-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIf You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
article has not abstract
Published in the journal: . PLoS Med 8(5): e32767. doi:10.1371/journal.pmed.1001030
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1001030Summary
article has not abstract
Summary Points
-
The Interagency Emergency Health Kit is a box with medicines and medical supplies designed to meet the expected primary health care needs of people exposed to major humanitarian emergencies.
-
Previous editions of the kit have been inadequate to help people with severe mental or neurological disorders.
-
The challenge to be addressed was to propose the inclusion of one medicine for each of five classes of psychotropic medicines.
-
Amitriptyline (tablets), haloperidol (tablets and injections), diazepam (tablets and injections), biperiden (tablets), and phenobarbital (tablets) will be included in the next edition of the kit.
-
A fundamental inequity has been addressed by ensuring that the availability of medicines for people with severe mental and neurological disorders will be on a par with that for other medical disorders in emergencies.
Introduction
The Interagency Emergency Health Kit (IEHK) [1] is a large, pre-packed box containing medicines and medical supplies (Table 1). The kit is designed to meet the expected primary health care (PHC) needs of persons exposed to acute humanitarian crises caused, for example, by forced displacement or major natural disaster such as an earthquake, cyclone, or tsunami. Such events often involve the partial or complete destruction of locally available medicines. The IEHK aims to provide sufficient medicines and supplies for medical care for a population of 10,000 people—located in one geographical area or place—for 3 months. The next version of the IEHK will be the fourth edition of the one originally developed three decades ago [2],[3]. While data on this new IEHK are not yet available, the third edition came in a very heavy, large box (1,045 kg, 4.6 m3) containing ten basic kits and one supplementary kit [4]. The IEHK is held in stock by major suppliers of generic medicines, most of whom ship it within 48 hours after being ordered by an aid agency (Figure 1).
Fig. 1. Cyclone Nargis response 2008: Interagency Emergency Health Kits en route to Myanmar. 
Photo credit: Fred Urlep, WHO. Tab. 1. Psychotropics in the next edition of the Interagency Emergency Health Kit [1]. ![Psychotropics in the next edition of the Interagency Emergency Health Kit <em class="ref">[1]</em>.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/f73655b0782d46a91abaa70bc19e2015.png)
The three anti-convulsants are phenobarbital (1,000 50-mg tablets), diazepam (200 injections, 5 mg/ml; 2 ml/ampoule), and magnesium sulfate (40 injections; 500 mg/ml; 10 ml/ampoule). The IEHK has become a core feature of international emergency response whenever medical facilities and pharmacies have been destroyed or populations have been displaced. Medical relief agencies tend to immediately allocate emergency funds to procure kits. These kits typically play a major role very early in sudden-onset emergencies when the exact medical needs of the population, the health services situation, and human resource capacities are largely unknown. Once local needs and resources have been assessed, standard orders of IEHKs tend to be replaced—as they should—with situation-specific orders of medicines and supplies.
The IEHK ensures continuity in the supply of medicines, most of which are life-saving. It addresses at least three emergency-relief management challenges: (a) managing the procurement, distribution, and logistics of supplies in an efficient manner; (b) focusing the good intent of donors of medicines and medical devices on a small variety of essential products rather than flooding systems with a large variety of unknown products, including expensive, non-essential, expired, or poorly labelled ones; and (c) harmonizing use of different medicines by different organizations for the same conditions across the health system [5],[6].
The IEHK is not a mini-pharmacy. Its purpose is to allow for a response in the acute phase of emergencies before any needs assessment results becomes available. Given that its size is limited to allow for easy transport, the kit involves a compromise, providing a limited range and number of medicines that are considered priority to meet the needs of populations with disrupted medical facilities. All medicines in the kit are on the WHO Model List of Essential Medicines [7] (WHO Model List). This is a prioritized list of over 350 medicines selected through a transparent, rigorous review of the evidence that considers the public health relevance, efficacy, safety, and cost-effectiveness of different medicines. The WHO Model List is used by many countries as a basis for deciding what medicines should be on their national list of essential medicines, which guides (amongst other things) procurement and supply of medicines for local PHC services. The IEHK, however, can only include only a fraction of the medicines on the WHO Model List. The 2011 version of the IEHK will contain 73 medicines.
Psychotropic Medicines in the IEHK
Medical care for people with severe mental or neurological disorders has, historically, not been a priority in humanitarian crises, but this is changing [8]. One major impetus for change has been the Inter-Agency Standing Committee (IASC) Guidelines on Mental Health and Psychosocial Support in Emergency Settings [9], which was developed through an extensive consultation process. The Guidelines were released by a committee of United Nations (UN) and non-UN international humanitarian agencies responsible for humanitarian policy according to UN General Assembly resolution 46/182. The Guidelines describe minimum responses during emergencies and cover a wide range of mostly social interventions for the population at large as well as psychological first aid for people experiencing acute distress. In addition, these IASC guidelines emphasize the protection of and care for people with severe mental disorders as a priority in acute emergencies.
The IEHK has been inadequate with respect to psychotropics. For example, the third (2006) edition included only three psychotropic medicines (chlorpromazine injections, diazepam injections, phenobarbital tablets), which do not cover first-line treatment of depression and psychosis. The IASC Guidelines stipulate that the minimum provision of medicines in emergency health kits should include—in tablet form—at least one anti-depressant, one anxiolytic, one anti-psychotic, one anti-Parkinsonian (to deal with extra-pyramidal side effects of anti-psychotic medicines), and one anti-epileptic medicine.
In 2009, the authors submitted an inter-agency proposal to suggest changes to the psychotherapeutic and antiepileptic medicines classes of the IEHK. This proposal was shaped by two considerations. First, given that the IEHK is meant to be a subset of medicines of the WHO Model List, the proposal was limited to selecting from the few psychotropic medicines on that list (Table 2). Second, given that the previous IEHK only had 67 medicines to address all priority health conditions relevant to PHC in emergency settings, the selection of additional medicines needed to be proportionate: one medicine for each of the classes of psychotropic medicines. The proposal—described in this article—was approved by the independent, inter-agency Review Committee for Updating the Interagency Emergency Health Kit, which consists of public health experts overseeing the IEHK update. Medicines from each of the aforementioned five classes of psychotropic medicines will be included in the next edition of the IEHK.
Tab. 2. The WHO Model List of Essential Medicines and the Interagency Emergency Health Kit (IEHK). 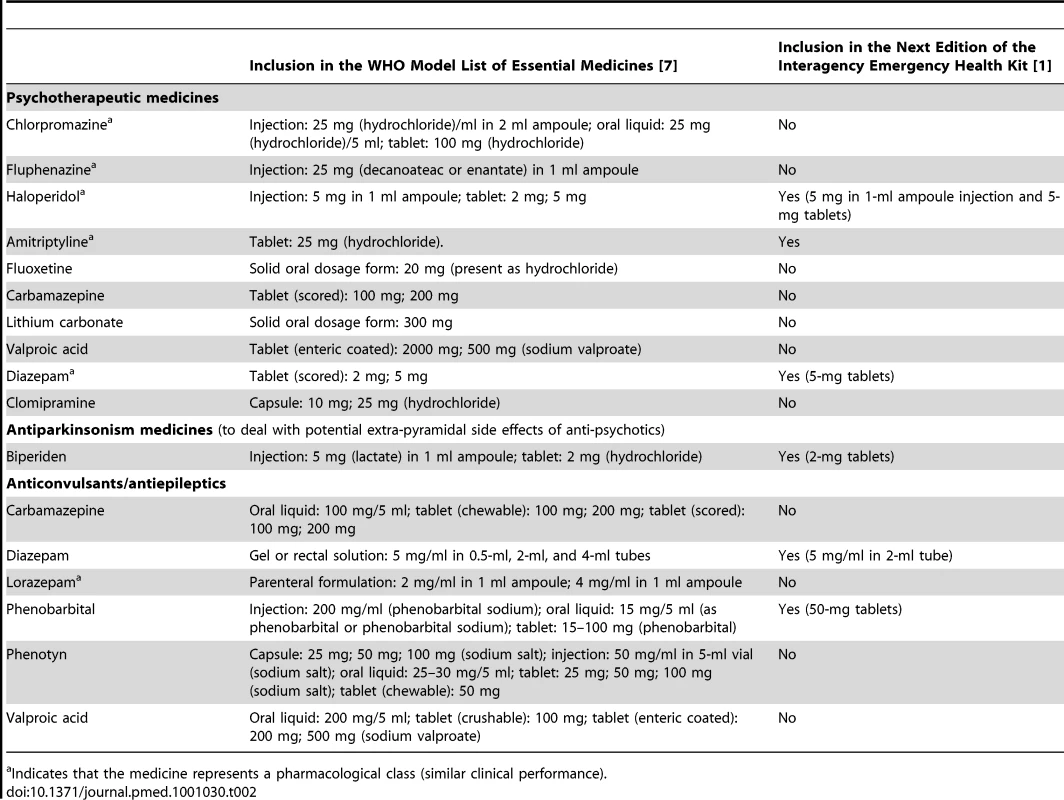
Indicates that the medicine represents a pharmacological class (similar clinical performance). Choice of Anti-Depressant
Fluoxetine and amitriptyline are the only anti-depressant medicines on the WHO Model List. After review of the advantages and disadvantages (Table 3) of these two similarly effective medicines [10],[11], amitriptyline was selected. The overriding consideration was the need to be consistent with what is most likely to be available currently in most low-income country PHC systems to maximize continuity of care and build on local PHC capacities. Compared to fluoxetine, amitriptyline is more widely available in PHC systems in emergency-prone Africa [12].
Tab. 3. Advantages and disadvantages of including fluoxetine versus amitriptyline in the Interagency Emergency Health Kit (IEHK). 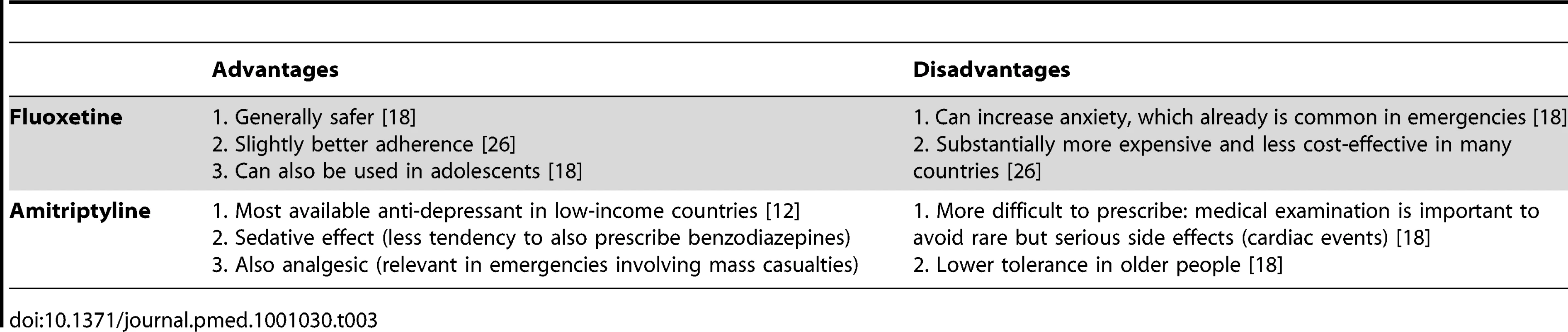
Selecting amitriptyline is consistent with the IASC Guidelines, which recommends the use of medicines that are on the country's essential medicines list [8]. Fluoxetine was only added to the WHO Model List in 2007. We expect that in future years fluoxetine will slowly be added to many countries' national list of essential medicines and accordingly will become widely available in Africa as well. We envision that future updates of the kit —possibly as early as the fifth edition in 2015—will involve replacing amitriptyline with fluoxetine, because fluoxetine is easier to prescribe and is more suitable for a broader range of age groups (e.g., from adolescence to old age). Of note, the evidence on these two medicines for use in post-traumatic stress disorder is inconclusive [13] and did not influence the selection.
Choice of Anxiolytic
A small supply of diazepam tablets will be added to the kit. The only anxiolytics on the WHO Model List are diazepam (in tablet and injectable form) and lorazepam (in injectable form). People presenting with normal (non-pathological), acute anxiety are numerous in the early phases of all emergencies. Non-pharmacological measures (e.g., psychological first aid) should be used. Psychological first aid involves basic, non-intrusive pragmatic care with a focus on listening but not forcing talk; assessing needs and concerns; ensuring that basic needs are met; encouraging social support from significant others; and protecting from further harm [9]. Anxiolytics in the form of benzodiazepines may slow down recovery from traumatic stress [14], can produce dependence, and tend to be prescribed indiscriminately in many emergencies. While precautions should be taken to prevent the routine prescription of benzodiazepines to people experiencing distress in emergencies [15], there are occasions when anxiolytics are indicated. For example, they are appropriate in cases of severe agitation or sleeplessness that interfere with a person's ability to address their own and their family's survival needs, and that do not respond to non-pharmacological interventions. The availability of injectable diazepam in previous editions of the kit has meant that on many occasions acutely anxious individuals were treated with injections of diazepam. This is not the first-line route of administration in anxiety reactions, and this encourages frequent returns to the already overburdened emergency health care provider. When combined with psychological first aid and culturally relevant relaxation methods, dispensing a few days of diazepam tablets can be humane and appropriate to lessen agitation and restore sufficient sleep to help a severely distressed person through a crisis. Injectable diazepam will be kept in the kit for the management of status epilepticus.
Choice of Anti-Psychotic
Injectable chlorpromazine—which was in the previous edition of the kit—will be replaced with haloperidol in both injectable and tablet form. Anti-psychotic tablets will be added for a number of reasons. Although injections can be used, oral administration is preferred in chronic psychosis, which may occur throughout an extended humanitarian emergency. Tablets also have the advantage that they can be provided by family members after prescription by very busy health staff. Oral haloperidol is preferred to oral chlorpromazine because, despite similar efficacy, randomized evidence suggests that the former is associated with better acceptability rates [16]. With respect to injectable anti-psychotics, National Institute for Health and Clinical Excellence (NICE) Guidelines recommend against rapid tranquillization using chlorpromazine, highlighting a number of side effects including acting as a local irritant if given intramuscularly [17]. Accordingly, chlorpromazine injections will be replaced with haloperidol tablets and injections.
Choice of Anti-Parkisoninan
Anti-cholinergic medicines need to be available to counteract movement disorders (such as Parkinsonism and acute dystonia) that can occur as side effects of anti-psychotic medicines [18]. A Cochrane review [19] reported that 33% of patients on haloperidol develop movement disorders and need anti-cholinergic medication. Biperiden and levodopa/carbidopa are the two anti-Parkinsonian medicines on the WHO Model List. Levodopa/carbidopa is generally not used in mental health to treat the side effects of anti-psychotics, so biperiden was suggested.
Choice of Anti-Epileptic
Phenobarbital continues to be in the kit, because it is by far the most commonly available anti-epileptic medicine in low-income countries [20], by far the least expensive, and by far the most cost-effective [21]. Tolerability of phenobarbital tends to be adequate [22], and it is promoted through the Global Campaign Against Epilepsy by WHO, the International League against Epilepsy, and the International Bureau for Epilepsy. However, the 100-mg tablet in the previous kit is too large for the treatment of children (even when one breaks the tablet), so the tablet weight will be reduced to 50 mg to make it available for children.
There is a caveat to the inclusion of phenobarbital and diazepam in the kit, and that is that these are controlled medicines requiring import authorization from national authorities [23]. This causes significant delays in delivery of the kit, as national authorities tend to be overwhelmed in emergencies. To address this challenge, suppliers of the IEHK tend to offer the option of buying the kit without controlled psychotropics or narcotics [4]. While some offer to substitute narcotics with tramadol (an analgesic that is not controlled), they do not offer substitutes for phenobarbital and diazepam. Although we are not aware of a good non-controlled alternative medicine to diazepam, drug suppliers may consider substituting phenobarbital with carbamazepine. The latter is much more expensive, but it is an effective anti-convulsant that is on the essential medicines list of almost all countries [20]. Carbamazepine should be importable given that it is not controlled.
Discussion
The changes to the IEHK described in this article have addressed a fundamental inequity. In emergencies, PHC professionals will now be in a position to provide medical treatment to people with severe mental or neurological disorders on a par with treatment for other medical conditions, because psychotropic medicines will be available in emergencies.
Some observers may argue that the IEHK should be limited to life-saving medicines, but such reasoning is not in line with current thinking. For example, the influential Sphere Project [24] promotes the principle of survival with dignity through its widely endorsed handbook. Although many people with severe mental disorders tend to not experience dignified life conditions even before a conflict or other disaster strikes (e.g., in terms of housing conditions or work opportunities), their life conditions tend to worsen dramatically during emergencies.
When left untreated, these disorders put people at substantial risk of death in the midst of an emergency [8]. People with psychosis have been known to be shot in conflict situations because of not comprehending instructions by soldiers. Alternatively, many have been abandoned by family members when there is population movement. Parents with severe depression in emergencies have neglected to take care of themselves or feed and provide care to their children. Also, people who were treated with anti-epileptics before the emergency face a fatal risk if there is a sudden discontinuation of supply of anti-epileptics due to the emergency. Finally, mental and neurological disorders are associated with elevated mortality rates [25].
Many specialists in psychiatry and neurology will likely argue that the inclusion of just one medicine of each of the classes of psychotropic medicines is insufficient. They may experience it as unacceptable that the vast arsenal of medicines available in modern specialist care is reduced to a handful of first-generation drugs. However, the IEHK has to be limited in size to be feasibly made available during acute emergencies.
In humanitarian emergencies that affect large numbers of people, it is necessary to apply a population-wide perspective to use all available means to address the health status of the maximum number of people. This requires priority setting, which involves making difficult choices. Including one medicine from each of five classes of psychotropic medicines in the IEHK has involved tough priority setting but has been a major step towards the agreed goal of providing minimum care for people with severe mental and neurological disorders in emergencies [9].
Zdroje
1. WHO 2011 The Interagency Emergency Health Kit 2011: medicines and medical devices for 10,000 people for approximately 3 months. Fourth edition. Geneva WHO In press
2. HogerzeilHV 1990 Emergency health kits. Lancet 336 1194
3. SimmondsSPWalkerGJA 1982 Essential drugs for primary health care standard packages. Lancet 1 8269 435 436
4. WHO 2007 Emergency kits used by WHO: description and specifications. Geneva WHO Available: http://www.who.int/hac/techguidance/ems/emergency_kits_21dec2007.pdf. Accessed 28 March 2011
5. PinheiroCP 2008 Drug donations: what lies beneath. Bull World Health Organ 86 580
6. SimmondsSMamdaniM 1988 Essential drug lists and health relief management. Trop Doct 18 155 158
7. WHO 2009 Model list of essential medicines. 16th edition. Geneva WHO
8. JonesLAsareJBEl MasriMMohanrajASheriefH 2009 Severe mental disorders in complex emergencies. Lancet 374 654 661
9. Inter-Agency Standing Committee (IASC) 2007 Guidelines on mental health and psychosocial support in emergency settings. Geneva IASC
10. CiprianiABrambillaPFurukawaTGeddesJGregisM 2005 Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst Rev 2005 CD004185
11. GuaianaGBarbuiCHotopfM 2007 Amitriptyline for depression. Cochrane Database Syst Rev 2007 CD004186
12. Health Action International 2010 Survey results. Available: http://www.haiweb.org/medicineprices/surveys.php. Accessed 28 March 2011
13. BissonJI 2010 Post-traumatic stress disorder. Clin Evid (Online) 02 1005
14. FreemanC 2003 Drugs and physical treatment after trauma. ØrnerRSchnyderU Reconstructing early intervention after trauma Oxford Oxford University Press 169 176
15. LeuchtCKitzmantelMChuaLKaneJLeuchtS 2008 Haloperidol versus chlorpromazine for schizophrenia. Cochrane Database Syst Rev 2008 CD004278
16. van OmmerenMSaxenaSSaracenoB 2005 Mental and social health during and after acute emergencies: emerging consensus? Bull World Health Organ 83 71 75
17. National Collaborating Centre for Nursing and Supportive Care 2005 Violence: the short-term management of disturbed/violent behaviour in psychiatric in-patient settings and emergency departments. London National Institute for Clinical Excellence (NICE)
18. WHO 2010 mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings. Geneva WHO Available: http://www.who.int/mental_health/evidence/mhGAP_intervention_guide/en/index.html. Accessed 28 March 2011
19. IrvingCBAdamsCELawrieS 2006 Haloperidol versus placebo for schizophrenia. ochrane Database Syst Rev 2006 CD003082
20. WHO 2004 Atlas: Country resources for neurological disorders. Geneva WHO
21. ChisholmD 2005 Cost-effectiveness of first-line antiepileptic drug treatments in the developing world: a population-level analysis. Epilepsia 46 751 759
22. KwanPBrodieMJ 2004 Phenobarbital for the treatment of epilepsy in the 21st century: a critical review. Epilepsia 45 1141 1149
23. International Narcotics Control Board 2003 List of psychotropic substances under international control. Vienna Vienna International Centre Available: http://www.incb.org/pdf/e/list/green.pdf. Accessed 28 March 2011
24. Sphere Project 2011 Humanitarian charter and minimum standards in disaster response. Geneva Sphere Project
25. PrinceMPatelVSaxenaSMajMMaselkoJ 2007 No health without mental health. Lancet 370 859 877
26. WHO 2006 Dollars, DALYs and decisions: economic aspects of the mental health system. Geneva WHO
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 5- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial
- Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Characterizing the Epidemiology of the 2009 Influenza A/H1N1 Pandemic in Mexico
- The Joint Action and Learning Initiative: Towards a Global Agreement on National and Global Responsibilities for Health
- Let's Be Straight Up about the Alcohol Industry
- Advancing Cervical Cancer Prevention Initiatives in Resource-Constrained Settings: Insights from the Cervical Cancer Prevention Program in Zambia
- The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in Africa
- Health Aspects of the Pre-Departure Phase of Migration
- Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature
- Threshold Haemoglobin Levels and the Prognosis of Stable Coronary Disease: Two New Cohorts and a Systematic Review and Meta-Analysis
- If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
- Migration and Health: A Framework for 21st Century Policy-Making
- Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study
- The Impact of Retail-Sector Delivery of Artemether–Lumefantrine on Malaria Treatment of Children under Five in Kenya: A Cluster Randomized Controlled Trial
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



