-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaValidity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: An observational study
Clara Menendez and colleagues examine the validity of a minimally invasive autopsy to determine cause of death in stillborn babies and neonates compared with complete diagnostic autopsy in Mozambique.
Published in the journal: . PLoS Med 14(6): e32767. doi:10.1371/journal.pmed.1002318
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002318Summary
Clara Menendez and colleagues examine the validity of a minimally invasive autopsy to determine cause of death in stillborn babies and neonates compared with complete diagnostic autopsy in Mozambique.
Introduction
Globally, progress in reducing child mortality has been significantly slower than the target annual rate of decline of 4.4% for children under 5 years established by the previous Millennium Development Goals [1–3]. Peri-neonatal deaths account for nearly half of all under-five child mortality [1,4,5], with nearly 3 million neonatal deaths (within 28 days after birth) and 2.6 million stillbirths (deaths occurring after 28 weeks of gestation) occurring annually. The vast majority of these deaths (99%) take place in low - and middle-income countries, where vital registration systems are inadequate or nonexistent and rarely medically certified [1–5]. This imposes the need to make sophisticated assumptions to establish the etiology in the majority of these deaths [6]. As a consequence, efforts to reduce the burden of peri-neonatal mortality are not always evidence driven, and it is extremely challenging to assess the extent of the progress towards the new Sustainable Development Goals and, thus, to adequately guide policy targeting this age group [7]. Because the causes of most peri-neonatal deaths still remain unknown, rigorous determination of the etiologies of mortality in this age group has been identified as a priority to achieve a significant reduction in this unacceptable burden [8].
In low - and middle-income countries, the 2 conventional sources of information on the causes of mortality, including peri-neonatal deaths, are verbal autopsies and clinical records, both having a significant level of imprecision [9,10]. Verbal autopsies have also been questioned because they are subject to a high degree of misclassification errors, especially for conditions with poor diagnostic specificity such as most causes of peri-neonatal deaths. In fact, although the current WHO verbal autopsy tool adequately identifies newborn deaths and stillbirths by time of death and distinguishes fresh from macerated stillborn babies, this method has many limitations in accurately establishing the cause of death in this group [8]. Clinical records are obtained only from those women who deliver at a health facility or attend it during the postpartum period or for babies who are admitted sick after birth. However, such cases are still a minority in many low - and middle-income countries, as most deliveries take place away from health services. Moreover, for those cases attended at health facilities, clinical diagnostic errors are not infrequent even in well-equipped, tertiary-level hospitals [11].
A reliable ascertainment of the causes of peri-neonatal mortality would require a complete diagnostic autopsy (CDA), a procedure that remains to date the gold standard for cause-of-death determination [12]. Recent studies in high-income countries have highlighted the importance of CDAs in peri-neonatal deaths, as they can correct clinical diagnostic errors and produce information that is additional to the clinical findings in up to 22%–76% of cases [13]. However, performing CDAs in low - and middle-income settings is challenging because of a lack of resources and trained personnel to perform them, the large proportion of deaths occurring outside the health system, and, potentially, cultural and/or religious apprehension about the practice of postmortem procedures, which often makes them unacceptable from the community perspective. In addition to these limitations, restricting the use of the autopsy to in-hospital deaths in these settings introduces an important bias, as the causes of death there may differ from the deaths occurring in the community not reaching the health facilities.
Thus, there is an urgent need to develop simpler and more feasible methods to ascertain accurately the most frequent causes of peri-neonatal death. In recent years, minimally invasive autopsy (MIA) techniques have been developed with this aim [11,14–17]. This method, which involves directed sampling using biopsy needles, provides fluids and tissue samples for pathological and microbiological analyses. The procedure is simple and could be easily conducted by trained technicians. We have recently validated the MIA against the CDA in a series of in-hospital adult deaths from Mozambique (http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002173) [18,19] and in a series of pediatric deaths in the same setting [20].
We are presenting here a study on the validity of the MIA to determine the cause of death in a series of stillbirths and neonatal deaths occurring at Maputo Central Hospital (MCH), Mozambique, by comparing the MIA diagnosis with the gold standard diagnosis obtained by the CDA. This study is part of a larger project that also validated the MIA to determine the cause of death in pediatric, maternal, and adult deaths.
Methods
Study setting and design
This observational study received the approval of the Clinical Research Ethics Committee of the Hospital Clinic of Barcelona (Spain; approved, File 2013/8677) and the National Bioethics Committee of Mozambique (Mozambique; approved, Ref. 342/CNBS/13). Verbal informed consent was obtained from the relatives of the patients included in the study.
This was a prospective observational study undertaken at the Department of Pathology of MCH, a 1,500-bed government-funded quaternary hospital in southern Mozambique. The complete validation study was carried out between November 2013 and March 2015; details of the study are reported elsewhere [18]. Between April and June 2014, all stillbirths and neonatal deaths fulfilling the inclusion criteria were included in the study; these were (1) a CDA requested by the clinician as part of the medical evaluation; (2) a verbal informed consent to perform the autopsy given by the relatives; (3) a definition of stillbirth (birth of a dead baby with birthweight ≥ 1,000 g, ≥28 completed weeks gestation, or body length ≥ 35cm) [21,22] or neonatal death (a death within 28 days of birth, which includes early neonatal deaths, from 1–7 days, and late neonatal deaths, from 8–28 days) [22,23]; and (4) a less than 24-hour period from death or delivery for neonates and stillborn babies, respectively. Deaths of traumatic origin in neonates were excluded. During this period, up to 2 coupled MIAs and CDAs were conducted per day.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement and the prospective analysis plan are included as supplementary information (S1 and S2 Text, respectively). All relevant data are within the paper and its Supporting Information files. Additional data are available upon request, in accordance with the consortium agreement signed by the CaDMIA project partnership. Data use and transfer are monitored by ISGlobal’s Biostatistics and Data Management Unit (contact email: ubioesdm@isglobal.org).
The overall study plan, indicating where the procedures were performed, the investigators involved, and the site and timing of each procedure, has been reported elsewhere [18].
Sample size calculation was done assuming an expected prevalence of infectious diseases as causes of death for all age groups in the overall validation study. A sample size was not estimated to assess the concordance between the MIA and the CDA for each age group and each category of disease. Instead, we included all cases available that met the inclusion criteria during the study period.
Autopsy procedures
Detailed MIA pathological methods have been reported elsewhere [24,25]. In brief, the procedure included an initial disinfection of the surface of the body, followed by the collection of blood and cerebrospinal fluid, aiming to obtain 10–20 mL of these fluids. Finally, solid organs (liver, lungs, central nervous system, heart, spleen, and kidneys) were punctured using biopsy needles (14–16G). Immediately after the MIA, a second pathologist performed the CDA. In brief, a dissection with macroscopic evaluation of all organs was performed following a standardized protocol for perinatal and pediatric autopsies [26]. Samples from the same viscera collected in the MIA and from any grossly identified lesions were taken during the CDA.
Histological and microbiological analyses
Two pathologists and 2 microbiologists reviewed and analyzed the samples from the MIA while blinded to any clinical information and to the results of the CDA. All samples collected for histology were stained with hematoxylin and eosin. Histochemical (e.g., PAS stain) and/or immunohistochemical staining (e.g., cytomegalovirus) were used, when needed, to confirm the diagnosis. Microbiological methods have been reported in detail elsewhere [25]. In brief, universal screening that included detection of Plasmodium falciparum (by PCR), antibodies against human immunodeficiency virus (HIV)-1/2 and against hepatitis C virus, hepatitis B surface antigen, pathogens (by real-time PCR) included in the TORCH group (Toxoplasma gondii, others [includes Treponema pallidum, parvovirus B19, and lymphocytic choriomeningitis virus], rubella virus, cytomegalovirus, and herpes virus), and respiratory viruses was performed on all cases, as well as culture of organisms (bacterial and fungal) using samples from the blood, cerebrospinal fluid (CSF), liver, lungs, and central nervous system. In addition, we performed a multiplex real-time PCR test for detection of Streptococcus pneumoniae, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae, and Escherichia coli using a commercial assay (Roche Modular assay). HIV viral load was determined in samples positive for antibodies against HIV. Other microorganisms were tested depending on the pathological findings observed in the MIA-obtained tissues.
Samples from the CDA were analyzed following the same strategy used for the analysis of the MIA samples. The microbiological results of the blood and CSF were also included in the CDA evaluation. The same team of experts evaluated the samples of the CDA without considering the results of the MIA in order to focus on the differences between the 2 methods while minimizing the potential for interobserver variation.
As described elsewhere [18], 2 scales were developed to grade the strength of the evidence of the findings: one was based on the severity of the pathological findings, and the other on the distribution and type of the microorganisms identified. These scales were used in the determination of the certainty of the cause-of-death attribution in both the MIA diagnosis and the CDA diagnosis. For stillborn babies and neonates, the following adaptations were applied to the scales in order to address the particularities of these 2 groups:
Body weight was considered as a pathological finding. In stillbirths, weight at birth < the 10th percentile for gestational age and sex (using the INTERGROWTH-21st standards in a stillborn baby [27–29]) was considered as level 2 pathological evidence of fetal growth restriction.
Cytomegalovirus and group B streptococcus needed to be always identified in at least 2 different samples to be considered as etiological agents. In the case of cytomegalovirus identification, real-time PCR Ct values ≥ 35 were considered as nonconclusive in the absence of histological evidence.
In stillborn babies, strong evidence was given to the identification of pathogens of the TORCH group (except cytomegalovirus), varicella-zoster virus, parvovirus B19, LCMV, group B streptococcus, and L. monocytogenes.
In neonates, strong evidence was given to the identification of S. pneumoniae, H. influenzae, N. meningitidis, Salmonella spp., and Bordetella pertussis.
Determination of the cause of death
Once all the analysis of the MIA samples had been completed, a panel composed of a pathologist, a microbiologist, an obstetrician, and a pediatrician with expertise in neonatology evaluated all the data of the MIA and assigned the MIA diagnosis. Investigators involved in the MIA diagnosis were aware of the external macroscopic examination, including anthropometrical measures, and the histological and microbiological results obtained in the MIA sampling but were blind to any other information, including the clinical records and the CDA results. After a washout period (minimum time 3 months, range 3–6 months), the same panel evaluated the data from the CDA and the clinical records and assigned the final diagnosis of the cause of death (CDA diagnosis), which was considered the gold standard. Investigators involved in the CDA diagnosis were aware of the macroscopic (external and internal), histological, and microbiological results and clinical information but were blind to the MIA results. Maternal clinical information was available in 16/18 (89%) stillborn babies and in 24/41 (59%) neonates.
According to the WHO application of the International Classification of Diseases, 10th revision (ICD-10) to deaths during the perinatal period (ICD-PM), a chain of conditions was established following the most probable chronological sequence of events leading to death [28]. When more than 1 severe pathological and/or microbiological diagnosis was identified, the disease most likely causing the death was considered the final diagnosis. Diseases contributing (or possibly contributing) to the death were classified as other diseases or conditions (e.g., prematurity) [28]. Other incidental conditions or concomitant infections not related to the chain of events leading to death were considered as other conditions not likely contributing to the death (e.g., otitis media). Maternal conditions or diseases (e.g., HIV infection) were considered as part of the pathway leading to fetal or infant death.
In each age group, the diseases causing death identified in the CDA or in the MIA were classified into major categories. Thus, in stillbirths the causes of death were classified into 5 categories: (1) fetal growth restriction, (2) infectious diseases, (3) intrauterine hypoxia, (4) intrapartum hypoxia, and (5) nonconclusive. Fetal growth restriction was considered as the cause of death when no other pathological and/or microbiological finding potentially causing death was identified. In neonates, the final cause-of-death diagnoses were classified as (1) infectious diseases, (2) intrapartum complications, (3) preterm complications, (4) congenital malformations, (5) other conditions, and (6) nonconclusive.
Definitions and statistical methods
The diagnosis of fetal growth restriction was applied to all stillborn babies who were small for gestational age, which was defined as weight at birth < the 10th percentile for gestational age and sex using the INTERGROWTH-21st standards in a stillborn baby [27–29]. Following the ReCoDe classification [30], fetal growth restriction was considered as the direct cause of death in stillbirths, whereas any underlying maternal condition was included in the chain of events. Intrauterine hypoxia and intrapartum hypoxia were defined as the presence of histological signs of hypoxia in the lungs (alveolar capillary dilatation and congestion with interstitial edema, focal hemorrhage, and alveolar ducts distended by squamous epithelial cells) [31] in a macerated or fresh stillborn baby, respectively, in the absence of fetal growth restriction. Intrapartum complications were diagnosed by the presence of signs of hypoxia in the histological examination of the lungs plus a history of intrapartum disorders (e.g., prolonged labor). Prematurity was defined as a gestational age less than 37 weeks, and low birth weight as less than 2.5 kg [27,32]. Maternal HIV infection was defined as the presence of HIV antibodies in fetal or neonatal blood.
The concordance between the categories of diseases obtained by the MIA and the CDA (gold standard) diagnoses was assessed by the Kappa statistic, and it was interpreted as suggested by Landis and Koch [33]. The diagnostic accuracy of the MIA to identify the categories established by the CDA diagnosis was evaluated as sensitivity, specificity, positive and negative predictive values, and the total percentage of cases correctly classified.
Results
Characteristics of stillbirths and neonatal deaths
Coupled MIA and CDA procedures were performed in 18 stillborn babies and 41 neonates. Twelve (67%) stillborn babies were macerated (3 grade I, 4 grade II, and 5 grade III). The median gestational age of the stillborn babies was 36 weeks (range 30 to 40). The range of weights at birth varied between 1,050 and 3,500 g. Twenty-nine (71%) neonates died during the early neonatal period, and 12 (29%) in the late neonatal period. Seven (39%) stillborn babies and 6 (15%) neonates tested positive for antibodies against HIV (all of them HIV-1). In addition, HIV RNA was detected by PCR in 1 neonate. In 3 (17%) stillborn babies, there was a history of malaria during pregnancy, while in 5 (12%) neonates P. falciparum was detected by PCR in blood collected onto filter paper. The sex of the babies, neonatal age at death, and the number of preterm and low-birth-weight babies are shown in Table 1.
Tab. 1. Characteristics of stillbirths and neonatal deaths. 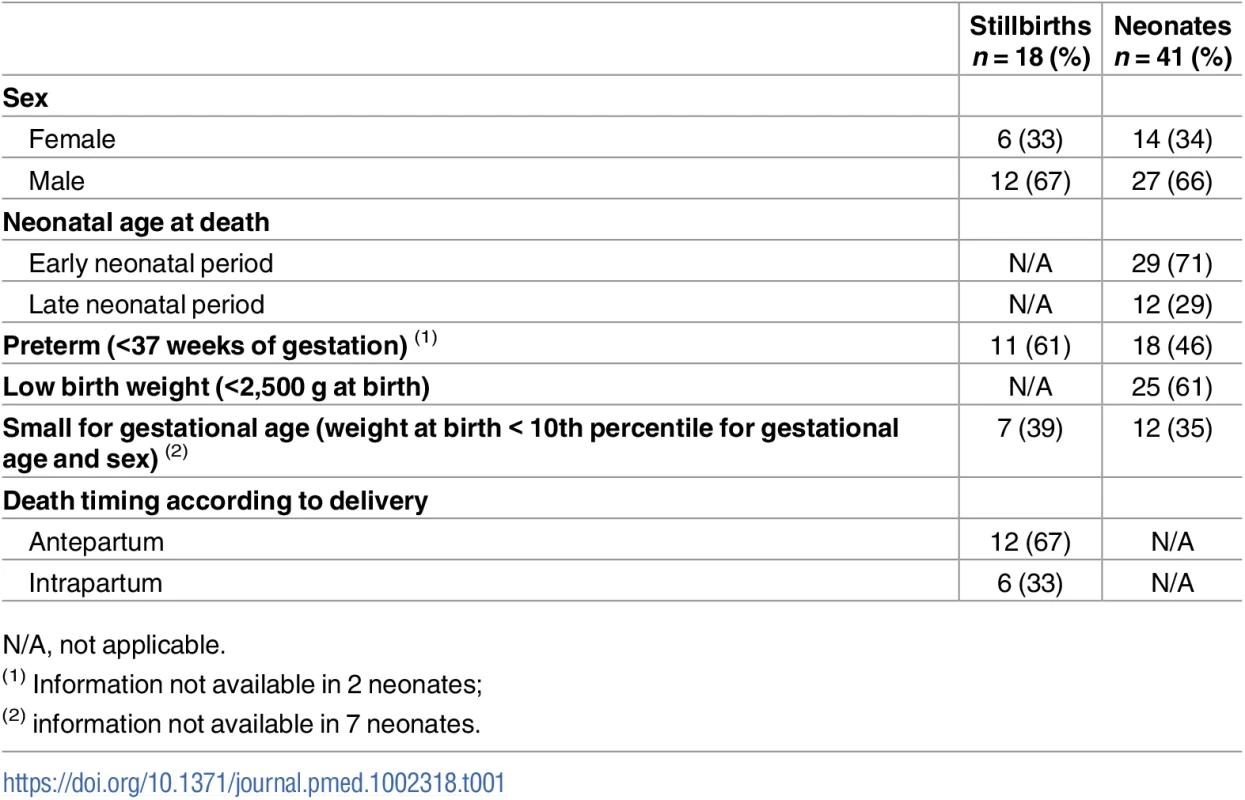
N/A, not applicable. Less than 10 mL of blood was obtained in 1 (6%) stillbirth and in 3 (7%) neonates. In all these cases, neither antibodies against HIV-1/2 nor viral load could be determined because of insufficient plasma volume. All the mothers of the study participants were known to be alive except in 3 cases for which this information was not available.
Concordance between the MIA diagnosis and the CDA diagnosis in stillborn babies
A cause of death was identified in the CDA (gold standard) in 16 out of 18 (89%) stillbirths. Fetal growth restriction accounted for 7 (39%), infectious diseases for 4 (22%), intrauterine hypoxia for 2 (11%), and intrapartum hypoxia for 3 (17%) of the deaths (Table 2). A MIA diagnosis of cause of death was identified in 15 out of 18 (83%) stillborn babies. Table 2 shows the concordance between the MIA and the CDA diagnoses of stillborn babies, grouped according to the major disease categories. The MIA categorization of disease showed a substantial concordance with the gold standard (Kappa = 0.78, 95% CI [0.56–0.99]) and agreed in 15/18 (83%) of the cases.
Tab. 2. Concordance of the categorization of the causes of death established by the complete diagnostic autopsy (CDA, gold standard) and the minimally invasive autopsy (MIA) in stillbirths. 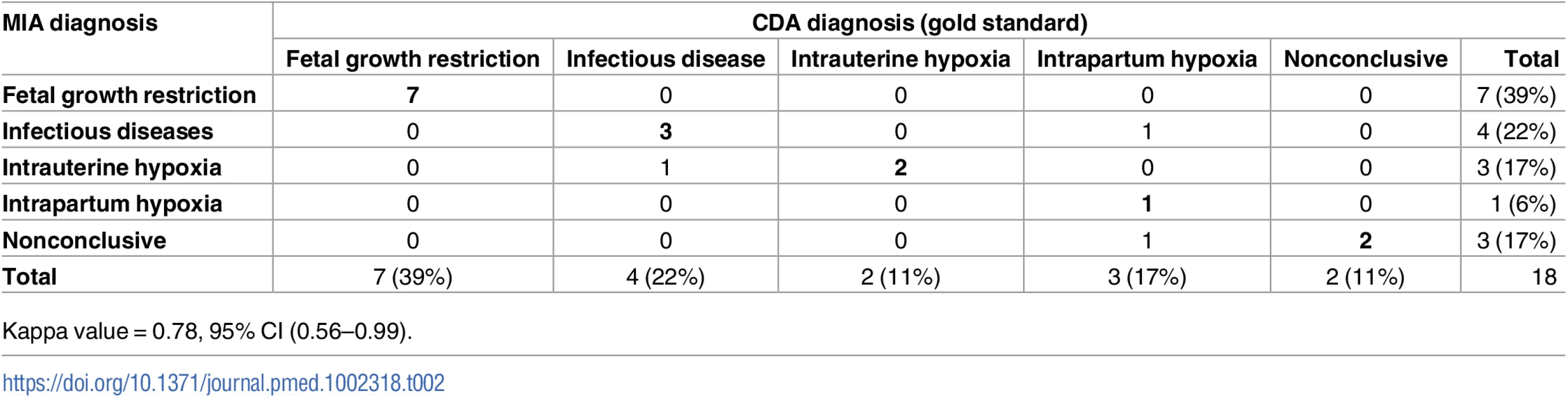
Kappa value = 0.78, 95% CI (0.56–0.99). S1 Table shows the diagnoses obtained in the CDA (gold standard) and the MIA for each case. In the 7 stillbirths with fetal growth restriction, the information registered in the obstetric clinical record identified a maternal condition likely to be associated with the intrauterine growth disorder as follows: a clinical history of eclampsia/preeclampsia was identified in 3 cases (one of the women also had a record of malaria infection and anemia during pregnancy, and another was also positive for HIV), maternal anemia alone was identified in 1 case, maternal HIV infection alone was detected in 2 cases, and 1 case was a multiple pregnancy (triplets). Maternal HIV infection was reported in the obstetric history of 7 stillborn babies; this infection was identified in the MIA through detection of HIV antibodies in fetal blood, with those making the identification blinded to the obstetric clinical record (S1 Table). Group B streptococcus (GBS) infection was identified in 3 stillborn babies in both the MIA and the CDA, but only in 2 of them was the GBS infection considered the cause of death in the CDA (in 1 of them, maternal HIV infection was detected in the MIA and reported in the obstetric record).
Concordance between the MIA diagnosis and the CDA diagnosis in neonates
The CDA identified a diagnosis in all cases (100%). Of them, 27 (66%) were diagnosed with infectious diseases, 5 (12%) had preterm complications, 4 (10%) had congenital malformations, 3 (7%) were classified as having birth asphyxia due to intrapartum complications, and 2 (5%) were classified as having other conditions (Table 3). Neonatal sepsis accounted for most of the infectious diseases (21/27, 78%). A MIA diagnosis was identified in 35/41 (85%) of neonatal deaths. S2 Table shows the diagnoses obtained by the CDA and the MIA for each case. Four (10%) neonates had GBS infection, and in 3 of them it was considered the cause of death in the CDA.
Tab. 3. Concordance of the categorization of the cause of death established by the complete diagnostic autopsy (CDA, gold standard) and the minimally invasive (MIA) diagnosis in neonates. 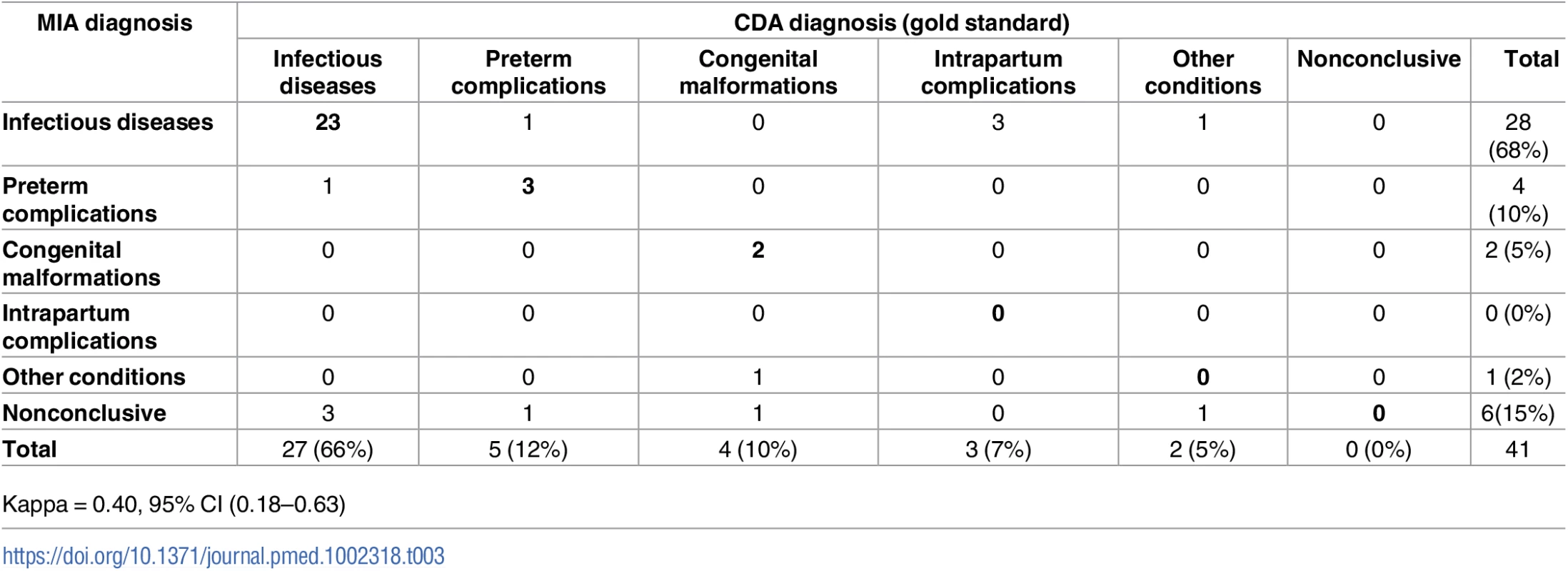
Kappa = 0.40, 95% CI (0.18–0.63) The categorization of cause of death according to the MIA diagnosis showed a moderate concordance with the categorization reached with the CDA (Kappa = 0.40, 95% CI [0.18–0.63]) and agreed in 28/41 (68%) of the neonatal deaths (Table 3). The concordance was higher for infectious diseases (23/27; 85%) and preterm complications (3/5; 60%) than for congenital malformations (2/4; 50%). None of the cases diagnosed in the CDA as intrapartum complications or other diseases were identified in the MIA.
Table 4 shows the list of etiologic agents identified in the CDA and the MIA and the number of cases in which the same etiologic agent was identified; for example, in the CDA, neonatal sepsis due to mixed infections of Enterobacteriaceae was diagnosed in 5 cases, while in the MIA this was diagnosed in 3 cases, and in 2 of these cases, the same etiologic agent was detected in both the MIA and CDA. The specific microorganisms causing death were identified in the CDA in 26/27 (96%) of the infectious deaths (Table 4). The MIA identified the same microorganism in 17/26 (65%) of the cases.
Tab. 4. List of microorganisms identified as the cause of death in the complete diagnostic autopsy (CDA, gold standard) and in the minimally invasive autopsy (MIA) in neonates. 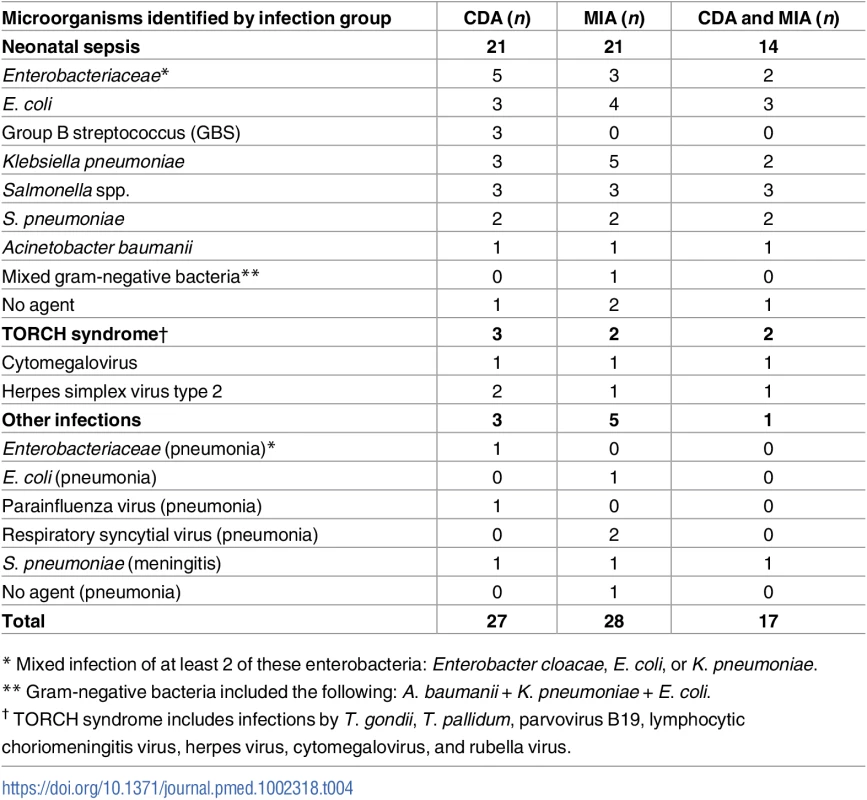
* Mixed infection of at least 2 of these enterobacteria: Enterobacter cloacae, E. coli, or K. pneumoniae. Diagnostic accuracy of the MIA
Table 5 shows the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the MIA for the different diagnostic categories in the CDA. Although the numbers are small, there was a high percentage of accuracy of the MIA for the different diagnostic categories both in stillbirths and neonatal deaths (>75%).
Tab. 5. Sensitivity, specificity, positive and negative predictive value (PPV and NPV), and accuracy of the minimally invasive autopsy (MIA) for the different diagnostic categories in stillbirths and neonates. 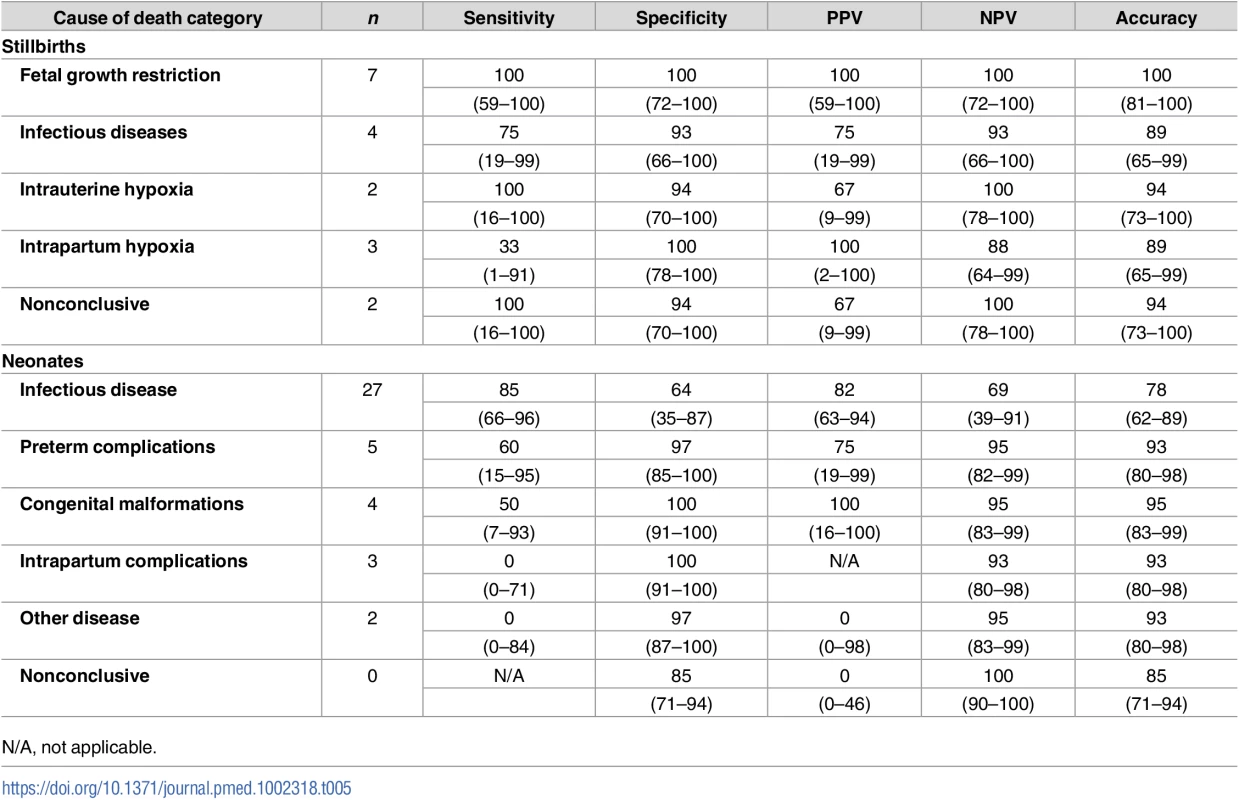
Figures are percentages and 95% confidence intervals. Other significant conditions contributing to death in stillborn babies and neonates
No other significant fetal condition contributing to death was detected in the series of stillbirths. A maternal condition contributing to death was identified in 14 out of 18 (78%) stillborn babies (S1 Table). Maternal HIV and preeclampsia (7/14, 50%, and 4/14, 29%, respectively) were the most frequent underlying maternal conditions. The other 3 cases were 1 maternal pneumonia, 1 multiple pregnancy (triplets), and 1 umbilical cord prolapse. Maternal malaria was identified as other significant condition leading to death in 3/18 cases (17%).
In neonates, other significant conditions contributing to death were identified in 27 out of 41 (66%) cases (S2 Table). The most frequent condition was prematurity, observed in 17/27 (63%) of the neonates. The remaining conditions consisted of 4 intrapartum complications, 2 cases of omphalitis, 1 case of omphalocele, 1 case of meningitis, 1 case of intra-uterine HIV infection, and 1 case of necrotizing enterocolitis. In 20/27 cases (74%), these contributory conditions were identified in the MIA procedure. Maternal HIV infection and congenital malaria (5/41,12%, and 1/41, 2%, respectively) were identified as maternal conditions contributing to death (S2 Table).
Discussion
To our knowledge, this is the first study to evaluate the validity of a minimally invasive postmortem procedure in stillbirths and neonatal deaths by comparing the results obtained with this approach with those achieved in the CDA, the gold standard for cause-of-death determination. The results show that the MIA diagnosis has a high concordance and percentage of agreement with the CDA diagnosis, and thus, the MIA could be a useful tool for cause of death determination in both stillborn babies and neonates. The MIA may contribute to obtaining more accurate information on the causes of mortality in these particular groups, which is essential in guiding the target and design of new interventions, as well as increasing the effectiveness of those already implemented [8].
Fetal growth restriction and infectious diseases were the most frequent causes of death in stillbirths, followed by intrauterine and intrapartum hypoxia. In 7 stillborn babies, the mother had HIV infection, which could be ascertained in the MIA through detection of HIV antibodies in the fetal blood. Thus, not only the cause of death but also some maternal medical conditions contributing to death could be identified in the MIA even in the absence of any clinical information from the obstetric record. Although the obstetric history is important in determining the cause of death, mainly in stillbirths, we were able to ascertain the cause of death in the MIA in the majority of stillbirths and neonates without making use of the obstetric clinical record. This is of relevance since in many high-burden settings the maternal record is not available because the delivery usually takes place outside the health facility or, if it exists, is often incomplete. However, some relevant maternal conditions known to be the underlying cause of fetal deaths, particularly those causing fetal growth restriction, such as preeclampsia/eclampsia and maternal anemia, will not be captured unless specific clinical data are obtained. In addition, relevant information could be also indirectly retrieved by including a careful placental examination as part of the MIA.
In 2 stillborn babies in whom the cause of death was related to intrauterine hypoxia both in the MIA and the CDA, only maternal HIV antibodies were detected. In addition, maternal HIV infection was observed in another 2 growth-restricted cases. Thus, maternal HIV infection was likely to be indirectly responsible for the death of more than a third of the stillbirths in this series. With the limitation intrinsic to small numbers, this figure is much higher than the recent estimation of the attribution of stillbirths to HIV in sub-Saharan Africa (0.7%) [8]. Although antiretroviral drugs and programs of prevention of mother-to-child transmission have been scaled up in the last decade, the prevalence of HIV infection remains high in Mozambique [34]. From these findings, it is clear that more efforts are needed to control this infection, which would have a significant impact in reducing a considerable proportion of preventable fetal deaths. In the same systematic review, it has been estimated that maternal malaria is associated with 20% of stillbirths in sub-Saharan Africa [8]. In our series, a history of malaria infection during pregnancy was reported in 3 stillborn babies (17%) whose final causes of death were determined as intrauterine hypoxia (2) and fetal growth restriction (1). Larger series of postmortem studies in rural areas with higher malaria endemicity than urban Maputo are needed to conclusively establish the impact of malaria in pregnancy as a cause of fetal deaths.
Infectious diseases were the second most important cause of death in stillbirths in this study, which is in agreement with previously reported estimations [8]. There were 2 cases of GBS infection, 1 case of chorioamnionitis, and 1 case of a disseminated E. coli infection. Though the numbers are small, the proportion (16%) of GBS-related stillbirths observed in this study is higher than that reported in a recent systematic review [35]. In 2 cases, the CDA and the MIA were concordant, while in the third case GBS infection was only considered as being the cause of death in the MIA. Two of these cases were observed in macerated stillbirths, indicating that death was likely to have occurred antepartum. Although GBS infection seems to be a more frequent cause of neonatal sepsis at least in high-income countries, GBS-associated stillbirths are not infrequent, and their prevention would require a different strategy than the currently recommended intrapartum antibiotic prophylaxis for colonized or at-risk women [36].
In stillborn babies, an external examination of the body determining whether it is fresh or macerated provides information on timing of demise, which is critical to classify the death as occurring ante - or intrapartum. In contrast with high-income countries, in low - and middle-income regions the majority of stillbirths are intrapartum [8,10,36]. In this study, more than a third of the stillbirths were intrapartum defined by the absence of skin maceration. However, it cannot be ruled out that in a number of macerated stillborn the death also occurred during labor if there was a delay in the access to adequate intrapartum care. Therefore, in contexts of scarce resources and limited access to high-quality care, it is difficult to get a precise estimate of the intrapartum stillbirth rate. This limits the use of this indicator as a marker of fetal death avoidable through improved care during labor, precisely in settings where it could be more helpful. Similarly, a challenge in resource-poor settings is distinguishing between intrapartum stillbirths and very early neonatal deaths. However, to our knowledge it is not possible to differentiate between the 2 conditions in the histological examination, and it was not part of the study to make this distinction; thus, we had to rely on the clinical judgment and the macroscopic examination.
In neonates, infectious diseases accounted for the majority of deaths (66%), followed by preterm complications, congenital malformations, and intrapartum-related complications. Most of the infections were sepsis, and congenital infections accounted for 3 cases of TORCH (2 herpesvirus type 2 and 1 cytomegalovirus), 2 cases of pneumonia, and 1 case of meningitis. As for stillbirths, GBS infection seemed to be an important cause of death in this group, either as the final cause (3 cases) or as a coinfection with CMV and HIV (1 case). Only in the latter case was there concordance between the MIA and the CDA. In neonates, the concordance between the CDA and the MIA was moderate, being the highest percentage of concordance (23/27, 85%) for infectious diseases. Of note, all intrapartum complications in the CDA examination were missed in the MIA. It is important to remember that as it is routine practice, in this study the clinical data, which include the obstetric record, were part of the information evaluated for cause-of-death attribution in the CDA, but not in the MIA. Therefore, the CDA alone without the clinical information would have probably missed these cases as well; on the other hand, inclusion of this information in the MIA cause-of-death attribution would have probably captured these cases. It is also important to notice that the microorganism could be identified in most deaths due to infectious diseases in the CDA but also in the MIA. This information is critical to improve the specificity and, therefore, the cost-effectiveness of targeted interventions and may also help to reduce the burden of antibiotic resistance in relation to the cause of death, a major global public health problem [37]. Although preterm labor is usually mentioned as a cause of neonatal death, this is usually a midfactor in the causal pathway. In this study, although prematurity was identified in over 40% of neonatal deaths, it was considered generally an underlying condition and not the final cause of death. Importantly, we could determine factors that may be causing prematurity (most of them infections), which are the ones that should be tackled by specific interventions. The proportion of male babies was higher than that of female babies both for stillbirths and neonatal deaths. The excess risk of death in male fetuses and newborns has been reported [38]. Although the reasons are unclear, it is suggested to be due to an increased risk of preterm birth and fetal growth restriction and X-linked congenital abnormalities, as well as to a higher incidence of placental vascular conditions [38].
The objective of this validation study was not to describe the causes of stillbirths and neonatal deaths. Thus, although the main causes of mortality identified in this study are similar to those previously reported so far, a conclusive and comprehensive description would need larger studies in different settings. Similarly, because of small numbers, the precision in the estimates of proportions is limited. Consequently, the sensitivity, specificity, positive predictive value, negative predictive value, and the percentage of cases correctly classified may vary with slight modifications in the classification of a few cases. In addition, a limitation of this study in the case of stillbirths is the lack of inclusion of early fetal deaths in the validation of the MIA against the CDA. This should be considered in future validations of this methodology. It is noteworthy that clinical findings, including maternal information, were essential to reach the final cause of death in a number of CDA diagnoses (6 cases). Thus, it is likely that the inclusion of clinical and obstetric information or, in its absence, data provided by the verbal autopsy could significantly improve the results of the MIA in these age groups. We did not include placental histologic/microbiologic examination in this MIA protocol, which may have enriched the ascertainment of diagnosis in some cases, since some maternal conditions such as preeclampsia and chorioamnionitis can be recognized in the placenta, while intrauterine hypoxia could be caused by placental abnormalities. Fetal growth restriction in stillborn babies was estimated using weight and gestational age. The latter was the only parameter obtained from the clinical record because it was necessary to classify the stillbirth. In the absence of this information, other anthropometric measurements such as foot length could be used [8,26,39]. In both stillborn babies and neonates, the MIA showed a high concordance with the CDA for infectious diseases but performed poorly in detecting congenital malformations. The blind sampling scheme of the MIA protocol does not allow macroscopic observation of the internal organs, but the observation of the entire body prior to the procedure or pictures of it could help to identify some external malformations.
It has been stated that better data are critical to accelerate progress towards the target of 12 or fewer stillbirths per 1,000 births and 12 or less neonatal deaths per 1,000 live births by 2030 [5,8,40]. Better statistics on the number of these deaths and, even more importantly, accurate information on the causes of mortality are essential for health planning, priority setting, designing effective health programs, and evaluating their impact, besides playing a role in the accountability of implemented interventions. The MIA approach may contribute to reducing peri-neonatal deaths from the more than 5 million annual deaths, by providing a tool that can be utilized by trained nurses and midwives in rural areas, where at least 60% of stillbirths and neonatal deaths occur, to establish the cause of death.
Conclusion
Most peri - and neonatal deaths occur in countries with very scarce resources to undertake more complex postmortem examinations, relying on verbal autopsies (VAs) to determine the cause of death in particular for births outside health facilities. However, VAs have many limitations in accurately establishing the cause of death in these groups [41]. The MIA approach, although still imperfect as compared to the gold standard (CDA plus clinical information), may provide unique information for health prioritization, which currently relies just on both indirect and inaccurate estimations and assumptions on the causes of mortality of stillborn babies and neonates in most parts of the world. This approach should also help at saving global health funds by directing expenditures to the most-effective health programs.
Supporting Information
Zdroje
1. Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, Bryce J, et al. Countdown to 2015 decade report (2000–10): taking stock of maternal, newborn, and child survival. Lancet. 2010 Jun 5;375(9730):2032–44. doi: 10.1016/S0140-6736(10)60678-2 20569843
2. Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010 Jun 5;375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1 20466419
3. United Nations Millennium Development Goals website. Background page retrieved 16 June 2009 [Internet]. 2009. http://www.un.org/millenniumgoals/bkgd.shtml
4. Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet. 2010 Apr 24;375(9724):1482–90. doi: 10.1016/S0140-6736(09)61712-8 20223514
5. Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, et al. Stillbirths: Where? When? Why? How to make the data count? The Lancet. 2011;377(9775):1448–1463.
6. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012 Jun 9;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1 22579125
7. Unated Nations. UN.Transforming our World: The 2030 Agenda for Sustainable Development. Resolution adopated by the General Assambly by September 25, 2015. New York [Internet]. 2015. http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E
8. Froen JF, Friberg IK, Lawn JE, Bhutta ZA, Pattinson RC, Allanson ER, et al. Stillbirths: progress and unfinished business. Lancet. 2016 Feb 6;387(10018):574–86. doi: 10.1016/S0140-6736(15)00818-1 26794077
9. Bartlett LA, Mawji S, Whitehead S, Crouse C, Dalil S, Ionete D, et al. Where giving birth is a forecast of death: maternal mortality in four districts of Afghanistan, 1999–2002. Lancet. 2005 Mar 5;365(9462):864–70. doi: 10.1016/S0140-6736(05)71044-8 15752530
10. Thonneau PF, Matsudai T, Alihonou E, De SJ, Faye O, Moreau JC, et al. Distribution of causes of maternal mortality during delivery and post-partum: results of an African multicentre hospital-based study. EurJObstetGynecolReprodBiol. 2004 Jun 15;114(2):150–4.
11. Breeze AC, Jessop FA, Set PA, Whitehead AL, Cross JJ, Lomas DJ, et al. Minimally-invasive fetal autopsy using magnetic resonance imaging and percutaneous organ biopsies: clinical value and comparison to conventional autopsy. Ultrasound ObstetGynecol. 2011 Mar;37(3):317–23.
12. Fligner CL, Murray J, Roberts DJ. Synergism of verbal autopsy and diagnostic pathology autopsy for improved accuracy of mortality data. Popul Metr. 2011 Aug 1;9 : 25. :25–9. doi: 10.1186/1478-7954-9-25 21806831
13. Gordijn SJ, Erwich JJ, Khong TY. Value of the perinatal autopsy: critique. PediatrDevPathol. 2002 Sep;5(5):480–8.
14. Breeze AC, Jessop FA, Whitehead AL, Set PA, Berman L, Hackett GA, et al. Feasibility of percutaneous organ biopsy as part of a minimally invasive perinatal autopsy. Virchows Arch. 2008 Feb;452(2):201–7. doi: 10.1007/s00428-007-0548-7 18087719
15. Sebire NJ. Towards the minimally invasive autopsy? Ultrasound ObstetGynecol. 2006 Dec;28(7):865–7.
16. Sebire NJ, Weber MA, Thayyil S, Mushtaq I, Taylor A, Chitty LS. Minimally invasive perinatal autopsies using magnetic resonance imaging and endoscopic postmortem examination (“keyhole autopsy”): feasibility and initial experience. JMaternFetal Neonatal Med. 2012 May;25(5):513–8.
17. Thayyil S, Chitty LS, Robertson NJ, Taylor AM, Sebire NJ. Minimally invasive fetal postmortem examination using magnetic resonance imaging and computerised tomography: current evidence and practical issues. PrenatDiagn. 2010 Aug;30(8):713–8.
18. Castillo P, Martinez MJ, Ussene E, Jordao D, Lovane L, Ismail MR, et al. Validity of a Minimally Invasive Autopsy for Cause of Death Determination in Adults in Mozambique: An Observational Study. PLoS Med. 2016;13(11):e1002171. doi: 10.1371/journal.pmed.1002171 27875530
19. Maixenchs M, Anselmo R, Zielinski-Gutierrez E, Odhiambo FO, Akello C, Ondire M, et al. Willingness to Know the Cause of Death and Hypothetical Acceptability of the Minimally Invasive Autopsy in Six Diverse African and Asian Settings: A Mixed Methods Socio-Behavioural Study. PLoS Med. 2016;13(11):e1002172. doi: 10.1371/journal.pmed.1002172 27875532
20. Bassat Q, Castillo P, Martínez MJ, Jordao D, Lovane L, Hurtado JC, et al. Validity of a minimally invasive autopsy tool for cause of death determination in pediatric deaths in Mozambique: An observational study. PLoS Med. 2017;14(6):e1002317. https://doi.org/10.1371/journal.pmed.1002317
21. March of Dimes P Save the Children,World Health Organization (WHO). Born too soon: the global action report on preterm birth. World Health Organ [Internet]. 2012; http://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf
22. World Health Organization. Making Every Baby Count. Audit and review of stillbirths and neonatal deaths. World Health Organ [Internet]. 2016; http://apps.who.int/iris/bitstream/10665/249523/1/9789241511223-eng.pdf?ua=1
23. Oza S, Cousens SN, Lawn JE. Estimation of daily risk of neonatal death, including the day of birth, in 186 countries in 2013: a vital-registration and modelling-based study. Lancet GlobHealth. 2014 Nov;2(11):e635–44.
24. Castillo P, Ussene E, Ismail MR, Jordao D, Lovane L, Carrilho C, et al. Pathological Methods Applied to the Investigation of Causes of Death in Developing Countries: Minimally Invasive Autopsy Approach. PLoS ONE. 2015;10(6):e0132057. doi: 10.1371/journal.pone.0132057 26126191
25. Martinez MJ, Massora S, Mandomando I, Ussene E, Jordao D. Infectious cause of death determination using minimally invasive autopsies in developing countries. Diagn Microbiol Infect Dis. 2016;84(1879–0070 (Electronic)):80–6.
26. Bove KE. Practice guidelines for autopsy pathology: the perinatal and pediatric autopsy. Autopsy Committee of the College of American Pathologists. ArchPatholLab Med. 1997 Apr;121(4):368–76.
27. Allanson ER, Tuncalp O, Gardosi J, Pattinson RC, Francis A, Vogel JP, et al. The WHO application of ICD-10 to deaths during the perinatal period (ICD-PM): results from pilot database testing in South Africa and United Kingdom. BJOG. 2016 Nov;123(12):2019–28. doi: 10.1111/1471-0528.14244 27527122
28. Villar J, Cheikh IL, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014 Sep 6;384(9946):857–68. doi: 10.1016/S0140-6736(14)60932-6 25209487
29. Mayer C, Joseph KS. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound ObstetGynecol. 2013 Feb;41(2):136–45.
30. Gardosi J. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ. 2005 Nov 12;331(7525):1113–7. doi: 10.1136/bmj.38629.587639.7C 16236774
31. Claireaux AE. Pathology of perinatal hypoxia. JClinPatholSuppl RCollPathol. 1977;11 : 142–8.:142–8.
32. World Health Organization. ICD-10 Volume 2 Instruction Manual [Internet]. 2016. http://apps.who.int/classifications/icd10/browse/Content/statichtml/ICD10Volume2_en_2016.pdf?ua=1
33. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–74. 843571
34. United Nations Programme on HIV/AIDS. UNAIDS Global Progress Report 2016, Mozambique [Internet]. 2016. http://www.unaids.org/sites/default/files/country/documents/MOZ_narrative_report_2016.pdf
35. Nan C, Dangor Z, Cutland CL, Edwards MS, Madhi SA, Cunnington MC. Maternal group B Streptococcus-related stillbirth: a systematic review. BJOG. 2015 Oct;122(11):1437–45. doi: 10.1111/1471-0528.13527 26177561
36. Di Renzo GC, Melin P, Berardi A, Blennow M, Carbonell-Estrany X, Donzelli GP, et al. Intrapartum GBS screening and antibiotic prophylaxis: a European consensus conference. JMaternFetal Neonatal Med. 2015 May;28(7):766–82.
37. Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013 Dec;13(12):1057–98. doi: 10.1016/S1473-3099(13)70318-9 24252483
38. Broere-Brown ZA, Schalekamp-Timmermans S, Hofman A, Jaddoe V, Steegers E. Fetal sex dependency of maternal vascular adaptation to pregnancy: a prospective population-based cohort study. BJOG. 2016 Jun;123(7):1087–95. doi: 10.1111/1471-0528.13519 26179828
39. Bukowski R, Hansen NI, Willinger M, Reddy UM, Parker CB, Pinar H, et al. Fetal growth and risk of stillbirth: A population-based case-control study. PLoS Med. 2014;11(4):e1001633. doi: 10.1371/journal.pmed.1001633 24755550
40. World Health Organization. Every Neonate Action Plan [Internet]. 2014. http://www.who.int/maternal_child_adolescent/topics/newborn/every-newborn-action-plan-draft.pdf
41. World Health Organization. Verbal autopsy standards—World Health Organization [Internet]. 2012. http://www.who.int/healthinfo/statistics/WHO_VA_2012_RC1_Instrument.pdf
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 6- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Vaccination to prevent human papillomavirus infections: From promise to practice
- Reducing US cardiovascular disease burden and disparities through national and targeted dietary policies: A modelling study
- Contribution of cognitive performance and cognitive decline to associations between socioeconomic factors and dementia: A cohort study
- Modelled health benefits of a sugar-sweetened beverage tax across different socioeconomic groups in Australia: A cost-effectiveness and equity analysis
- Risk factors and short-term projections for serotype-1 poliomyelitis incidence in Pakistan: A spatiotemporal analysis
- The US President’s Malaria Initiative and under-5 child mortality in sub-Saharan Africa: A difference-in-differences analysis
- Estimating the causal influence of body mass index on risk of Parkinson disease: A Mendelian randomisation study
- Low-intensity cognitive-behaviour therapy interventions for obsessive-compulsive disorder compared to waiting list for therapist-led cognitive-behaviour therapy: 3-arm randomised controlled trial of clinical effectiveness
- Population-level impact of an accelerated HIV response plan to reach the UNAIDS 90-90-90 target in Côte d’Ivoire: Insights from mathematical modeling
- Validity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: An observational study
- Malaria control adds to the evidence for health aid effectiveness
- Effectiveness and equity of sugar-sweetened beverage taxation
- A Collection on the prevention, diagnosis, and treatment of sexually transmitted infections: Call for research papers
- Pathways and progress to enhanced global sexually transmitted infection surveillance
- Elimination of mother-to-child transmission of HIV and Syphilis (EMTCT): Process, progress, and program integration
- Assessing process, content, and politics in developing the global health sector strategy on sexually transmitted infections 2016–2021: Implementation opportunities for policymakers
- Validity of a minimally invasive autopsy tool for cause of death determination in pediatric deaths in Mozambique: An observational study
- Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide
- Vaccination to prevent human papillomavirus infections: From promise to practice
- A Collection on the prevention, diagnosis, and treatment of sexually transmitted infections: Call for research papers
- Elimination of mother-to-child transmission of HIV and Syphilis (EMTCT): Process, progress, and program integration
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



