-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control
Lopman and colleagues outline key findings from the PLOS Norovirus Collection. The global burden falls hardest on children, and efforts to develop vaccines focus primarily on this vulnerable group.
Published in the journal: . PLoS Med 13(4): e32767. doi:10.1371/journal.pmed.1001999
Category: Collection Review
doi: https://doi.org/10.1371/journal.pmed.1001999Summary
Lopman and colleagues outline key findings from the PLOS Norovirus Collection. The global burden falls hardest on children, and efforts to develop vaccines focus primarily on this vulnerable group.
Summary Points
Diagnostic improvements have fundamentally changed our understanding of norovirus. The current evidence suggests that disease burden of norovirus is high, second only to rotavirus as a cause of severe acute gastroenteritis in children in developed countries, and that it is a key cause of diarrhea-associated morbidity and mortality worldwide.
Young children experience the highest incidence of disease; severe outcomes are most common among young children and the elderly.
Immunity is of limited duration and is strain - or genotype-specific, with little or no protection conferred across genogroups.
Innate susceptibility to noroviruses is determined by the host’s genetics of glycan expression; individuals with a functional FUT2 gene (known as secretors) have greater susceptibility to certain common viruses.
Recent progress has been made in the development of in vitro cell culture for norovirus as well as in the identification of candidate immune correlates of protection.
Norovirus vaccines are steadily moving through the development pipeline. All of these products are based on the production of virus like particles (VLPs) or P particle subunit in expression systems. Initial human challenge studies have demonstrated safety, immunogenicity, and efficacy.
One of the challenges for developing targeted interventions, including a norovirus vaccine, is that many distinct population groups, based on demographics (e.g., children, elderly) or risk (e.g., food handlers, military, travelers, health care workers), are affected.
Introduction
Significant progress has been made towards the control of diarrheal diseases. Global diarrheal deaths for all ages have declined dramatically, from an estimated 2.6 million annually in 1990 to approximately 1.3 million in 2013 [1]. Over the same period, improvements specifically for children under 5 years of age have been most impressive, with rates of decline in diarrheal deaths at around 5% per year, in absolute numbers, to an estimate of 578,000 deaths in 2013 [2]. Diarrheal disease is now the fourth most common cause of mortality overall, but morbidity has not changed at the same pace, with diarrheal disease remaining the second most common cause of morbidity worldwide in children under the age of 5 years [3]. Many bacterial and parasitic pathogens are controllable through improvements in water, sanitation, and hygiene, facilitated by economic development, as is evidenced by the rapid declines in mortality; rotavirus disease has been significantly impacted through vaccination in virtually every setting where it has been introduced, and can be further impacted with additional introduction of rotavirus vaccines in countries [4].
Norovirus is ubiquitous, associated with 18% (95% CI: 17%–20%) of diarrheal disease worldwide, with significant burden of disease in high-, middle-, and low-income settings. It is estimated to cause 212,000 deaths annually worldwide; approximately 99% of these are estimated to occur in middle - and high-mortality countries [5]. According to these estimates, norovirus is the most common cause of diarrheal cases across for all ages, the second most common cause of diarrheal death in children under the age of 5 years, and the most common cause of diarrheal death over 5 years of age, with similar patterns across WHO regions. So while the overall mortality risks are likely to be much lower in high-income settings, high incidence of disease appears universal. For example, the Malnutrition and Enteric Disease Study (MAL-ED), conducted in eight low - and middle-income countries, found norovirus to be the first and second most common cause of diarrheal disease in the first and second year of life, respectively [6]. And in high - and middle-income countries with successful rotavirus vaccination programs, norovirus is the most common cause of pediatric gastroenteritis [7,8].
The increase in norovirus-related research over the last 15 years has been tremendous, concurrent with improvements in and more widespread availability of diagnostic methods. Included in this body of work are fundamental advances in our understanding, ranging from better burden-of-disease estimates [5,9,10], to the first demonstration of in vitro cell culture [11], to elucidation of key interactions that noroviruses have with other agents in the microbiome [11–13], to the demonstration that vaccination against human norovirus infection and disease is possible [14,15]. While many challenges remain before norovirus can be considered “controllable,” the confluence of recent advances gives us optimism. To this end, in February 2015, Centers for Disease Control and Prevention (CDC) and the Bill & Melinda Gates Foundation (BMGF), led by a scientific organizing committee from the areas of government, academia, and philanthropy, convened a symposium in Atlanta, Georgia, United States, with representatives from all these sectors in addition to vaccine developers. Our charge was to review the most up-to-date knowledge on norovirus with the aim of identifying key gaps and detailing the most critical studies to address them, all with the ultimate goal of guiding the development of a norovirus vaccine for the populations that stand to benefit most: children in the developing world. The full report from the convening can be found at http://www.cdc.gov/norovirus/downloads/global-burden-report.pdf. Many of the papers in this PLOS Collection were either presented at or inspired by the meeting.
Critical Knowledge Gaps
Basic epidemiological and disease burden data are lacking, especially from developing countries. There remains uncertainty and some scientific controversy in defining the norovirus disease burden.
With sensitive real-time quantitative PCR (RT-qPCR) assays, norovirus is frequently detected in stool of healthy individuals, complicating the interpretation of diagnostic results and disease attribution.
Understanding of natural immunity to norovirus remains incomplete.
The implications of genetic susceptibility for population health, viral evolution, and vaccines remains unclear.
Genotype-specific immune responses and antigenic variation of norovirus suggest that a polyvalent vaccine will be needed and may require updating when new pandemic strains emerge.
There is limited understanding of the relative roles that different age groups play in virus transmission. Better data coupled with appropriate models could help to devise vaccination strategies that lead to the greatest benefits at the population level.
Insights from this Collection
Global Economic Burden
In a recent PLOS Collection, the World Health Organization’s Global Estimates of the Burden of Foodborne Disease in 2010 were published. These estimates position norovirus as the most common cause of cases of and deaths from foodborne diarrhea disease and the fourth greatest burden in terms of disability-adjusted life years (DALYs). Norovirus was estimated to cause 684 million (95% Uncertainty Interval 491–1,112 million) episodes of diarrheal disease and 212,000 deaths, annually, for all ages and from all modes of transmission [5,10].
In our current Collection, Bartsch et al. extend those findings to consider global economic impacts of norovirus [16]. They estimate that globally, norovirus results in an economic burden of US$4.2 billion (95% UI: US$3.2–US$5.7 billion) in direct health system costs and US$60.3 billion (95% UI: US$44.4–US$83.4 billion) in societal costs annually. Two-thirds of that burden is a result of disease in children under the age of five years. Low-, middle-, and high-income countries all have a considerable economic burden, indicating that norovirus gastroenteritis is a truly global economic problem.
Local Burden Data
While there is clearly a lack of local, national, and regional studies from developing countries on the epidemiology and molecular diversity of norovirus, those gaps are beginning to be filled. In their systematic review, Mans et al. included data on 19 studies from 14 African countries. Overall, in these studies, norovirus was associated with 13.5% of diarrheal disease in children, and GII.4 strains predominated in the majority of studies. The authors identified lack of data in older children and adults as a critical gap in Africa [17]; the same gap exists for low - and middle-income settings globally [9].
A number of papers in this collection also offer new epidemiological data from specific populations from Asia, Africa, and North and South America. Shioda et al. present some of the first age-specific community and outpatient incidence rates of diarrheal disease associated with norovirus (as well as sapovirus and astrovirus) in Kenya [18]. Their community-based incidence estimate is about twice that of estimates from the US, United Kingdom, and the Netherlands, suggesting higher overall incidence in this developing country.
Grytdal et al. provide some of the first estimates of age-specific incidence rates for norovirus-associated acute gastroenteritis (AGE) in outpatient and community settings for the US [19]. In a population served by a managed care organization, norovirus gastroenteritis incidence in the community was estimated at 6% per year, with substantially higher rates among children under 5 years of age, particularly in outpatient settings (i.e., medically attended disease). Also for the US, Rha et al. estimated medically attended norovirus AGE rates for active-duty military personnel and their beneficiaries [20]. Like Grytdal et al., they reported that outpatient rates were approximately five times higher among children under 5 years of age compared to the rest of the population. Overall, rates of medically attended norovirus in this military population were considerably higher than estimates for the civilian population from Grytdal et al. [19] or previously published estimates [21–24], perhaps owing to more frequent exposure for military personnel.
Molecular Epidemiology
A signature biological feature of norovirus is its genetic diversity and rapid, immune selection-driven evolution. A number of papers in this collection highlight this diversity and give insight into its mechanistic underpinnings and implications for health and disease. Most of our understanding of the molecular epidemiology of norovirus comes from outbreak samples. However, Allen et al. examined samples from sporadic cases in diverse settings: the UK and Malawi [25]. Certain GII.4 strains that caused global increases in outbreak activity could be found in sporadic samples in both of these settings many years before becoming globally predominant. Based on this, the authors suggest the importance of surveillance of sporadic disease. Such data, especially from high incidence settings in the developing world, may be crucial for understanding and anticipating strain emergence and for predicting the potential strains for vaccines.
Fumian et al. and Lee Kim et al. present data on the molecular epidemiology of norovirus outbreaks from Brazil and Australia/New Zealand, respectively [26,27]. Despite their obvious geographical differences, the observations are remarkably consistent. GII.4 viruses were identified in 72% and 63% of outbreaks in Brazil and Australia/New Zealand, respectively. Of the non-GII.4 outbreaks, the majority in both settings were inter-genotype recombinant viruses, highlighting this important evolutionary mechanism, especially for less common strains.
Natural History, Host–Pathogen Interactions
Vomiting is a cardinal symptom of norovirus and key to its transmission, but it has also been a challenge to study directly. Using data from a collection of intentional-exposure volunteer studies, Kirby et al. show that over two-thirds of participants experience vomiting and that virus can be detected in most emesis samples from these participants [28]. Cases with vomiting but without diarrhea are typically excluded from studies of acute gastroenteritis; Kirby et al.’s study reminds us that in doing so, we exclude an important syndrome caused by norovirus infection and therefore underestimate its disease burden.
It is from these and other volunteer studies that much of our knowledge of norovirus immunity is derived. More recently, clinical trials using virus-like particles (VLPs) as candidate vaccines have further advanced our knowledge. Ramani et al. highlight two recently identified potential correlates of protection against norovirus gastroenteritis: norovirus-specific salivary IgA and norovirus-specific memory IgG cells [29]. Currently, however, serum antibody that blocks the binding of norovirus VLPs to histo-blood group antigens (HBGAs) is the best-studied and leading candidate correlate of protection.
In their Pearls article, Nordgren et al. discuss the implications of heterogeneity in innate susceptibility to norovirus in terms of burden of disease, viral genetic diversity as a function of human host diversity, and what the implications of this might be for norovirus vaccines and clinical trial design [30].
Quantifying Disease Burden and the Need for a Vaccine
The current evidence suggests that norovirus disease burden is great, but there remains considerable uncertainty and some scientific controversy in defining the precise role of norovirus in severe pediatric gastroenteritis. Epidemiological and etiological data are lacking, especially from developing countries. Routine testing is rarely performed in ongoing surveillance platforms, in part because molecular diagnostics are the standard reference for norovirus detection, and have only recently been widely available. Real-time quantitative reverse-transcription PCR (RT-qPCR) is the most sensitive and specific diagnostic for noroviruses, but use of these assays is mainly restricted to public health and research laboratories in middle - and high-income settings.
A robust estimate of the disease burden is critical to establishing a public health case to guide interventions and norovirus vaccine development. A vexing problem is how to identify when norovirus is disease-causing and then to attribute a fraction of the acute gastroenteritis disease “envelope” to norovirus. For norovirus, attribution has been particularly challenging since detection is based on highly sensitive RT-qPCR and virus is frequently detected in stool of individuals with gastroenteritis, but also in stool of healthy controls. Reinfection is common and sometimes asymptomatic; viral shedding can persist for weeks or months after symptoms and, fundamentally, we lack a diagnostic that readily discriminates between disease-causing and asymptomatic infection.
The recent Global Enterics Multi-Center Study (GEMS) is the largest systematic assessment for understanding the etiology of childhood diarrhea in developing countries [31]. GEMS and other case-control studies use the odds ratio of a microbe being present in cases versus healthy controls to calculate an attributable or etiologic fraction. When detected as frequently in healthy controls as in cases, some study authors have concluded that norovirus is a minor pathogen. We think that the conclusion is inaccurate for a virus that commonly causes reinfection and has long excretion patterns. An alternative explanation is that high levels of asymptomatic infection are a result of frequent exposure, some of which will result in asymptomatic infection because of acquired immunity [32]. Therefore, high prevalence of norovirus detection in healthy controls may be characteristic of “hyper-endemicity” where burden is higher, not lower. Quantitative and multiplex diagnostics may be important tools for ascribing etiological fractions for norovirus and other enteric pathogens, especially when coupled with rigorous field studies, such as the multi-center MAL-ED study [33]. Indeed, in that study, norovirus was identified as the pathogen with the first - and second-highest attributable fraction for diarrhea in the first and second year of life, respectively, but still only about 5% of disease could be attributed to norovirus [6].
Developing a Vaccine and Addressing Biological Challenges
Noroviruses are a genetically and antigenically diverse group of ssRNA viruses, which presents serious challenges both for creating broadly reactive diagnostics and eliciting a broadly protective immune response, following either natural infection or vaccination. The norovirus strains that infect humans are found among 29 genotypes among genogroup (G) I (n = 9), GII (n = 19), and GIV (n = 1) [34]. Among this array of different noroviruses are the GII.4 strains, which rapidly evolve in a boom-and-bust cycle, with novel viruses emerging every 2–4 years and replacing previous dominant ones, a process driven by evasion of immunity in the human population [35]. In addition to their evolutionary dynamics, there are public health reasons that a successful norovirus vaccine must provide protection against GII.4 viruses: they are the predominant cause of pediatric infections worldwide [36], they predominate overwhelmingly as a cause of disease amongst the elderly in health care–associated outbreaks, and they result in more severe illness and death [37]. Studies published in this Collection are consistent with the view that GII.4 viruses predominate globally [17,25,26], with rare exception in the last 20 years [38].
Understanding of natural immunity to norovirus is far from complete, but the current view is that immunity is strain - or genotype-specific, with little or no protection conferred across genogroups. Immunity is not lifelong, with estimates of duration ranging from 6 months to 9 years [32,39–42]. Accordingly, genotype-specific immune responses and antigenic variation suggest that a polyvalent vaccine will be needed and may require updating when new strains emerge. To date, vaccine trials and challenge studies have been conducted among adults, leaving much to learn about how “unprimed” children who have not experienced (as many) norovirus exposures develop immunity and therefore respond to vaccination.
The lack of a robust in vitro cell culture system for human norovirus has hampered the development of assays to measure protective neutralizing antibodies conferred by either natural or vaccine-induced immunity. However, important progress has been achieved very recently in this area [11] as has identification of candidate immune correlates of protection (e.g., [43]). These are important breakthroughs that may accelerate vaccine development.
Overcoming Logistical and Programmatic Challenges
Many distinct population groups are affected by norovirus, which could complicate the formulation of a research agenda and clinical development plan for specific products (Tables 1 and 2). But, if harnessed, this diversity in disease burden could also serve to stimulate development and generate demand from difference sources. A development plan for a target population of young children will look quite different than for older adults, or for a specific risk group, such as travelers, military personnel, or health care workers. From a public health perspective, it is clear that young children experience the highest overall incidence of disease [21], and severe disease outcomes are most common among young children and, at least in developed countries, the elderly [24]. Young children also seem to be the most important group in driving transmission in the community [44]. Accordingly, vaccinating young children would likely be most efficient for directly preventing disease burden, and would offer the greatest potential for impact at the population level through indirect benefits resulting from reduced transmission. Defining the relative roles of different age groups in transmission is challenging and may well differ in high - and low-income settings, but a combination of empirical (e.g., household) studies and mathematical modeling analysis may help to optimize the direct and population-level effect of vaccinating through targeting different groups.
Tab. 1. Epidemiological and economics characteristics of various age groups for considering norovirus vaccines1,2. 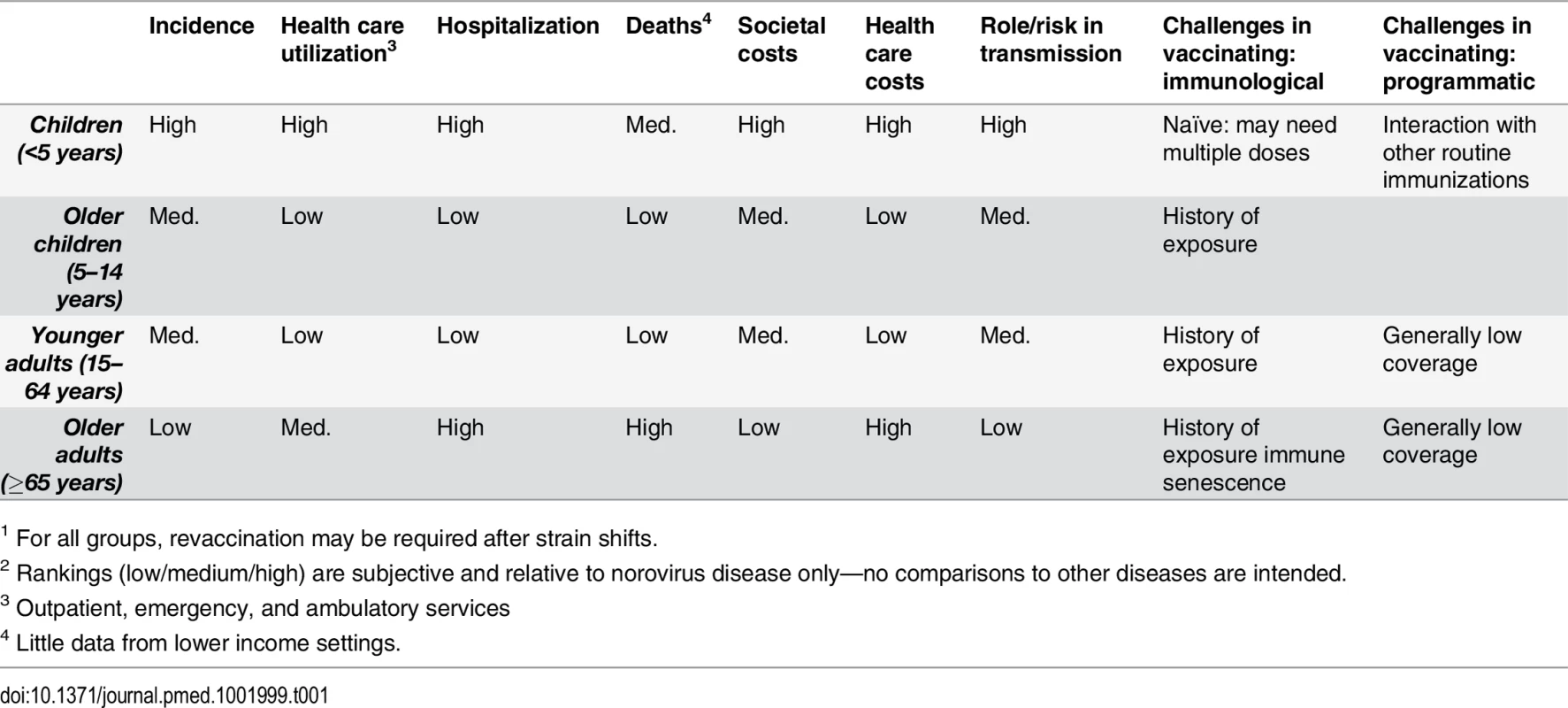
1 For all groups, revaccination may be required after strain shifts. Tab. 2. Epidemiological, economic, and programmatic considerations for specific subpopulation risk groups. 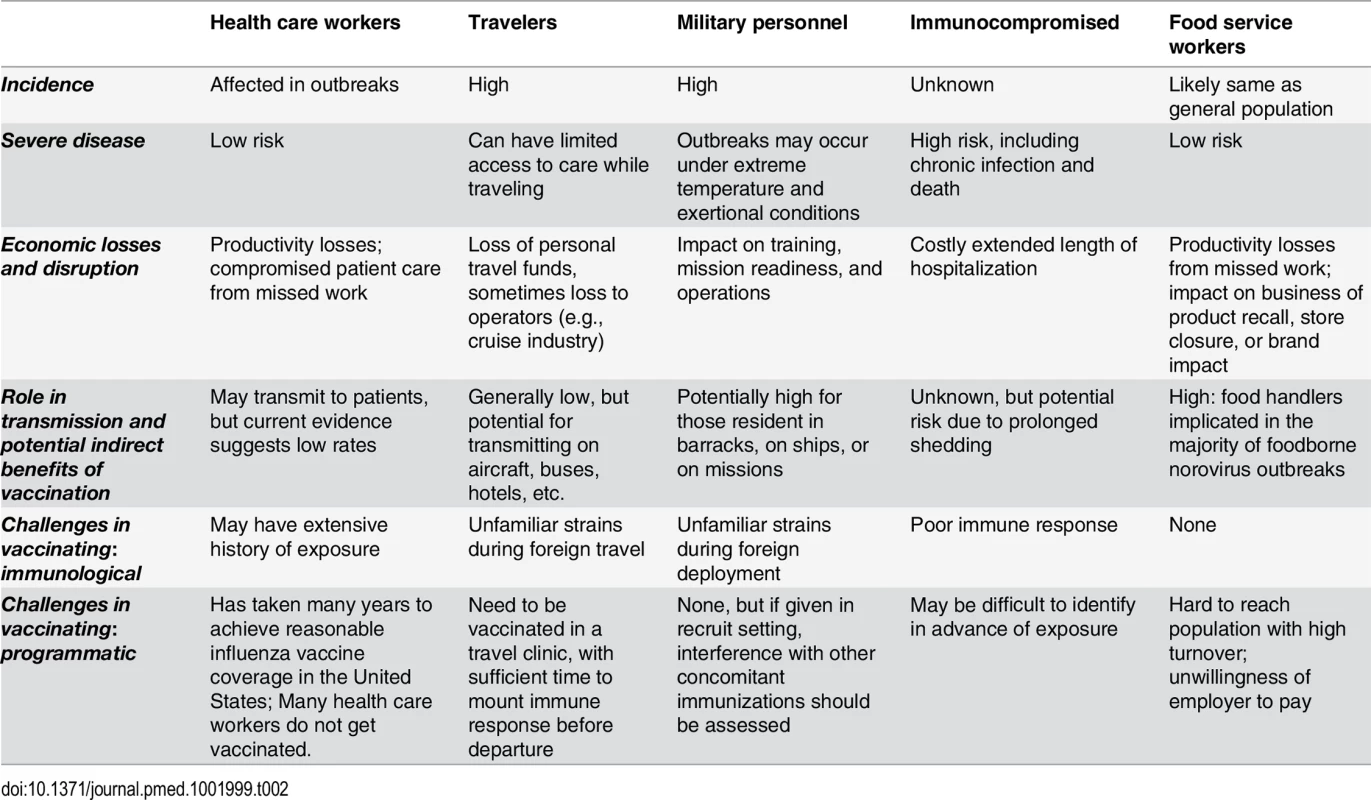
In the absence of an outside stimulus, such as major global public health donors, developed world markets are likely to provide the initial economic impetus for private industry to develop norovirus vaccines. To date, early-phase trials have been conducted among adults in high-income settings, and there have been no clinical studies in children, so we see a need for a targeted pediatric development plan. Such a plan should include studies to generate data on the compatibility of a norovirus vaccine with current routine childhood immunizations, and in particular the Expanded Program on Immunization (EPI). Adding a vaccine to the EPI schedule involves great effort to demonstrate the added value of the vaccine, on both economic and health grounds. The economics of a norovirus vaccine requiring multiple doses and/or periodic reformulation will be scrutinized carefully by policy makers. At the earliest stage, clinical development plans should define a target product profile (TPP) that will maximize public health gains by focusing on young children, with the aim of developing a vaccine that can be incorporated into the logistical arrangements of current immunization programs.
Future Directions
Norovirus as a target for vaccination is unique in many ways. First, the public health need is not restricted to a specific region, income level, or even age range, so the groups with a stake in vaccine development are many and diverse. Second, much of the totality of economic and health burden results from relatively mild disease. But even if the proportion of cases with severe outcomes is relatively low, the sheer incidence of norovirus still results in a considerable severe disease burden. Third, given the current state of knowledge, we expect that progress in understanding of disease burden and epidemiology, human–virus interactions, and vaccine development will be interdependent. More robust estimates of the disease burden of children in low-income settings should stimulate more research in vaccines that will benefit those populations; vaccine trials themselves can be used to better characterize the disease burden and also to identify correlates of protection, which have the potential to make subsequent vaccine evaluations faster and less costly; mathematical modeling studies can serve as a framework to integrate this variety of data and to predict the impact of vaccination strategies, and to objectively identify the most critical gaps in our knowledge (Table 3). Addressing these key issues will be vital to accelerate and achieve the development and implementation of interventions such as vaccines to control and prevent the tremendous global morbidity and mortality from norovirus.
Tab. 3. Critical studies to be performed and questions to be answered to advance vaccine development. 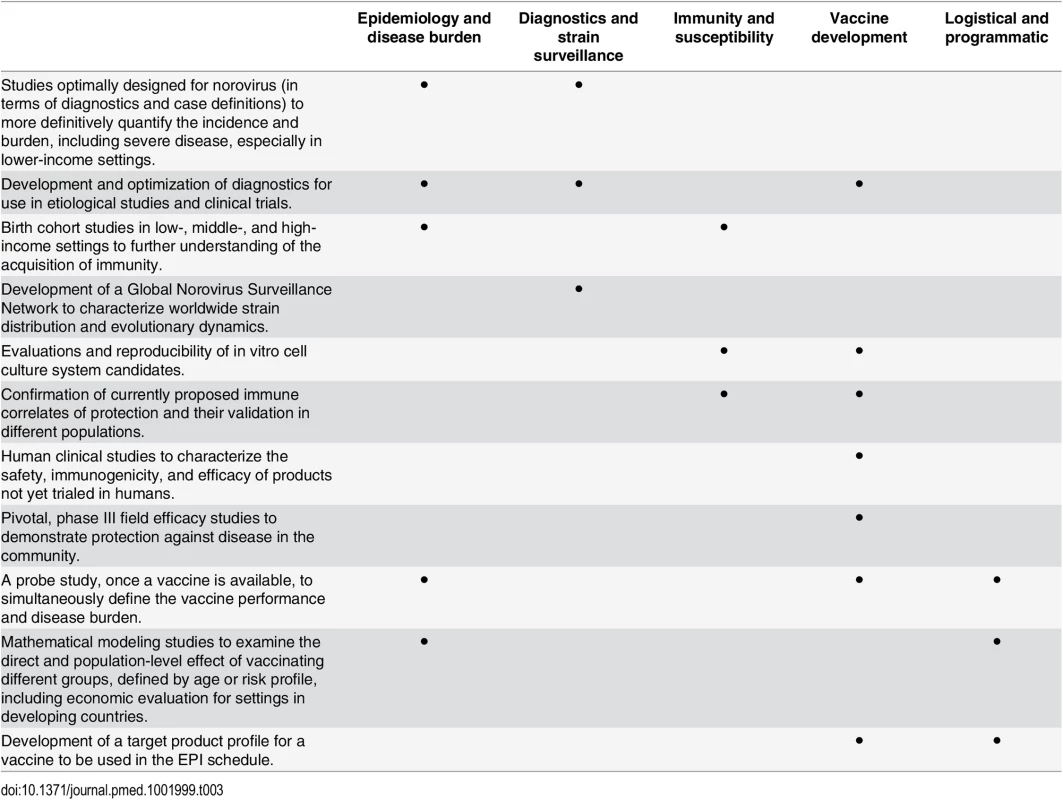
Zdroje
1. GBD 2013 Mrtality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2 25530442; PubMed Central PMCID: PMCPMC4340604.
2. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6 25280870.
3. Global Health Data Exchange: Institute for Health Metrics and Evaluation; 2013. http://vizhub.healthdata.org/irank/arrow.php.
4. Yen C, Tate JE, Hyde TB, Cortese MM, Lopman BA, Jiang B, et al. Rotavirus vaccines: current status and future considerations. Human vaccines & immunotherapeutics. 2014;10(6):1436–48. doi: 10.4161/hv.28857 24755452.
5. Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, et al. Aetiology-Specific Estimates of the Global and Regional Incidence and Mortality of Diarrhoeal Diseases Commonly Transmitted through Food. PLoS ONE. 2015;10(12):e0142927. doi: 10.1371/journal.pone.0142927 26632843; PubMed Central PMCID: PMCPMC4668836.
6. Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). The Lancet Global health. 2015;3(9):e564–75. doi: 10.1016/S2214-109X(15)00151-5 26202075.
7. Bucardo F, Reyes Y, Svensson L, Nordgren J. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS ONE. 2014;9(5):e98201. doi: 10.1371/journal.pone.0098201 24849288; PubMed Central PMCID: PMCPMC4029982.
8. Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368(12):1121–30. doi: 10.1056/NEJMsa1206589 23514289; PubMed Central PMCID: PMCPMC4618551.
9. Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):725–30. doi: 10.1016/S1473-3099(14)70767-4 24981041.
10. Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12(12):e1001921. doi: 10.1371/journal.pmed.1001921 26633831; PubMed Central PMCID: PMCPMC4668831.
11. Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346(6210):755–9. doi: 10.1126/science.1257147 25378626; PubMed Central PMCID: PMCPMC4401463.
12. Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, et al. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science. 2015;347(6219):266–9. doi: 10.1126/science.1258025 25431490; PubMed Central PMCID: PMCPMC4409937.
13. Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, et al. Coinfection. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science. 2014;345(6196):578–82. doi: 10.1126/science.1256942 25082704; PubMed Central PMCID: PMCPMC4548887.
14. Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365(23):2178–87. Epub 2011/12/14. doi: 10.1056/NEJMoa1101245 22150036.
15. Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis. 2015;211(6):870–8. doi: 10.1093/infdis/jiu497 25210140.
16. Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. Global Economic Burden of Norovirus Gastroenteritis. PLoS ONE. 2016;11(4):e0151219. doi: 10.1371/journal.pone.0151219
17. Mans J, Armah GE, Steele AD, Taylor MB. Norovirus Epidemiology in Africa: A Review. PLoS ONE. 2016;11(4):e0146280. doi: 10.1371/journal.pone.0146280
18. Shioda K, Cosmas L, Audi A, Gregoricus N, Vinjé J, Parashar UD, et al. Population-Based Incidence Rates of Diarrheal Disease Associated with Norovirus, Sapovirus, and Astrovirus in Kenya. PLoS ONE. 2016;11(4):e0145943. doi: 10.1371/journal.pone.0145943
19. Grytdal SP, DeBess E, Lee LE, Blythe D, Ryan P, Biggs C, et al. Incidence of Norovirus and Other Viral Pathogens That Cause Acute Gastroenteritis (AGE) among Kaiser Permanente Member Populations in the United States, 2012–2013. PLoS ONE. 2016;11(4):e0148395. doi: 10.1371/journal.pone.0148395
20. Rha B, Lopman BA, Alcala AN, Riddle MS, Porter CK. Incidence of Norovirus-Associated Medical Encounters among Active Duty United States Military Personnel and Their Dependents. PLoS ONE. 2016;11(4):e0148505. doi: 10.1371/journal.pone.0148505
21. Phillips G, Tam CC, Conti S, Rodrigues LC, Brown D, Iturriza-Gomara M, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol. 2010;171(9):1014–22. Epub 2010/04/03. doi: 10.1093/aje/kwq021 20360244.
22. Verhoef L, Koopmans M, VANP W, Duizer E, Haagsma J, Werber D, et al. The estimated disease burden of norovirus in The Netherlands. Epidemiol Infect. 2013;141(3):496–506. Epub 2012/05/19. doi: 10.1017/S0950268812000799 22595489.
23. Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61(1):69–77. doi: 10.1136/gut.2011.238386 21708822; PubMed Central PMCID: PMC3230829.
24. Hall AJ, Lopman BA, Payne DC, Patel MM, Gastanaduy PA, Vinje J, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013;19(8):1198–205. doi: 10.3201/eid1908.130465 23876403; PubMed Central PMCID: PMCPMC3739528.
25. Allen DJ, Trainor E, Callaghan A, O'Brien SJ, Cunliffe NA, Iturriza-Gómara M. Early Detection of Epidemic GII-4 Norovirus Strains in UK and Malawi: Role of Surveillance of Sporadic Acute Gastroenteritis in Anticipating Global Epidemics. PLoS ONE. 2016;11(4):e0146972. doi: 10.1371/journal.pone.0146972
26. Fumian TM, da Silva Ribeiro de Andrade J, Leite JPG, Miagostovich MP. Norovirus Recombinant Strains Isolated from Gastroenteritis Outbreaks in Southern Brazil, 2004–2011. PLoS ONE. 2016;11(4):e0145391. doi: 10.1371/journal.pone.0145391
27. Lim KL, Hewitt J, Sitabkhan A, Eden J - S, Lun J, Levy A, et al. A Multi-Site Study of Norovirus Molecular Epidemiology in Australia and New Zealand, 2013–2014. PLoS ONE. 2016;11(4):e0145254. doi: 10.1371/journal.pone.0145254
28. Kirby AE, Streby A, Moe CL. Vomiting as a Symptom and Transmission Risk in Norovirus Illness: Evidence from Human Challenge Studies. PLoS ONE. 2016;11(4):e0143759. doi: 10.1371/journal.pone.0143759
29. Ramani S, Estes MK, Atmar RL. Correlates of Protection against Norovirus Infection and Disease—Where Are We Now, Where Do We Go? PLoS Pathog. 12(4): e1005334. doi: 10.1371/journal.ppat.1005334
30. Nordgren J, Sharma S, Kambhampati A, Lopman BA, Svensson L. Innate Resistance and Susceptibility to Norovirus Infection. PLoS Pathog. 12(4): e1005385. doi: 10.1371/journal.ppat.1005385
31. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2 23680352.
32. Lopman B, Simmons K, Gambhir M, Vinje J, Parashar U. Epidemiologic implications of asymptomatic reinfection: a mathematical modeling study of norovirus. Am J Epidemiol. 2014;179(4):507–12. doi: 10.1093/aje/kwt287 24305574.
33. Platts-Mills JA, McCormick BJ, Kosek M, Pan WK, Checkley W, Houpt ER, et al. Methods of analysis of enteropathogen infection in the MAL-ED Cohort Study. Clin Infect Dis. 2014;59 Suppl 4:S233–8. doi: 10.1093/cid/ciu408 25305292; PubMed Central PMCID: PMCPMC4204610.
34. Vinje J. Advances in laboratory methods for detection and typing of norovirus. J Clin Microbiol. 2015;53(2):373–81. doi: 10.1128/JCM.01535-14 24989606; PubMed Central PMCID: PMCPMC4298492.
35. Siebenga JJ, Vennema H, Renckens B, de Bruin E, van der Veer B, Siezen RJ, et al. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J Virol. 2007;81(18):9932–41. doi: 10.1128/JVI.00674-07 17609280; PubMed Central PMCID: PMCPMC2045401.
36. Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol. 2013;56(3):185–93. doi: 10.1016/j.jcv.2012.11.011 23218993.
37. Desai R, Hembree CD, Handel A, Matthews JE, Dickey BW, McDonald S, et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis. 2012;55(2):189–93. doi: 10.1093/cid/cis372 22491335; PubMed Central PMCID: PMCPMC3491774.
38. de Graaf M, van Beek J, Vennema H, Podkolzin AT, Hewitt J, Bucardo F, et al. Emergence of a novel GII.17 norovirus—End of the GII.4 era? Euro Surveill. 2015;20(26). 26159308.
39. Baron RC, Greenberg HB, Cukor G, Blacklow NR. Serological responses among teenagers after natural exposure to Norwalk virus. JInfectDis. 1984;150(4):531–4.
40. Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis. 1990;161(1):18–21. 2153184.
41. Parrino TA, Schreiber DS, Trier JS, Kapikian AZ, Blacklow NR. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med. 1977;297(2):86–9. doi: 10.1056/NEJM197707142970204 405590.
42. Wyatt RG, Dolin R, Blacklow NR, DuPont HL, Buscho RF, Thornhill TS, et al. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis. 1974;129(6):709–14. Epub 1974/06/01. 4209723.
43. Atmar RL, Bernstein DI, Lyon GM, Treanor JJ, Al-Ibrahim MS, Graham DY, et al. Serological Correlates of Protection against a GII.4 Norovirus. Clin Vaccine Immunol. 2015;22(8):923–9. doi: 10.1128/CVI.00196-15 26041041; PubMed Central PMCID: PMCPMC4519714.
44. de Wit MA, Koopmans MP, van Duynhoven YT. Risk factors for norovirus, Sapporo-like virus, and group A rotavirus gastroenteritis. Emerg Infect Dis. 2003;9(12):1563–70. doi: 10.3201/eid0912.020076 14720397; PubMed Central PMCID: PMCPMC3034344.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Why Are Some Population Interventions for Diet and Obesity More Equitable and Effective Than Others? The Role of Individual Agency
- Risk of Bias in Systematic Reviews of Non-Randomized Studies of Adverse Cardiovascular Effects of Thiazolidinediones and Cyclooxygenase-2 Inhibitors: Application of a New Cochrane Risk of Bias Tool
- The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control
- The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials
- Disentangling the Association between Statins, Cholesterol, and Colorectal Cancer: A Nested Case-Control Study
- Gender Differences in Homicide of Neonates, Infants, and Children under 5 y in South Africa: Results from the Cross-Sectional 2009 National Child Homicide Study
- Mobile Phones As Surveillance Tools: Implementing and Evaluating a Large-Scale Intersectoral Surveillance System for Rabies in Tanzania
- Building Learning Health Systems to Accelerate Research and Improve Outcomes of Clinical Care in Low- and Middle-Income Countries
- The Future of the RTS,S/AS01 Malaria Vaccine: An Alternative Development Plan
- Birth “Out-of-Hours”: An Evaluation of Obstetric Practice and Outcome According to the Presence of Senior Obstetricians on the Labour Ward
- A Nested Case–Control Study of Metabolically Defined Body Size Phenotypes and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC)
- Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma
- Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries
- The Impact of Hotspot-Targeted Interventions on Malaria Transmission in Rachuonyo South District in the Western Kenyan Highlands: A Cluster-Randomized Controlled Trial
- Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
- Is There Evidence of Poorer Birth Outcomes for Mothers and Babies When the Most Senior Obstetrician Is Not On Site?
- Clinical Implications of Cancer Genomics: A Call for Papers
- The ITA.LI.CA Staging System: A Novel Staging System for Hepatocellular Carcinoma
- Observational Evidence of For-Profit Delivery and Inferior Nursing Home Care: When Is There Enough Evidence for Policy Change?
- Child Homicide: A Global Public Health Concern
- The Chernobyl Disaster and Beyond: Implications of the Sendai Framework for Disaster Risk Reduction 2015–2030
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Observational Evidence of For-Profit Delivery and Inferior Nursing Home Care: When Is There Enough Evidence for Policy Change?
- Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
- The Chernobyl Disaster and Beyond: Implications of the Sendai Framework for Disaster Risk Reduction 2015–2030
- Is There Evidence of Poorer Birth Outcomes for Mothers and Babies When the Most Senior Obstetrician Is Not On Site?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



