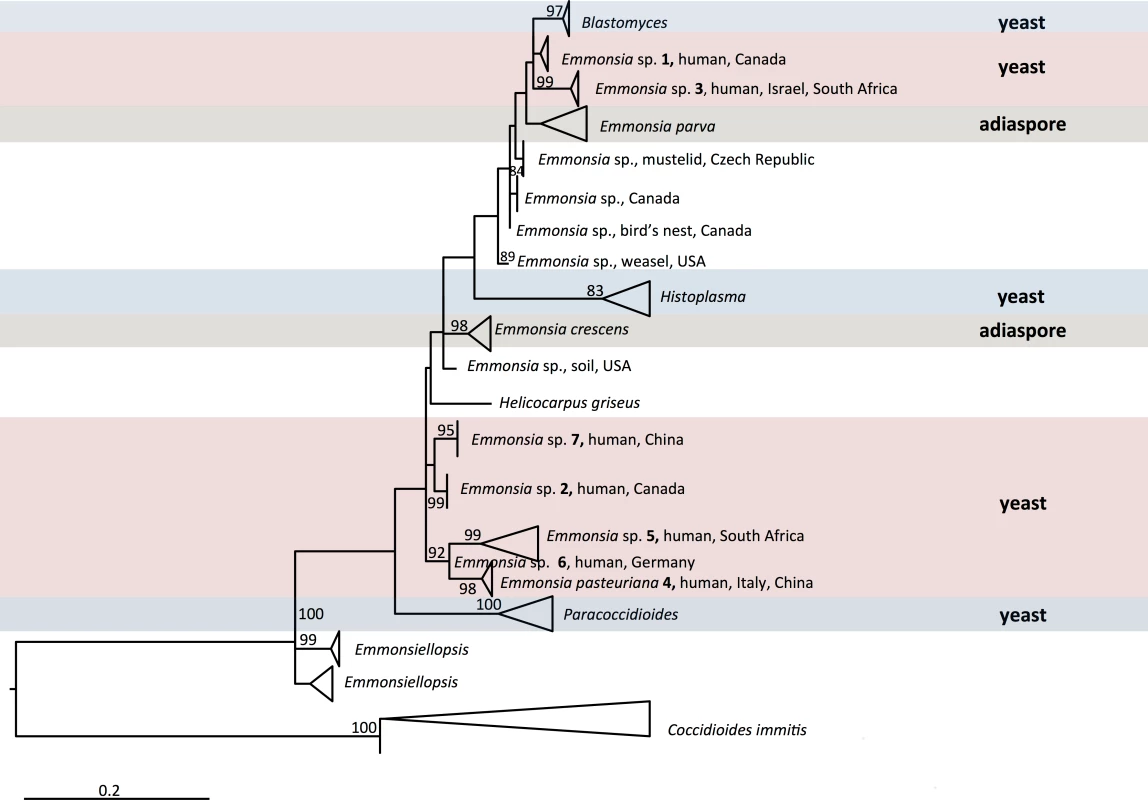
Bootstrap branch support above 80% is shown. Multiple sequences in the same species are collapsed. Genera included in the family Ajellomycetaceae are shown, with the exception of Lacazia loboi, which could not meaningfully be aligned. Occasionally, humans can also be affected. The first human case of adiaspiromycosis was reported in 1964 [7], and cases have since been reported worldwide [2,3]. E. crescens has been implicated in the vast majority of these infections. Unusual cases of infection by E. parva have also been reported in immunocompromised hosts [8,9]; however, clinical and histopathological findings were so atypical that, in the absence of molecular confirmation, the identification of the pathogen has been questioned [2,3]. Additionally, superficial adiaspiromycosis was reported to cause granulomatous conjunctivitis in 99 of 5,084 children (1.9%) screened in the Amazon basin, and histopathological examination of ocular nodules identified adiaspore-like structures in two of 14 cases [10]. The investigators identified diving in a nearby river as a risk factor, and surmised that conjunctival irritation from spicules of freshwater sponges provided a portal of entry. However, the identification of the putative pathogen remains unclear: the unusual exposure history and clinical features suggest that Rhinosporidium seeberi rather than Emmonsia species might have been involved.
A Leap from Obscurity to Global Medical Importance
Over the last four decades, reports have emerged of patients with unusual mycoses: in the laboratory, cultures have isolated molds with asexual reproductive structures that resembled Emmonsia, but clinical and histopathological pictures were more compatible with blastomycosis or histoplasmosis than adiaspiromycosis (Fig 2). Molecular sequencing has since confirmed that these fungi belong to a cluster of novel Emmonsia-like species (Fig 1). It remains unclear whether this cluster of Emmonsia-like species only emerged recently as human pathogens, or whether previous infections were merely underestimated. Support for the latter hypothesis comes from South Africa, where the introduction of molecular identification tools resulted in a dramatic increase in the number of cases of disseminated Emmonsia disease, commensurate with a decline in the number of cases of confirmed histoplasmosis [11]. The timing of human cases is illustrated in Fig 3.
Fig. 2. Clinical, pathological, and mycological facets of a novel Emmonsia-like fungus reported from South Africa. 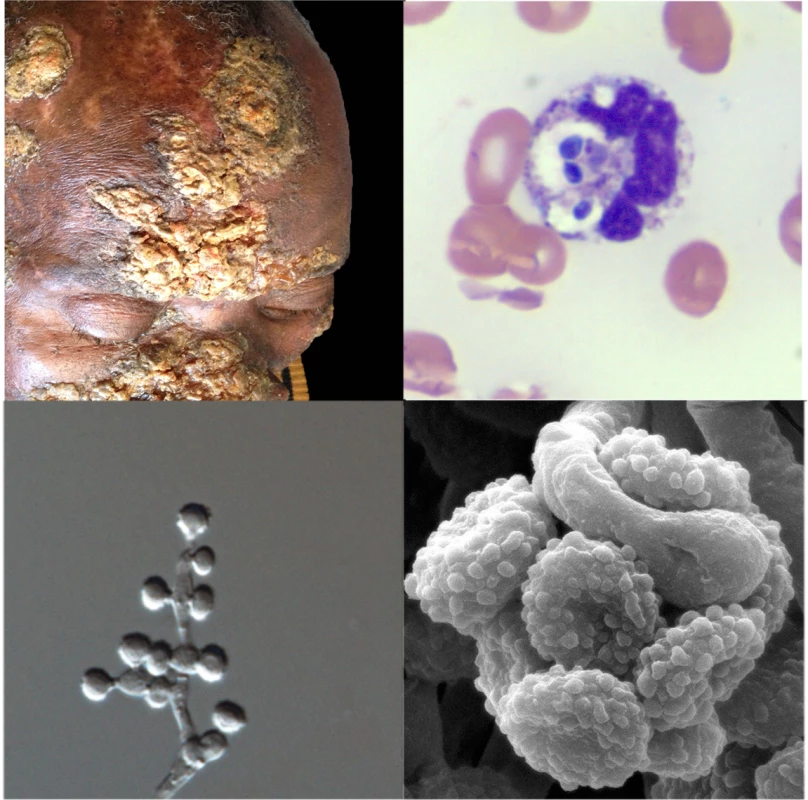
Top left: Hyperkeratotic skin lesions in a patient with disseminated Emmonsia disease (published with patient consent; courtesy of Dr. Tabie Greyling, Stellenbosch University). Top right: Peripheral blood smear showing neutrophils with multiple phagocytosed yeast-like cells (Wright-Giemsa staining x1,000). Bottom right: Electron microscopy image of conidia (courtesy of Dr. Monica Birkhead, National Institute of Communicable Diseases). Bottom left: Light microscopy image of conidiophores and conidia (x1,000). Fig. 3. Timeline of human cases of disease caused by classical Emmonsia species (with adiaspores at 37°C) and novel Emmonsia-like species (with yeast cells at 37°C). 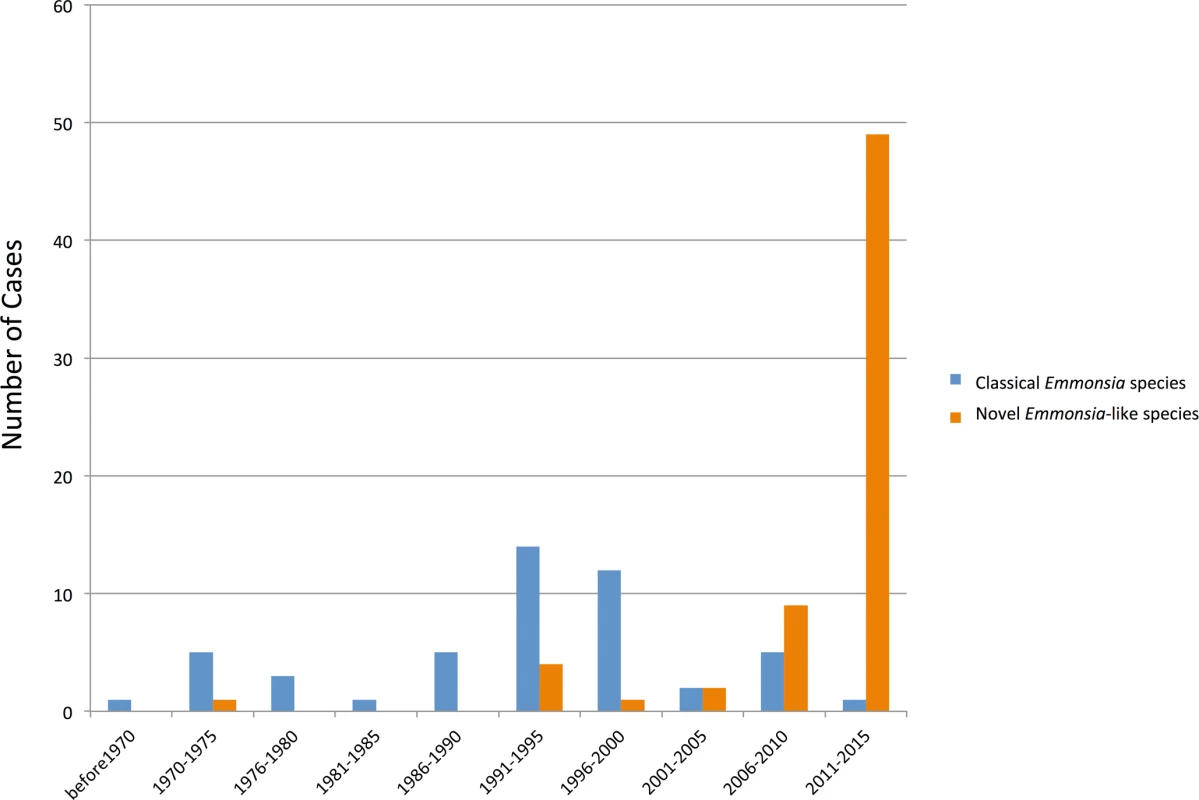
Vertical bars represent number of cases during 5-year intervals, as determined by literature review. The increase in cases of diseases caused by novel Emmonsia-like species occurring between 2006 and 2015 is primarily driven by recognition of HIV-associated cases in South Africa following the introduction of molecular identification protocols for dimorphic fungal infections in 2008. The earliest recorded case, from 1970, was reported as an unusual case of blastomycosis in a patient from Alberta, Canada, a region considered non-endemic for this disease [12]. The patient presented with neurological symptoms; at autopsy, histopathology sections revealed atypical yeast-like cells in lung and brain, and a fungus resembling Blastomyces dermatitidis was isolated from cerebral spinal fluid and lung. However, the organism failed to produce typical conidia or thick-walled yeast cells in culture [12]. Retrospective examination of the culture, including sequencing of large ribosomal subunit and internal transcribed spacers (ITS) loci, determined that this isolate represented a novel Emmonsia-like fungus (Emmonsia sp. 1, Fig 1) [6].
The next recorded case of disseminated infection, in 1992, was an HIV-infected male from Saskatchewan, Canada, with cutaneous lesions. Skin biopsy culture grew a mold resembling Emmonsia; retrospective genetic analysis demonstrated the novelty of this species (Emmonsia sp. 2, Fig 1) [6,13]. A second case from Saskatchewan occurred in 2003, in an Asian renal transplant recipient with atypical pneumonia. An Emmonsia-like fungus was cultured from blood and respiratory specimens [14].
A third novel Emmonsia-like fungus (Emmonsia sp. 3, Fig 1) was found in 1993, causing granulomatous mucocutaneous lesions in an immunocompetent man in Israel [13]. The yeast-like cells seen on skin biopsy were originally thought consistent with blastomycosis [13], but the fungal cells were smaller, and an Emmonsia-like fungus was isolated [15]. A second case of infection was recently identified in an immunocompetent South African patient with a mycotic brain abscess [16]. Histopathology of brain biopsy was suggestive of blastomycosis [17], but the ITS sequence of an Emmonsia-like fungus isolated from brain tissue was nearly identical to that of the fungus cultured from the Israeli case [18], suggesting these isolates belong to the same species.
In 1994, an HIV-infected woman from Italy was diagnosed with a disseminated mycosis [19]. Biopsies of cutaneous lesions revealed small, yeast-like cells in tissue, and a thermally dimorphic Emmonsia-like fungus was isolated in culture [19]. This was described as the novel species E. pasteuriana (E. pasteuriana 4, Fig 1) [20]. An additional case was reported in 2011, in a liver transplant recipient with HIV infection from Spain, with pulmonary and cutaneous lesions [21,22]. Recently, two additional cases of E. pasteuriana were reported from Guangzhou, China, in a renal transplant recipient [23] and in a patient treated with high-dose corticosteroids [24].
In 1995, a fifth novel Emmonsia-like fungus (Emmonsia sp. 5, Fig 1) was isolated from a skin biopsy of an HIV-infected man in South Africa with widespread skin lesions [11]. The fungus had yeast-like structures instead of adiaspores. Another case was not identified until 2008, when routine molecular identification of dimorphic fungi was adopted at several microbiology laboratories in South Africa. Over the next 3 years, 13 cases of disseminated disease were diagnosed among HIV-infected adults [11]. By 2015, 55 cases had been diagnosed [17,25]. Among these, 53 were HIV-infected, and another was a renal transplant recipient. Pulmonary disease was common and cutaneous involvement near universal. The case fatality rate was 48% [17].
In 2000, a sixth novel Emmonsia-like fungus (Emmonsia sp. 6, Fig 1) was implicated as the cause of isolated, necrotizing pneumonia in a German farmer with rheumatoid arthritis treated with corticosteroids. Yeast-like cells were present in a transbronchial biopsy [26]. And finally, in 2005, infection due to a seventh novel Emmonsia-like fungus (Emmonsia sp. 7, Fig 1) occurred in a diabetic patient from Beijing, China [27], who had pulmonary and cutaneous disease.
Key Emergent Traits of Disseminated Emmonsia Disease
The newly recognized Emmonsia species differ significantly from the agents of adiaspiromycosis, most notably in displaying thermal dimorphism, and growing as a mold at 25°C and as yeast-like cells (rather than producing adiaspores) at 37°C. Consequently, major differences exist in the pathogenesis, epidemiology, disease spectrum, diagnostic findings, course, and management.
The primary route of infection, conserved among Emmonsia spp., is presumed to be inhalation of airborne conidia released from saprophytic mycelia in soil [2]. Similarities in pathogenesis between classical and emerging Emmonsia-like species end there. Once in the mammalian (human) host tissue, rather than swelling to sterile, static adiaspores, conidia of newer Emmonsia-like species convert to yeast-like cells capable of replication and extra-pulmonary dissemination. Disease results from tissue invasion [28], although a contribution of host response to pathogenesis is suggested by apparent unmasking immune reconstitution inflammatory syndrome in some HIV-infected patients who develop stigmata of disease upon initiating antiretroviral treatment [17].
Most reported patients with adiaspiromycosis have been immunocompetent [2,4], although exceptions exist [3,4]. In contrast, nearly all reported patients with disease due to emerging Emmonsia-like species have had profound impairment of cell-mediated immunity. These have included HIV infection in the vast majority (among whom the median CD4+ T-lymphocyte count was 16 cells/μL) [11,17,19,21,25]. Other associated conditions include solid organ transplantation [14,16,21,23] and corticosteroid use [23,26].
Results of histopathological and microbiological investigations of adiaspiromycosis and disseminated Emmonsia disease are unmistakably different. The sine qua non of adiaspiromycosis is the presence of adiaspores in tissue; the causative agents have rarely been cultured from humans [2,13]. On the other hand, the histopathological hallmark of disseminated Emmonsia disease is yeast-like cells in tissue [11,12,15,17,19], which may be mistaken for Histoplasma [17] or Blastomyces [12,15,17]. Fungi may be isolated from clinical specimens, particularly with prolonged incubation on routine fungal culture media [2,12,14,17,19,21,23,25,26].
The natural histories of adiaspiromycosis and disseminated Emmonsia disease are quite different and, consequently, principles of management differ, although evidence to guide therapy is anecdotal. Adiaspiromycosis is generally self-limiting, and fatalities are exceptional [29–31]. Because this disease results from host response, corticosteroids have been advocated in severe cases; the role of antifungals remains uncertain [3,31,32]. In contrast, disseminated Emmonsia disease appears to be a progressive disease in many patients, particularly immunocompromised hosts, in whom case-fatality rates approach 50%; among these patients, antifungals appear to be imperative [17].
Disentangling the Ajellomycetaceae
Even before the discovery of newer Emmonsia spp., the taxonomy of these species was a matter of debate among mycologists [2,33]. The sexual stage (teleomorph) of E. crescens belongs to Ajellomyces, the same genus as the teleomorphs of B. dermatitidis and H. capsulatum [34], suggesting a close relatedness. Genetic studies have also demonstrated a close phylogenetic relationship between Emmonsia and Blastomyces [13,35]; in fact, E. parva is more closely related to Blastomyces than to E. crescens [13]. Members of both genera produce budding, yeast-like cells and share genetic similarities, perhaps even justifying the assignment of Blastomyces and Emmonsia species to a single genus [13,35,36]. Full genome sequencing of multiple isolates of all Emmonsia-like species will provide greater resolution of phylogenetic relationships, and may help to clarify taxonomic boundaries. Recently, genome sequences for two animal-associated Emmonsia strains were made publicly available in NCBI (isolated from lungs of a rodent and from a weasel, and catalogued under Bioprojects PRJNA178252 and PRJNA178178, respectively), and submissions are in progress for additional human-associated, pathogenic strains shown in Fig 1. Preliminary phylogenetic analyses suggest that most of the new human-associated Emmonsia-like fungi form a single, derived clade in the Ajellomycetaceae, while agents of adiaspiromycosis appear to be polyphyletic (Fig 1).
Zdroje
1. Emmons CW, Jellison WL. Emmonsia crescens sp. n. and adiaspiromycosis (haplomycosis) in mammals. Ann N Y Acad Sci. 1960;89 : 91–101. 13696711
2. Sigler L. Adiaspiromycosis and other infections caused by Emmonsia species. In: Hay RJ, Merz WG, editors. Topley and Wilson’s Microbiology and Microbial Infections. 10th ed. London, U.K.: Arnold Hodder; 2005. pp. 809–824.
3. Anstead GM, Sutton DA, Graybill JR. Adiaspiromycosis causing respiratory failure and a review of human infections due to Emmonsia and Chrysosporium spp. J Clin Microbiol. 2012;50 : 1346–1354. doi: 10.1128/JCM.00226-11 22259200
4. England DM, Hochholzer L. Adiaspiromycosis: an unusual fungal infection of the lung. Report of 11 cases. Am J Surg Pathol. 1993;17 : 876–86. 8352373
5. Borman AM, Simpson VR, Palmer MD, Linton CJ, Johnson EM. Adiaspiromycosis due to Emmonsia crescens is widespread in native British mammals. Mycopathologia. 2009;168 : 153–63. doi: 10.1007/s11046-009-9216-6 19533414
6. Sigler L, Peterson SW. Molecular genetic analysis supports recognition of new species among Emmonsia and Blastomyces isolates. International journal of antimicrobial agents. 2009. p. S593.
7. Doby-Dubois M, Chevrel ML, Doby JM, Louvet M. [1st human case of adiaspiromycosis, caused by Emmonsia crescens, Emmons and Jellison, 1960]. Bull Soc Pathol Exot Filiales. 1964;57 : 240–4. 14184774
8. Echavarria E, Cano EL, Restrepo A. Disseminated adiaspiromycosis in a patient with AIDS. J Med Vet Mycol. 1993;31 : 91–97. 8483061
9. Turner D, Burke M, Bashe E, Blinder S, Yust I. Pulmonary adiaspiromycosis in a patient with acquired immunodeficiency syndrome. Eur J Clin Microbiol Infect Dis. 1999;18 : 893–5. 10691202
10. Mendes MO, Moraes MAP, Renoiner EIM, Dantas MHP, Lanzieri TM, Fonseca CF, et al. Acute conjunctivitis with episcleritis and anterior uveitis linked to adiaspiromycosis and freshwater sponges, Amazon region, Brazil, 2005. Emerg Infect Dis. 2009;15 : 633–9. doi: 10.3201/eid1504.081281 19331759
11. Kenyon C, Bonorchis K, Corcoran C, Meintjes G, Locketz M, Lehloenya R, et al. A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med. 2013;369 : 1416–1424. doi: 10.1056/NEJMoa1215460 24106934
12. Sekhon AS, Jackson FL, Jacobs HJ. Blastomycosis: report of the first case from Alberta Canada. Mycopathologia. 1982;79 : 65–69. 6813742
13. Peterson SW, Sigler L. Molecular genetic variation in Emmonsia crescens and Emmonsia parva, etiologic agents of adiaspiromycosis, and their phylogenetic relationship to Blastomyces dermatitidis (Ajellomyces dermatitidis) and other systemic fungal pathogens. J Clin Microbiol. 1998;36 : 2918–25. 9738044
14. Sanche S, Wong A, Sigler L, Angel S, Peterson S. Invasive infection caused by a novel Emmonsia species in a renal transplant patient. Focus on Fungal Infections. Miami; 2005. p. Abstr 87.
15. Kemna ME, Weinberger M, Sigler L, Zeltser R, Polachek I, Salkin. IF. A primary oral blastomycosis-like infection in Israel, abstr. F-75. 94th General Meeting of the American Society for Microbiology. Washington, DC; 1994. p. 601.
16. Heys I, Taljaard J, Orth H. An emmonsia species causing disseminated infection in South Africa. N Engl J Med. 2014;370 : 283–284.
17. Schwartz IS, Govender NP, Corcoran C, Dlamini S, Prozesky H, Burton R, et al. Clinical characteristics, diagnosis, management and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis. 2015.; 61(6):1004–12. doi: 10.1093/cid/civ439 26060283
18. Stielow JB, Moreno L, Kenyon C, Botha A, Lerm B, Govender N, et al. From ITS to genomes: two novel Emmonsia species with fully annotated genomes. Genomics of Neglected Pathogens—CBS Symposium Week. Utrecht: CBS-KNAW Fungal Biodiversity Centre; 2015. http://www.blackyeast.org/GenomicsNeglectedPathogens/files/Benjamin_Stielow.pdf
19. Gori S, Drouhet E. Cutaneous disseminated mycosis in a patient with AIDS due to a new dimorphic fungus. J Mycol Med. 1998;8 : 57–63.
20. Drouhet E, Gueho E, Gori S, Huerre M, Provost F, Borgers M, et al. Mycological, ultrastructural and experimental aspects of a new dimorphic fungus Emmonsia pasteuriana sp. nov. isolated from a cutaneous disseminated mycosis in AIDS. J Mycol Med. 1998;8 : 64–77.
21. Pelegrín I, Ayats J, Xiol X, Cuenca-Estrella M, Jucglà A, Boluda S, et al. Disseminated adiaspiromycosis: case report of a liver transplant patient with human immunodeficiency infection, and literature review. Transpl Infect Dis. 2011;13 : 507–514. doi: 10.1111/j.1399-3062.2011.00611.x 21323828
22. Pelegrín I, Alastruey-Izquierdo A, Ayats J, Cuenca-Estrella M, Cabellos C. A second look at Emmonsia infection can make the difference. Transpl Infect Dis. 2014;0 : 1–2.
23. Feng P, Yin S, Zhu G, Li M, Wu B, Xie Y, et al. Disseminated infection caused by Emmonsia pasteuriana in a renal transplant recipient. J Dermatol. Epub 2015 Jun 24.
24. Tang XH, Zhou H, Zhang XQ, Han J De, Gao Q. Cutaneous Disseminated Emmonsiosis Due to Emmonsia pasteuriana in a Patient With Cytomegalovirus Enteritis. JAMA dermatology. 2015;151(9):1026–1028
25. Lochan H, Naicker P, Maphanga T, Ryan A, Pillay K, Govender NP, et al. A case of emmonsiosis in an HIV-infected child. S Afr J HIV Med. 2015;16 : 2–5.
26. Wellinghausen N, Kern W V, Haase G, Rozdzinski E, Kern P, Marre R, et al. Chronic granulomatous lung infection caused by the dimorphic fungus Emmonsia sp. Int J Med Microbiol. 2003;293 : 441–5. 14760976
27. Dukik K, Feng P, Freeke J, Nasrabadi, Azadeh Jamalian; Stielow B, Gerrits van den Ende B, de Hoog S. Redefining the genus Emmonsia: emergence of new dimorphic human pathogens. Genomics of Neglected Pathogens—CBS Symposium Week. Utrecht: CBS-KNAW Fungal Biodiversity Centre; 2015. http://www.blackyeast.org/GenomicsNeglectedPathogens/files/Karolina_Dukik.pdf
28. Drouhet E, Huerre M. Yeast tissue phase of Emmonsia pasteuriana inoculated in golden hamster by intratesticular way. Mycoses. 1999;42 Suppl 2 : 11–8. 10865897
29. Moraes MA, de Almeida MC, Raick AN. [A fatal case of human pulmonary adiaspiromycosis]. Rev Inst Med Trop Sao Paulo. 1989;31 : 188–94. 2694306
30. Peres LC, Figueiredo F, Peinado M, Soares FA. Fulminant disseminated pulmonary adiaspiromycosis in humans. Am J Trop Med Hyg. 1992;46 : 146–50. 1539748
31. Barbas Filho J V, Amato MB, Deheinzelin D, Saldiva PH, de Carvalho CR. Respiratory failure caused by adiaspiromycosis. Chest. 1990;97 : 1171–5. 2331914
32. Watts JC. Adiaspiromycosis. An uncommon disease caused by an unusual pathogen. CHEST J. American College of Chest Physicians; 1990;97 : 1030.
33. Carmichael JW. Chrysosporium and some other aleuriosporic hyphomycetes. Can J Bot. 1962;40 : 1137–1174.
34. Sigler L. Ajellomyces crescens sp. nov., taxonomy of Emmonsia spp., and relatedness with Blastomyces dermatitidis (teleomorph Ajellomyces dermatitidis). J Med Vet Mycol. 1996;34 : 303–14. 8912163
35. Guého E, Leclerc MC, de Hoog GS, Dupont B. Molecular taxonomy and epidemiology of Blastomyces and Histoplasma species. Mycoses. 1997;40 : 69–81. 9375491
36. Leclerc MC, Philippe H, Guého E. Phylogeny of dermatophytes and dimorphic fungi based on large subunit ribosomal RNA sequence comparisons. J Med Vet Mycol. 1994;32 : 331–41. 7844699
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



