-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
Background:
Clinical practice guidelines (CPGs) aim to improve professionalism in health care. However, current CPG development manuals fail to address how to include ethical issues in a systematic and transparent manner. The objective of this study was to assess the representation of ethical issues in general CPGs on dementia care.Methods and Findings:
To identify national CPGs on dementia care, five databases of guidelines were searched and national psychiatric associations were contacted in August 2011 and in June 2013. A framework for the assessment of the identified CPGs' ethical content was developed on the basis of a prior systematic review of ethical issues in dementia care. Thematic text analysis and a 4-point rating score were employed to assess how ethical issues were addressed in the identified CPGs. Twelve national CPGs were included. Thirty-one ethical issues in dementia care were identified by the prior systematic review. The proportion of these 31 ethical issues that were explicitly addressed by each CPG ranged from 22% to 77%, with a median of 49.5%. National guidelines differed substantially with respect to (a) which ethical issues were represented, (b) whether ethical recommendations were included, (c) whether justifications or citations were provided to support recommendations, and (d) to what extent the ethical issues were explained.Conclusions:
Ethical issues were inconsistently addressed in national dementia guidelines, with some guidelines including most and some including few ethical issues. Guidelines should address ethical issues and how to deal with them to help the medical profession understand how to approach care of patients with dementia, and for patients, their relatives, and the general public, all of whom might seek information and advice in national guidelines. There is a need for further research to specify how detailed ethical issues and their respective recommendations can and should be addressed in dementia guidelines.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(8): e32767. doi:10.1371/journal.pmed.1001498
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001498Summary
Background:
Clinical practice guidelines (CPGs) aim to improve professionalism in health care. However, current CPG development manuals fail to address how to include ethical issues in a systematic and transparent manner. The objective of this study was to assess the representation of ethical issues in general CPGs on dementia care.Methods and Findings:
To identify national CPGs on dementia care, five databases of guidelines were searched and national psychiatric associations were contacted in August 2011 and in June 2013. A framework for the assessment of the identified CPGs' ethical content was developed on the basis of a prior systematic review of ethical issues in dementia care. Thematic text analysis and a 4-point rating score were employed to assess how ethical issues were addressed in the identified CPGs. Twelve national CPGs were included. Thirty-one ethical issues in dementia care were identified by the prior systematic review. The proportion of these 31 ethical issues that were explicitly addressed by each CPG ranged from 22% to 77%, with a median of 49.5%. National guidelines differed substantially with respect to (a) which ethical issues were represented, (b) whether ethical recommendations were included, (c) whether justifications or citations were provided to support recommendations, and (d) to what extent the ethical issues were explained.Conclusions:
Ethical issues were inconsistently addressed in national dementia guidelines, with some guidelines including most and some including few ethical issues. Guidelines should address ethical issues and how to deal with them to help the medical profession understand how to approach care of patients with dementia, and for patients, their relatives, and the general public, all of whom might seek information and advice in national guidelines. There is a need for further research to specify how detailed ethical issues and their respective recommendations can and should be addressed in dementia guidelines.
Please see later in the article for the Editors' SummaryIntroduction
Clinical practice guidelines (CPGs) are meant to improve standards of clinical competence and professionalism by referring explicitly to evidence-based information on benefits and harms [1]. Their development increasingly includes measures to strengthen their validity and accountability: patient participation [2], explicit procedures to grade the strength of recommendations [3], and the requirement to disclose and manage conflicts of interest [4]. While all these developments in guideline methodology are laudable, particularly from an ethical perspective, CPG development manuals worldwide still fail to address how to include disease-specific ethical issues (DSEIs): a search of leading CPG development manuals for the term “ethics” or “ethical” does not yield any information about how to identify and address clinical ethical situations that are relevant to the management of specific diseases [1],[5]–[8]. What has been addressed in some manuals is the need to realize that (a) different groups value outcomes differently and (b) values and preferences should be considered when making guideline recommendations.
How does a DSEI arise? Widely shared frameworks for medical professionalism and common approaches to morality in bioethics are all based on a set of prima facie binding ethical principles: respect for patient autonomy, beneficence, non-maleficence, and justice [9]–[11]. Once we accept that these ethical principles are relevant to health-related decision-making, a DSEI can arise from (a) neglect of one or more ethical principles, for example, “Insufficient consideration of patient autonomy and patient preferences in dementia care decisions”, or (b) conflicts between two or more ethical principles, for example, “Balancing the do-no-harm principle (non-maleficence) versus the freedom-to-move-at-will principle (patient autonomy) in decision-making for or against physical restraints on account of inappropriate patient behaviour.”
Awareness not only of the four general ethical principles but especially of relevant DSEIs, and competency in managing these DSEIs, are deeply intertwined with the concepts of clinical competence and professionalism of health care workers [9]. Furthermore, awareness of and competence with respect to DSEIs is important also for other caregivers, such as relatives of persons with dementia [12].
Although no current CPG development manual explains how to identify and integrate specific DSEIs, it is unclear whether and to what extent existing CPGs already address DSEIs. While some research has been conducted on the prevalence, content, and quality of ethical guidelines [13]–[15], to our knowledge no study has yet investigated in a systematic manner how CPGs vary in addressing DSEIs. Because ethical issues pervade dementia care, and much has been written on this topic [16]–[18], existing dementia care guidelines provide a good starting point to look more specifically at whether and how DSEIs are addressed.
The objective of this study was to assess the representation of DSEIs in national evidence-based CPGs on dementia care.
Methods
Identification of Clinical Practice Guidelines on Dementia Care
To maintain comparability, we restricted our analysis to national, general CPGs on dementia care and therefore did not analyze guidelines that address a specific aspect of dementia care. Further reasons to restrict our analysis to national guidelines addressing the whole spectrum of dementia care were the following: First, national CPGs (from institutions such as National Institute for Health and Clinical Excellence [NICE] in the United Kingdom or the American Psychiatric Association [APA] in the United States) must meet high standards for guideline development. Second, we believe that national CPGs should be most interested in addressing DSEIs as they often also address patients and relatives as potential readership.
We restricted our search to national guidelines written in English or German. To identify national CPGs we searched the following five guideline databases: (1) National Guideline Clearinghouse (United States), (www.guideline.gov), (2) National Library for Health on Guidelines Finder (United Kingdom), (www.library.nhs.uk/GuidelinesFinder/), (3) Canadian Medical Association Infobase (Canada), (www.cma.ca/index.php/ci_id/54316/la_id/1.htm), (4) G-I-N International Guideline Library (www.g-i-n.net), (5) AWMF (Germany) (www.awmf.org/leitlinien/leitlinien-suche.html). The date of the search was August 15, 2011. We complemented the search by contacting national psychiatric associations and societies in Switzerland and Austria, because no guidelines could be identified for these two German-speaking countries in the above-mentioned databases. We repeated the guideline search on June 10, 2013, but found neither additional national guidelines nor changes in the already-retrieved guidelines. Because a revised version of the Canadian dementia guideline (from 2012) currently undergoes a public consultation process this study assessed the still official guideline from 2007.
Development of a Matrix of Ethical Issues in Dementia Care
Prior to this guideline assessment study we conducted a systematic review in Medline (restricted to English and German literature published between 2001 and 2011) and Google Books (restricted to the first 100 hits) to identify the full spectrum of ethical issues in dementia care. More detailed methodological information and the findings of this systematic review are published elsewhere [19]. In total, this systematic review retrieved 92 references that together mentioned a spectrum of 56 DSEIs in dementia care. The DSEIs were grouped under 33 mid-level categories that were themselves grouped under seven main categories.
For the purpose of this study the 56 DSEIs were reduced to 31 broader DSEIs (grouped under the same seven main categories). In most cases we broadened the content of a DSEI by referring to the original mid-level categories, e.g., the narrow DSEIs, (a) “Insufficient consideration of the patient as a person” and (b) “Insufficient consideration of existing preferences of the patient” were reduced to the broader DSEI “Adequate appreciation of the patient.” We further reduced the number of DSEIs where mid-level categories were sufficiently related (e.g., we synthesized the DSEIs “Adequate amount and manner of information” and “Consideration of cultural aspects” into the broader DSEI “Adequate consideration of the complexity of informing patients with dementia”). While the wording of some DSEIs is generic (e.g., “Responsible handling of costs and allocation of limited resources”), the corresponding text examples always highlight specific challenges related to dementia care. Text examples for all DSEIs have been published elsewhere [19].
This spectrum of 31 DSEIs was used as a framework for the assessment of ethical content in each CPG on dementia care.
The concept of DSEIs is rooted in the ethical theory of principlism [10] that forms the basis of many ethical and medical professionalism frameworks [9],[11] (see also the Introduction). Broadly drawn, the term covers all clinical (diagnostic or therapeutic) decisions that need to balance potential benefits and harms. In this regard almost all disease-specific clinical issues automatically become disease-specific ethical issues. We addressed this (unavoidable) challenge of “over-inclusiveness” by only considering a dementia-specific clinical issue to be a DSEI when we identified examples from the literature indicating an important controversy on how to balance benefits and harms. For example, the clinical decision on whether or not antipsychotic drugs should be used in dementia care was included as an example for the DSEI “Medication” because we found several text passages highlighting specific controversies and practice variations on how to balance benefits and harms regarding this clinical issue.
Assessment of the Representation of Ethical Issues in Clinical Practice Guidelines on Dementia Care
The representation of all 31 DSEIs in each identified CPG was assessed according to standards in thematic text analysis [20]. All researchers were experienced in thematic text analysis and medical ethics. The academic background of the five researchers included training (degrees) and at least two years' practice in clinical psychiatry (DS), internal medicine (GN), philosophy (DS, MM, MS), public health (HK), social sciences (MM), and physiotherapy (HK). First, CPGs were read in full by at least two researchers independently to identify and extract text passages corresponding to one of the 31 DSEIs in dementia care. Secondly, for every individual CPG the same researchers independently assigned one of the following four possible ratings to each of the 31 DSEI: N, DSEI not addressed; I, DSEI implicitly addressed; E, DSEI explicitly addressed; and R, DSEI explicitly addressed with recommendations. For rating examples see Table 1.
Tab. 1. Text examples illustrating the ratings presented in Tables 3 and 4. 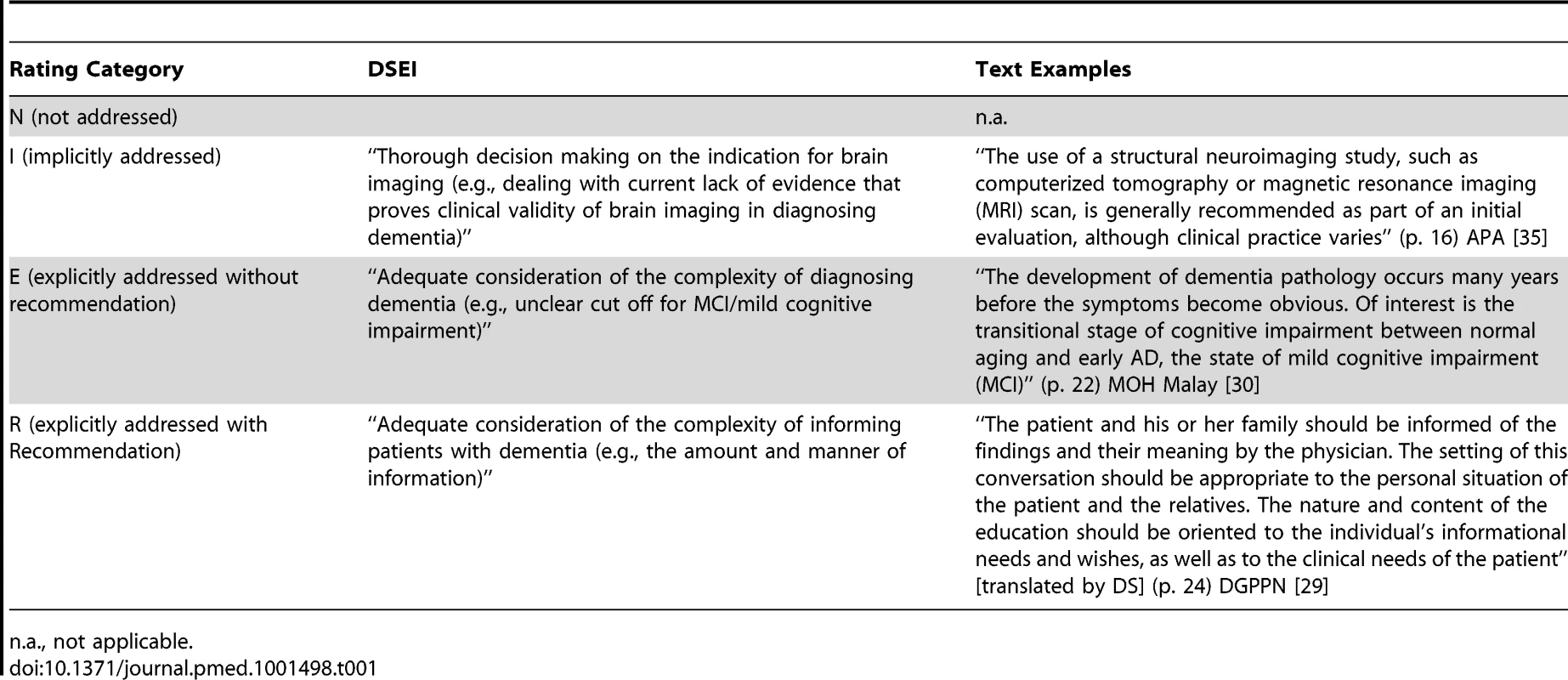
n.a., not applicable. CPGs in category R were further assessed for whether the recommendation was justified in the text or not and whether citations supporting the recommendation were given or not.
After the independent text extraction and rating the researchers compared their results. Discrepancies between the resulting spreadsheets were identified in 51 (13.7%) of 372 ratings. These discrepancies were discussed and resolved by including at least one other researcher with training in both clinical medicine and medical ethics (DS, GN). The latter researchers also supported the validity check for another 32 ratings where both independent reviewers rated equally but still felt some uncertainty concerning the validity of their rating. For text examples from CPGs and their respective ratings, see the Findings section and Table S1.
Results
In total we included 12 national CPGs from 12 countries in four continents (Table 2).
Tab. 2. Characteristics of included clinical practice guidelines. 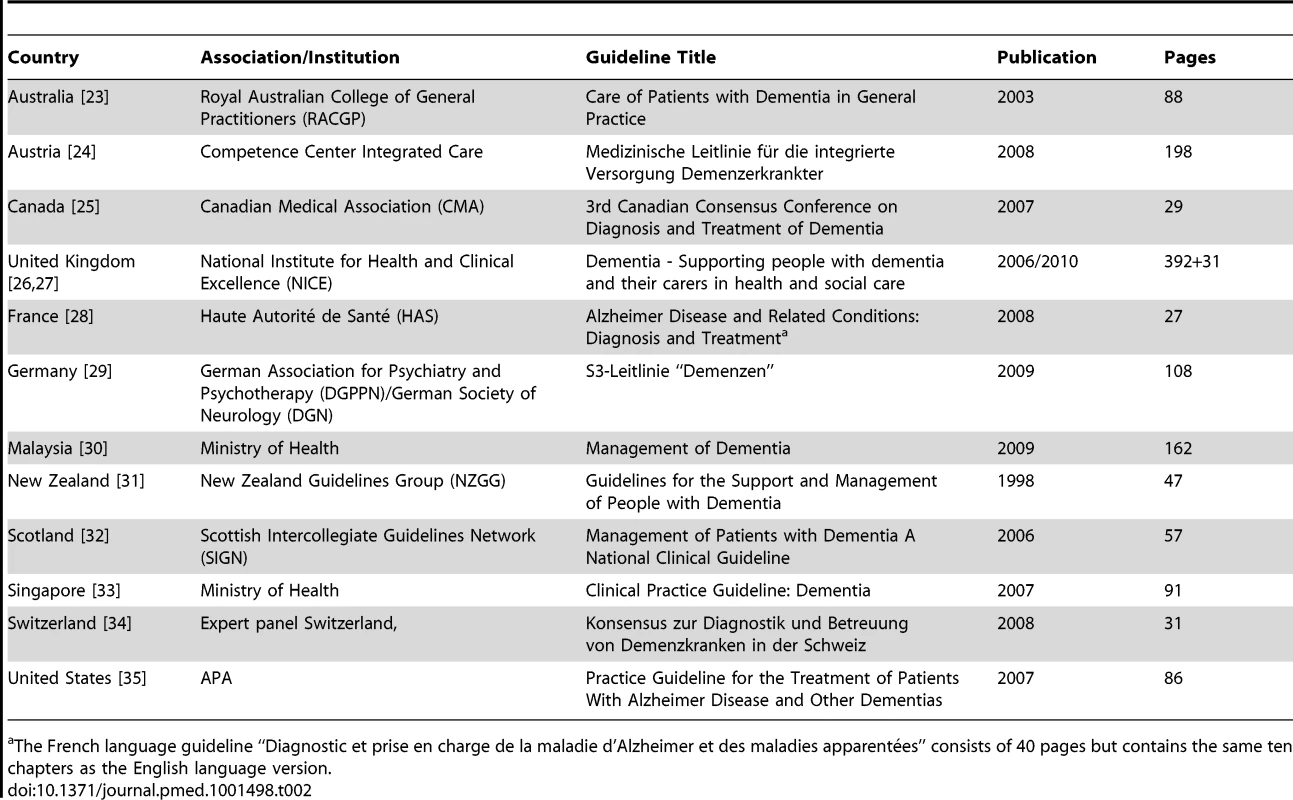
The French language guideline “Diagnostic et prise en charge de la maladie d'Alzheimer et des maladies apparentées” consists of 40 pages but contains the same ten chapters as the English language version. Six CPGs are published or certificated by a central governmental institution (Australia, France, Malaysia, New Zealand, Singapore, United Kingdom), four by a medical association (Canada, Germany, Scotland, United States), one by a statutory health insurance body (Austria), and one by an expert panel (Switzerland). All guidelines explicitly acknowledged the involvement of experts from different specialties (most often from psychiatry, neurology, gerontology, and family medicine). Only one guideline (from New Zealand) did not describe explicitly which specialties were involved.
All assessed guidelines on dementia care already address clinical ethical issues to some extent. However, CPGs differed considerably as to the number of DSEIs addressed, implicitly or explicitly. The rate of DSEIs that were explicitly addressed by each CPG (calculated as the sum of E and R ratings for each guideline) ranged from 20% (Switzerland) to 77% (United States) with a median of 49.5% (Table 3).
Tab. 3. Quantitative representation of the 31 DSEIs. 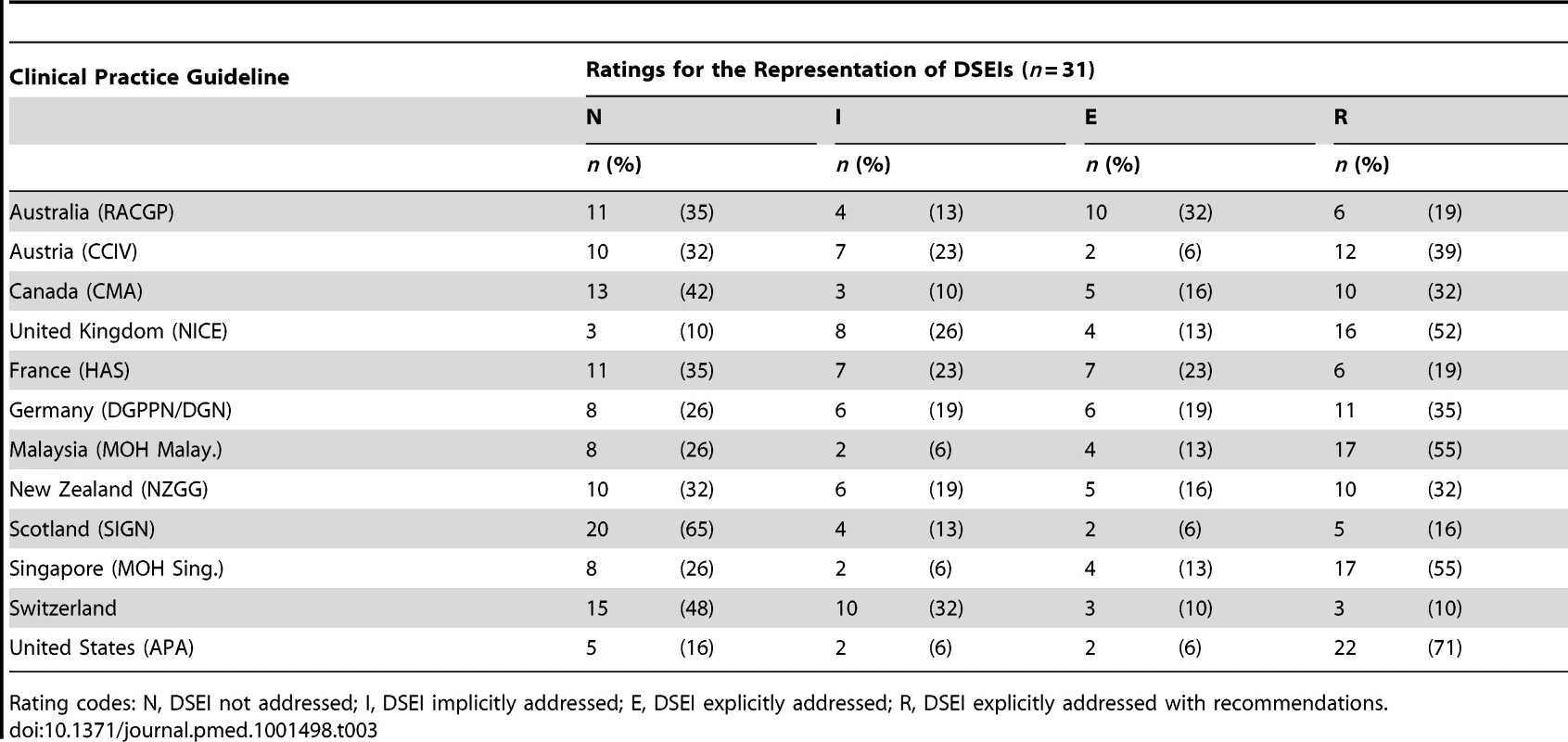
Rating codes: N, DSEI not addressed; I, DSEI implicitly addressed; E, DSEI explicitly addressed; R, DSEI explicitly addressed with recommendations. When adding all implicitly addressed DSEIs (I, E or R ratings) the rate of DSEIs ranged from 35% (Scotland) to 91% (United Kingdom) with a median of 67.5%. However, the inclusion of recommendations (R rating) on how to deal with the 31 DSEIs (per CPG) ranged from 10% to 71% with a median of 32% (table 3).
Four DSEIs (13%) were neither implicitly nor explicitly addressed in at least 11 out of 12 CPGs: “Adequate consideration of existing advance directives in medical decision making,” “Usage of GPS and other monitoring techniques,” “Covert medication,” and “Dealing with suicidality” (Table 4).
Tab. 4. Representation of 31 DSEIs in 12 national clinical practice guidelines on dementia care. 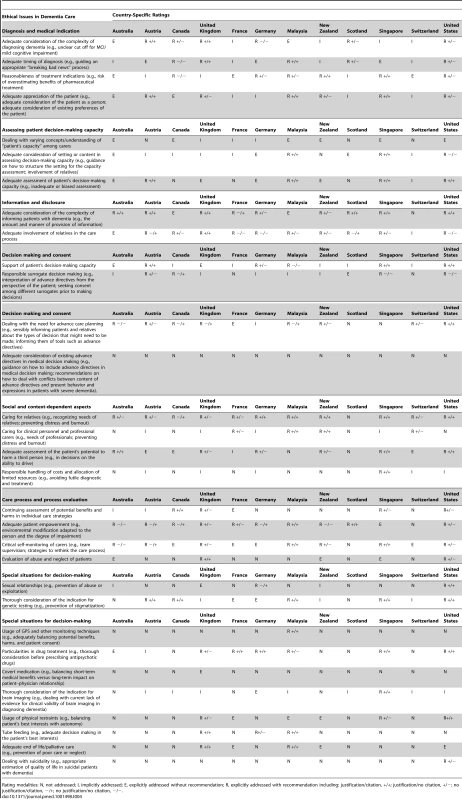
Rating modalities: N, not addressed; I, implicitly addressed; E, explicitly addressed without recommendation; R, explicitly addressed with recommendation including: justification/citation, +/+; justification/no citation, +/−; no justification/citation, −/+; no justification/no citation, −/−. Five DSEIs (16%) were addressed explicitly (E or R ratings) in at least 11 out of 12 CPGs, for example “Adequate consideration of complexity of informing patients with dementia” and “Caring for relatives.” When adding all implicit mentions of DSEIs (I, E, or R ratings) 11 DSEIs (35%) were addressed in at least 11 out of 12 CPGs (Table 4).
Though some DSEIs were explicitly addressed in several CPGs, the CPGs varied regarding which aspects of each DSEI were highlighted and in how much detail each was described. As an example, see the original wording examples for the DSEI “Adequate involvement of relatives in the care process” (Table S2). As the wording examples demonstrate, CPGs differed in the specification of (a) whether permission to disclose the diagnosis to carers should or must be sought, (b) which care decisions should be discussed with relatives, and (c) how specific the advice and support for relatives should be.
CPGs further differed in whether a justification and/or citations were given to support a recommendation made in the guideline (Table 5). While the CPGs from Malaysia and Singapore both provided justifications and citations for 71% and 76% of all DSEIs with ethical recommendations (n = 12 and n = 13), the CPGs from the United Kingdom and the United States provided justifications and citations for only 44% and 45% of all DSEIs with ethical recommendations (n = 7 and n = 10).
Tab. 5. Rating results for how often a CPG gave a recommendation (R-Rating) along with justification and/or citations with respect to the 31 DSEIs presented. 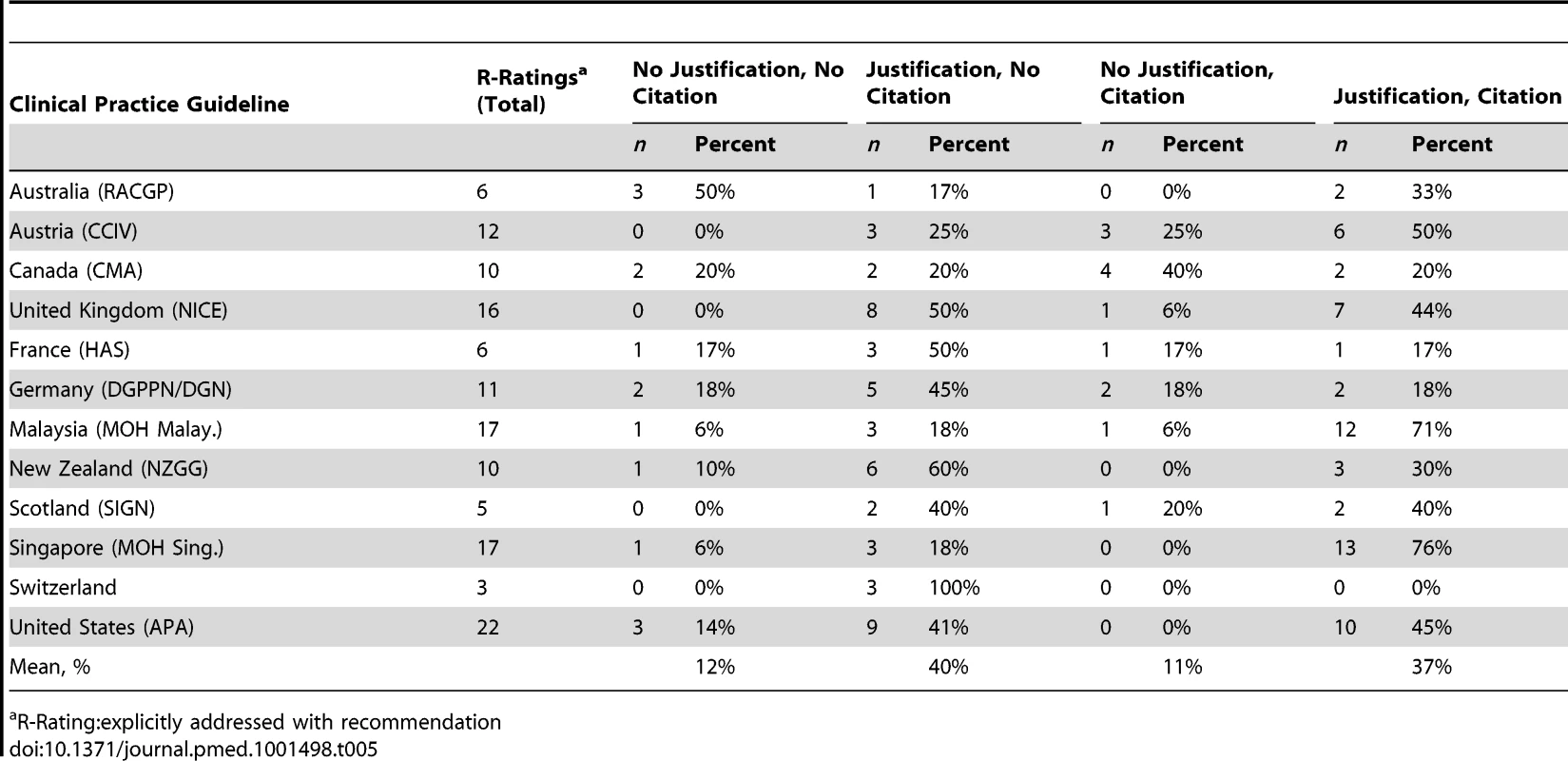
R-Rating:explicitly addressed with recommendation In line with the varying wordings and specifications for the description of a DSEI (Table S2), the arguments chosen for justification of specific ethical recommendations also differed substantially between CPGs. A more in-depth analysis of justification and support patterns for different types of ethical recommendations in CPGs is beyond the scope of this paper. Here we present two rare examples where more or less “precise” justifications were given in a CPG.
For the DSEI “Reasonableness of treatment indications (e.g., risk of overestimating benefit of pharmaceutical treatment)”, the CPG from Singapore recommends:
“Non-pharmacological methods to manage behavioural and psychological symptoms of dementia should be instituted, prior to consideration of pharmacological measures. […] The reason for considering [non-pharmacological treatment] NPT first and as an enduring endeavour in addressing difficult behaviour is two-fold. First, NPTs […] address the underlying reasons for the behaviour. Second, medications carry adverse side-effects and often mask and suppress the behaviour that actually serves to communicate the need of the person with dementia.”
Another example is from the APA guideline regarding the DSEI “Dealing with the need for advanced care planning (e.g., sensibly informing patients and relatives about the types of decision that might need to be made; informing them of tools such as advance directives)”:
“Patients with dementia usually lose the ability to make medical, legal, and financial decisions as the disorder progresses, and consequently these functions must be taken over by others (ref. 97). Clinical evaluation, including cognitive testing when needed, can assist in determining whether a patient with Alzheimer's disease has the capacity to make medical decisions (ref. 98–100). If family members act while the patient is still able to participate, they can seek his or her guidance regarding long-term plans. This approach can help in incorporating the patient's own wishes and values into the decision-making process, as well as in avoiding future conflict.”
Discussion
This study demonstrates that national CPGs on dementia care (in English and German) already address clinical ethical issues. However, the extent to which the full spectrum of ethical issues in dementia care is represented varies significantly within and among the 12 included CPGs. National guidelines vary in different ways, according to: (a) which ethical issues are represented and which not (for example, the DSEIs “Adequate consideration of existing advance directives in medical decision making,” “Usage of GPS and other monitoring techniques,” and “Dealing with suicidality” were only addressed in one out of 12 CPGs); (b) whether ethical issues are addressed implicitly or explicitly; (c) whether a recommendation on how to deal with a specific ethical issue is included or not; (d) whether a rationale and/or references are provided to explain and justify a specific recommendation; and (e) how thoroughly the ethical issue and/or the respective recommendation is explained.
Recommendations on how to deal with DSEIs often lack the sort of evidence health care professionals employ to justify clinical decisions, such as whether to recommend a specific diagnostic or therapeutic intervention. Nevertheless, ethical recommendations also need appropriate justifications (see the examples given in the Results section). Original literature on dementia ethics tends (with good reason) to employ more argumentation and explanation to justify a specific ethical recommendation [16]–[18]. While CPGs could quite easily be improved with respect to at least basic standards for justification of ethical recommendations, there is a need for further research into how reductive and simplistic the justification of ethical recommendations in future CPGs can and should be [21]. On the one hand, oversimplification can render the content meaningless or unhelpful. On the other hand, in-depth analyses of DSEIs might not fit into the format of CPGs. Many of these challenges are comparable with general challenges in the development of practice-oriented recommendations and guidance [15]. The integration of DSEIs into guidelines, therefore, could use quality assessment tools and standard procedures for guideline development such as the AGREE or GRADE instruments [3],[22].
The Nuffield Council of Bioethics report on ethical issues in dementia care demonstrates how some of the complex issues captured in our DSEI spectrum can be addressed by providing a set of criteria that guide the process of ethical decision-making in dementia care [16]. A good example is the DSEI “Adequate appreciation of the patient (e.g., problems concerning understanding and handling of patient autonomy)”. The Nuffield Council addresses this DSEI as follows: “Wellbeing factors, such as the person's general level of happiness are also important but again cannot automatically take precedence over the person's interests in having their autonomy respected” [16]. In the following, the Nuffield Council suggests factors which should be taken into account when weighing up the conflicting ethical principles in dementia care (wellbeing versus respect of autonomy): “(i) How important is the issue at stake?, (ii) How much distress or pleasure is it causing now?, (iii) Have the underlying values or beliefs on which the earlier preferences were based genuinely changed or can they be interpreted in a new light?, (iv) Do the apparent changes in preferences or values result from psychosocial factors (such as fear) or directly from the dementia (such as sexually disinhibited behaviour), or are they linked with a genuine pleasure in doing things differently?” [16].
The more CPGs are claimed to be key to fostering medical professionalism, and the more these CPGs are broadly accepted as a key resource (by physicians, patients, insurers, hospital managers, and health policy decision makers), the more they should address practice-oriented DSEIs and offer recommendations on how to deal with them. However, it is important to realize that the 31 DSEIs that comprise the matrix for this guideline assessment are only potentially relevant ethical issues for dementia guidelines. The prior systematic review that identified all DSEIs for dementia care was purely descriptive [19]. It cannot be inferred (without further normative justification) that all these DSEIs should be explicitly addressed in every dementia guideline, but the mere fact that the issues have been discussed controversially in analysis papers, editorials, and textbooks is evidence that decision makers need guidance concerning these DSEIs. Guideline development groups need to select the most relevant DSEIs, just as they need to select the most relevant clinical issues.
Guideline development manuals currently fail to address how DSEIs should be included in CPGs. As with any information gathered for inclusion, DSEIs should be incorporated in a systematic, transparent and comprehensible manner. All the usual steps of information retrieval and appraisal must be performed and documented: (a) identification of DSEIs; (b) assessment of relevance; (c) selection of key ethical issues that should be addressed in the CPG; (d) drafting, agreeing and grading text sections that address the DSEI and provide recommendations on how to deal with it.
Our study had the following limitations. The identification and rating of text passages in CPGs that deal with one of the 31 DSEIs unavoidably involves interpretative tasks, which could affect the validity and reliability of the results. We addressed this by having at least two researchers (with education in medicine/public health or bioethics or both) identify and rate text passages independently. In cases of different ratings a third researcher was involved. However, even when this problem is taken into account, we believe that the core findings of this study remain valid and reliable, namely, the low mean rates of explicit coverage of ethical issues in dementia guidelines and the variations in how these guidelines addressed ethical issues. Furthermore, we restricted our analysis to CPGs listed in databases of guidelines, which often employ quality criteria for inclusion. The addition of further guidelines that follow lower standards for evidence-based policy making and stakeholder involvement might result in even lower mean rates for the coverage of DSEIs.
In conclusion, ethical issues were inconsistently addressed in national dementia guidelines, with some guidelines including most and some including few ethical issues (which can be life-determining or important to quality of life). Ethical issues and how to deal with them are important for guidelines to address, for the medical profession to understand how to approach care of patients with dementia, and for patients, their relatives, and the general public, all of whom might seek information and advice in national guidelines.Finally, from a methodological point of view there is a need for further research to specify in how much detail CPGs should address DSEIs and justify recommendations for their handling.
Supporting Information
Zdroje
1. IOM (2011) Clinical practice guidelines we can trust. Washington (D.C.): National Academies Press, Institute of Medicine (IOM).
2. HardingE, PettinariCJ, BrownD, HaywardM, TaylorC (2011) Service user involvement in clinical guideline development and implementation: learning from mental health service users in the UK. Int Rev Psychiatr 23 : 352–357.
3. GuyattGH, OxmanAD, VistGE, KunzR, Falck-YtterY, et al. (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 : 924–926.
4. GuyattG, AklEA, HirshJ, KearonC, CrowtherM, et al. (2010) The vexing problem of guidelines and conflict of interest: a potential solution. Ann Intern Med 152 : 738–741.
5. NICE (2009) The guidelines manual. London: National Institute for Health and Clinical Excellence (NICE), Available: www.nice.org.uk.
6. WHO (2008) WHO Handbook for guideline development. Geneva: World Health Organization (WHO).
7. SIGN (2008) SIGN 50. A guideline developer's handbook. Edinburgh: Scottish Intercollegiate Guidelines Network.
8. CMA (2007) Handbook on clinical practice guidelines. Ottawa: Canadian Medical Association.
9. ABIM Foundation, ACP–ASIM Foundation, European Federation of Internal Medicine (2002) Medical professionalism in the new millennium: a physicians' charter. Lancet 359 : 520–522.
10. Beauchamp TL, Childress JF (2008) Principles of Biomedical Ethics. New York: Oxford University Press.
11. American Psychiatric Association (2010) The Principles of medical ethics: with annotations especially applicable to psychiatry. Washington (D.C.): American Psychiatric Association.
12. HughesJC, HopeT, ReaderS, RiceD (2002) Dementia and ethics: the views of informal carers. J Roy Soc Med 95 : 242–246.
13. GiacominiM, CookD, DeJeanD, ShawR, GedgeE (2006) Decision tools for life support: a review and policy analysis. Crit Care Med 34 : 864–870.
14. LemiengreJ, de CasterleBD, Van CraenK, SchotsmansP, GastmansC (2007) Institutional ethics policies on medical end-of-life decisions: a literature review. Health Policy 83 : 131–143.
15. StrechD, SchildmannJ (2011) Quality of ethical guidelines and ethical content in clinical guidelines: the example of end-of-life decision-making. J Med Ethics 37 : 390–396.
16. Nuffield Council on Bioethics (2009) Dementia: ethical issues. London: Nuffield Council on Bioethics.
17. Hughes JC, Baldwin C (2008) Ethics and the old age psychiatry. Jacoby R, Oppenheimer C, Dening T, editors. Oxford textbook of old age psychiatry. New York: Oxford University Press. pp. 709–729.
18. Oppenheimer C (2009) Ethics in old age psychiatry. Bloch S, Green S, editors. Psychiatric ethics. New York: Oxford University Press. pp. 409–433.
19. StrechD, MertzM, KnuppelH, NeitzkeG, SchmidhuberM (2013) The full spectrum of ethical issues in dementia care: systematic qualitative review. Brit J Psychiatr 202 : 400–406.
20. Dixon-WoodsM, AgarwalS, JonesD, YoungB, SuttonA (2005) Synthesising qualitative and quantitative evidence: a review of possible methods. J Health Serv Res Policy 10 : 45–53.
21. ErikssonS, HoglundAT, HelgessonG (2008) Do ethical guidelines give guidance? A critical examination of eight ethics regulations. Camb Q Healthc Ethics 17 : 15–29.
22. BrouwersMC, KhoME, BrowmanGP, BurgersJS, CluzeauF, et al. (2010) AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 182: E839–842.
23. Royal Australian College of General Practitioners (2003) Care of patients with dementia in general practice: guidelines. Sydney: Royal Australian College of General Practitioners, NSW Dept. of Health.
24. Dorner T, Rieder A, Stein KV (2011) Besser Leben mit Demenz - Medizinische Leitlinie für die integrierte Versorgung Demenzerkrankter. Wien: Competence Center Integrierte Versorgung.
25. CCCDTD3 (2007) 3rd Canadian Consensus Conference on Diagnosis and Treatment of Dementia. Montreal: 3rd CCCDTD
26. National Institute for Health and Clinical Excellence, National Collaborating Centre for Mental Health (2006) Dementia: the NICE-Scie guideline on supporting people with dementia and their carers in health and social care. London: National Institute for Health and Clinical Excellence (NICE). 978-1-85433-451-0 978-1-85433-451-0.
27. National Institute for Health and Clinical Excellence (2010) End of life care for people with dementia. Commissioning guide - implementing NICE guidance. London: National Institute for Health and Clinical Excellence (NICE).
28. Haute Autorité de Santé (2008) Diagnosis and treatment of Alzheimer's disease and related conditions. Saint-Denis La Plaine: Haute Autorité de Santé (HAS), Professional Guidelines Department.
29. DGN, DGPPN (2009) S3-Leitlinie “Demenzen”. Bonn: Deutsche Gesellschaft für Psychiatrie Psychotherapie und Nervenheilkunde (DGPPN), Deutsche Gesellschaft für Neurologie (DGN).
30. Ministry of Health Malaysia (2009) Management of dementia. Putrajaya: Ministry of Health Malaysia, CPG Secretariat, Health Technology Assessement Section, Guideline Development Group (Chair: Suraya Yusoff).
31. Ministry of Health New Zealand (1997) Guidelines for the support and management of people with dementia. Ministry of Health New Zealand.
32. Scottish Intercollegiate Guidelines Network (2006) Management of patients with dementia - a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, Guideline Development Group (Chair: Peter Connelly).
33. Ministry of Health Singapore (2007) Clinical practice guideline: dementia. Singapore: Ministry of Health, Singapore. 978-981-05-8095-7 978-981-05-8095-7.
34. Expertengruppe der Schweiz (2008) Konsensus zur Diagnostik und Betreuung von Demenzkranken in der Schweiz. Muttenz: Alzheimer Forum Schweiz, Schweiz Med Forum.
35. American Psychiatric Association (2007) Practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Arlington (VA): American Psychiatric Association (APA), Work Group on Alzheimer's Disease and Other Dementias (Chair: Peter V. Rabins).
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 8- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Better Reporting of Scientific Studies: Why It Matters
- Towards Universal Voluntary HIV Testing and Counselling: A Systematic Review and Meta-Analysis of Community-Based Approaches
- Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
- Public Engagement in Health Priority Setting in Low- and Middle-Income Countries: Current Trends and Considerations for Policy
- Expanding Disease Definitions in Guidelines and Expert Panel Ties to Industry: A Cross-sectional Study of Common Conditions in the United States
- Pediatric AIDS in the Elimination Agenda
- Country Contextualization of the Mental Health Gap Action Programme Intervention Guide: A Case Study from Nigeria
- Reproductive and Maternal Health in the Post-2015 Era: Cervical Cancer Must Be a Priority
- Effect of Household-Based Drinking Water Chlorination on Diarrhoea among Children under Five in Orissa, India: A Double-Blind Randomised Placebo-Controlled Trial
- First Diagnosis and Management of Incontinence in Older People with and without Dementia in Primary Care: A Cohort Study Using The Health Improvement Network Primary Care Database
- Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis
- Inclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
- Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis
- Inclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
- Country Contextualization of the Mental Health Gap Action Programme Intervention Guide: A Case Study from Nigeria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



