-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe BCG World Atlas: A Database of Global BCG Vaccination Policies
and Practices
article has not abstract
Published in the journal: . PLoS Med 8(3): e32767. doi:10.1371/journal.pmed.1001012
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1001012Summary
article has not abstract
Summary Points
-
Despite nearly a century of use, the Bacille Calmette-Guérin (BCG) vaccine continues to be controversial, with known variations in BCG substrains and vaccine efficacy.
-
Because vaccination policies and practices vary across time and countries, we created the first searchable, online, open access database of global BCG vaccination policy and practices, the BCG World Atlas (http://www.bcgatlas.org/), which contains detailed information on current and past BCG policies and practices for over 180 countries.
-
The Atlas is for clinicians, policymakers, and researchers and provides information that may be helpful for better interpretation of tuberculosis (TB) diagnostics as well as design of new TB vaccines.
Tuberculosis: A Global Threat
Tuberculosis (TB) remains one of the major causes of infectious morbidity and mortality globally, claiming millions of lives every year. Approximately one-third of the world's population is estimated to be infected with Mycobacterium tuberculosis, giving rise to 9.4 million new cases of active TB disease each year [1]. The majority of the TB burden exists in 22 high-burden countries, but with immigration and global travel TB is difficult to eliminate from any one country [1]–[4].
The Bacille Calmette-Guérin (BCG) vaccine, first introduced in 1921, continues to be the only vaccine used to prevent TB [5],[6]. Despite nearly a century of use, BCG remains controversial, with known variations in BCG substrains, vaccine efficacy, policies, and practices across the world. Global information on BCG policies and practices may be useful for clinical interpretation of diagnostic tests as well as in the design of novel TB vaccines that are under development.
BCG: A Range of Global Policies
While most experts agree that BCG is efficacious against severe forms of childhood TB, its efficacy against TB in adults is highly variable [7]. As a result of the uncertain efficacy of the BCG vaccine, countries have developed very different BCG vaccination policies. Some countries, such as the United Kingdom, have or have had universal BCG vaccination programs, while others (including Canada and the United States) either only recommended BCG for high-risk groups or did not advocate BCG countrywide. The Canadian situation was further complicated by differing policies across provinces, where some provinces underwent mass vaccination programs and others did not. In addition, BCG vaccination policies have varied by the number of doses used, the age at which vaccination was given, and the methods used to deliver the vaccine (although most countries today use only the intradermal route) [8]. Vaccination practices also have changed within and across countries over the years, reflecting changes in evidence, health policy, public perception, increasing or decreasing TB incidence, and HIV incidence. As a result of these changes to the BCG policies in various countries, it is necessary to not only know the current BCG vaccination policies but also past policies and applicable changes when dealing with adults who received BCG vaccination in childhood.
Since the publication of the M. tuberculosis genome, comparative genomic studies have documented that BCG vaccine strains have evolved and differ from each other and from the original BCG first used in 1921 [9]. Because these genetic differences affect antigenic proteins, these changes may translate into differences in efficacy and effect on the tuberculin skin test (TST) [10],[11]. Work done by Ritz and Curtis looking at global BCG strain variations demonstrates the diversity of strains used by different countries and even within the same countries [12]. They found that 44% (83/188) of countries reported using more than one BCG strain type during an interval of only 5 years. This highlights the importance of documenting BCG vaccination practices for both clinical and research purposes.
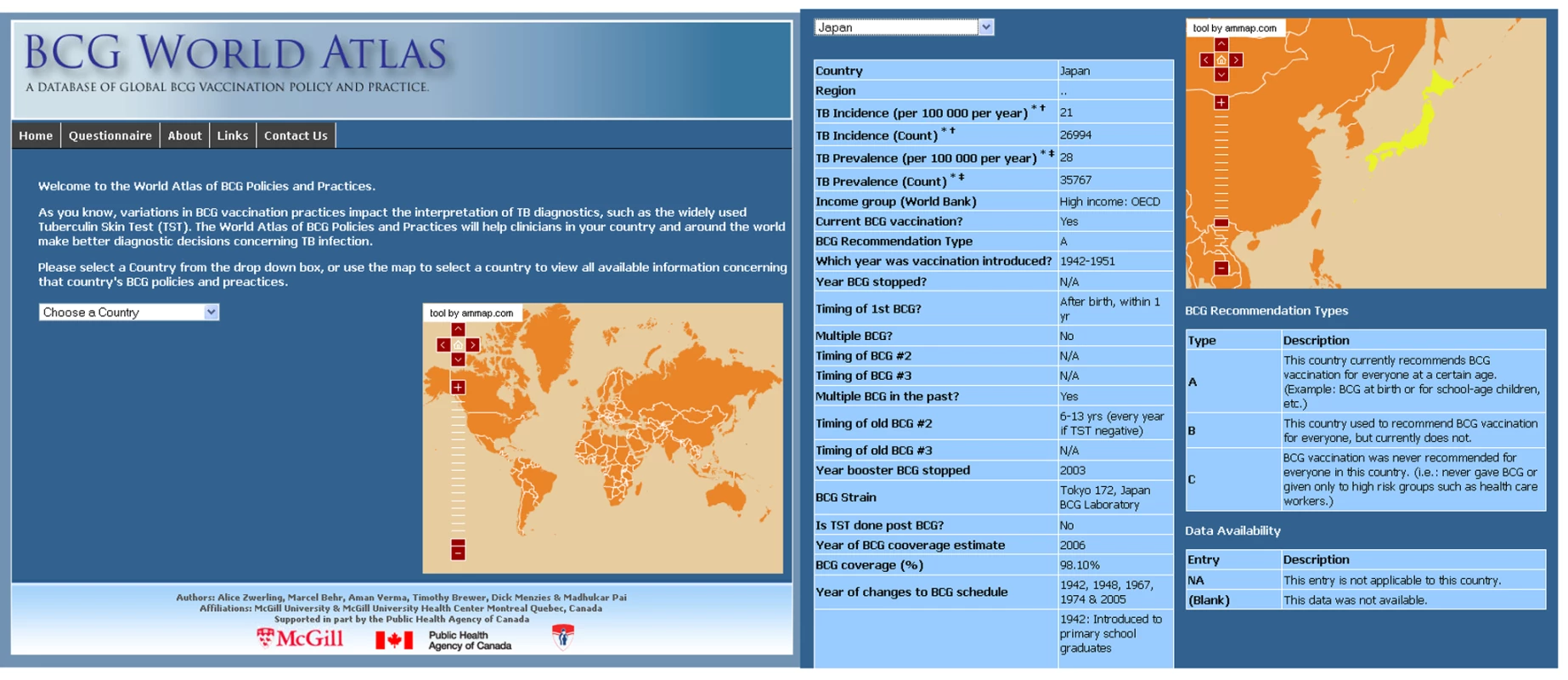
Clinicians cannot be expected to know BCG practices in all countries, and immigrants themselves may not know about the vaccination policies in their countries of birth (most adult immigrants are unlikely to retain childhood vaccination records). Information on diversity of BCG policies between countries and across time may be helpful for better interpretation of TB diagnostics as well as design of new TB vaccines. To our knowledge, there is no single, comprehensive, searchable database of BCG policies and practices (past and present) across the world. We developed the “BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices,” a database containing BCG information from each country across all world regions (http://www.bcgatlas.org/). Figure 1 shows the homepage of the Atlas. To make this resource practical and useful for clinicians, public health practitioners, and researchers alike, our database captures both present and previous policy and practices within a country, as well as any applicable changes.
Assembling the Database
Detailed information on past and present BCG vaccination policies and practices were collected from as many countries as possible by one of three methods. First, short respondent-completed questionnaires were sent out to at least two individuals in each country. Questionnaires were sent to experts in TB research, TB control programs, or public health/vaccination programs. Whenever possible, an attempt was made to collect two completed questionnaires from each country in order to validate the data. Questionnaires were available in English, French, and Spanish, and were designed to capture both current policy and actual BCG practices, as well any applicable changes that had occurred over the last 25 years. Detailed questions asked for information concerning past as well as current practices, the timing and nature of changes to the policies and or practices, repeat, multiple, or booster shots, information concerning tuberculin skin testing in conjunction with BCG vaccination, influence of HIV on the decision to vaccinate, and vaccine strain differences. A total of 89 completed questionnaires were received over a 2-year period. Second, data were abstracted from published papers, reports, and available government policy documents retrieved through literature searches on PubMed and via the World Wide Web. Third, we used immunization data available from the World Health Organization Vaccine Preventable Diseases Monitoring System (http://apps.who.int/immunization_monitoring/en/globalsummary/ScheduleSelect.cfm), which provide basic information on all vaccines currently in use in each country [13].
Based on the data generated by all methods, countries were grouped into three main categories: A), the country currently recommends universal BCG vaccination at a certain age; B), the country used to recommend universal BCG vaccination but currently does not; or category C), BCG vaccination is recommended only for selected high-risk groups or was never recommended.
Summary of Findings
The beta version of the Atlas went live in the fall of 2008 with completed questionnaires on BCG vaccination from 62 countries. Since that time, more data have been added and several improvements have been made. As of October 2010, we have collected data concerning BCG vaccination policies and practices for 180 of 209 (86%) countries worldwide that we approached. The database is available as an interactive Web site at http://www.bcgatlas.org/, where information for a particular country's BCG policy, along with its estimated World Health Organization (WHO) TB incidence statistics, can be viewed alongside a graphical map. Among the 180 countries with available data, 157 countries currently recommend universal BCG vaccination, while the remaining 23 countries have either stopped BCG vaccination (due to a reduction in TB incidence), or never recommended mass BCG immunization and instead favored selective vaccination of “at risk” groups (Figure 2). Complete questionnaire data were available for 77 countries, while remaining data were extracted from published sources.
Fig. 2. Map displaying BCG vaccination policy by country. 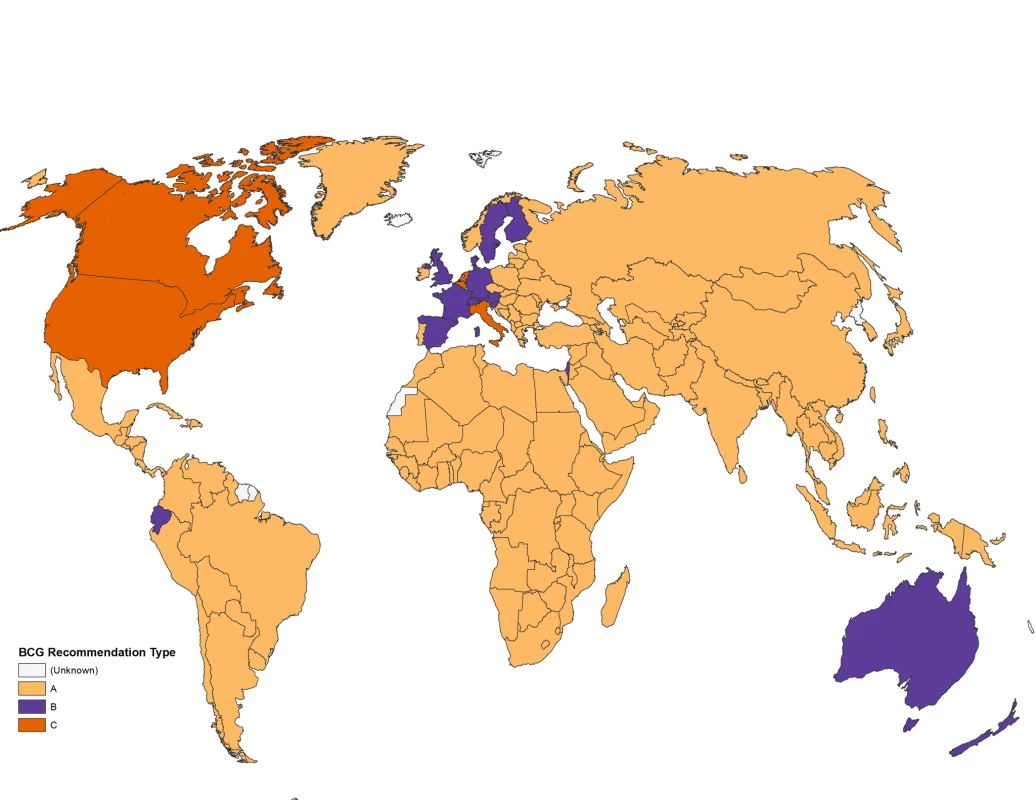
Many countries began BCG vaccination programs in the 1940s–1980s, though some countries such as Romania and Uzbekistan report vaccination campaigns as early as 1928 and 1937, respectively, while some sub-Saharan African nations such as Nigeria and Sierra Leone only began BCG vaccinations in 1991 and 1990. Nine countries have ceased universal BCG vaccination programs; Spain and Denmark were among the first, stopping in 1981 and 1986, respectively, while Austria and Germany had stopped by 1990 and 1998. The remaining countries, including the Isle of Man, Slovenia, UK, Finland, and France, all ceased their BCG vaccination campaigns between 2005 and 2007. While these countries may have ceased mass universal vaccination programs, many do continue to provide BCG vaccination selectively to high-risk individuals, including those involved in high TB risk occupations and/or travel, and infants born into high TB risk environments.
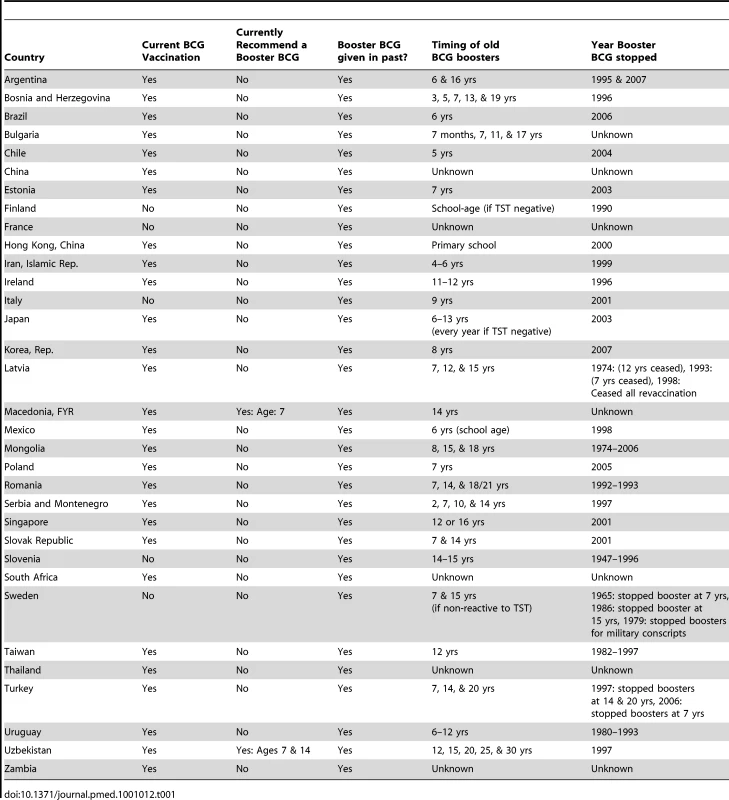
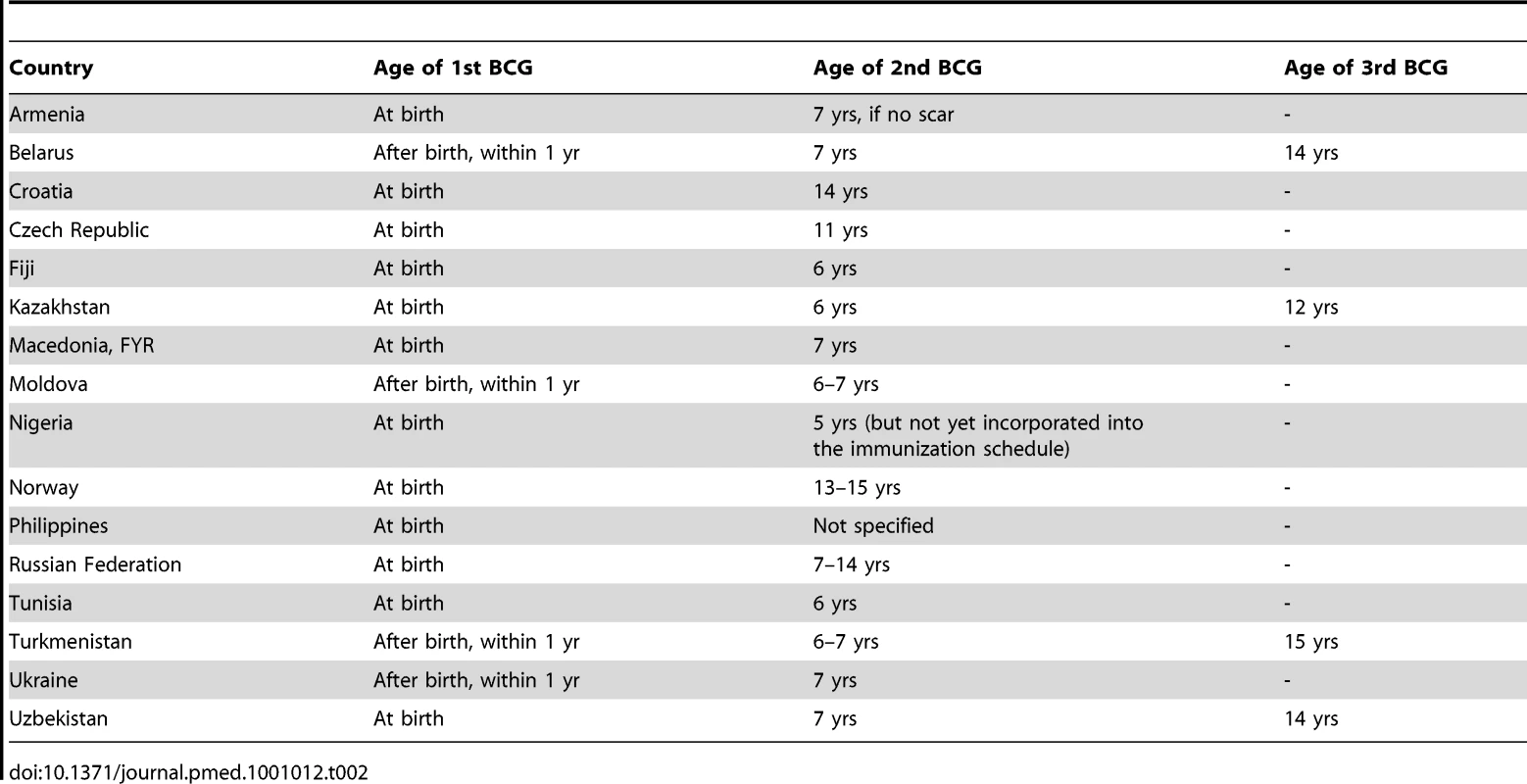
We identified 49 countries that reported changes to their BCG vaccination policy in the past 20 years. Twenty-seven countries reported major changes to their BCG policy within the last 10 years. Of particular interest is the large number (n = 33) of countries that had multiple vaccination programs in the past, but have since ceased revaccination, and now use a single BCG vaccination schedule (Table 1). These revaccination policy changes were as recent as 2007. Sixteen countries continue to give an additional BCG vaccination after the initial BCG, known as a booster vaccination (Table 2), while Kazakhstan, Belarus, Uzbekistan, and Turkmenistan continue to recommend three BCG vaccinations, with the third given between the ages of 12 and 15. Multiple revaccinations may lead to a delayed hypersensitivity reaction also known as the Koch response (phenomenon), where a person previously infected with M. tuberculosis is reinfected intracutaneously, resulting in a local inflammatory reaction marked by necrotic lesions that develops rapidly and heals quickly [14].
Changes in vaccine strain were the most frequent type of change reported (n = 42), and many countries have employed several strains over the course of their BCG vaccination program's existence, with countries reporting strain changes as recently as 2008.
Additional variations in BCG vaccination administration are seen across countries. Currently, eight countries recommend TST post–BCG vaccination, and two other countries had this policy but have since ceased. Estimated national BCG coverage ranged from 70% to 100%; however, frequently these estimates were not available or were several years out of date. Finally, 19 countries that did not recommend universal BCG vaccination did report BCG vaccination for certain at-risk groups, most frequently health care workers and infants living in high-risk TB settings. These variations highlight the importance of mapping these differences across regions both for clinical purposes and research.
Open Access through an Interactive Web Site
The Atlas is an interactive Web site that allows users to select and view information concerning a country's past and current BCG vaccination policy either by clicking on an interactive map or by selecting the country of interest from a drop-down list (Figure 1). The Web site is available to the public and is free of charge. Over the past year (during its beta phase), we have recorded over 6,000 visits to the site, with a steady increase in traffic over time.
Implications for Diagnosis of TB
While novel diagnostics have been developed for latent TB infection (LTBI) [15]–[19], the TST continues to be the most widely used diagnostic test worldwide [20]. False positives can occur in BCG-vaccinated individuals, complicating interpretation of test results [21]. However, research suggests the timing of vaccination plays an important role [9],[10]; in a meta-analysis, Farhat et al. found BCG vaccination at infancy has only a minimal effect on TST specificity, particularly if the TST is done more than 10 years after the BCG was administered, whereas BCG later in life or if given more than once led to more frequent, larger, and pronounced TST reactions [21]. The Atlas may help clinicians interpret TST by providing the information necessary to assess whether the TST is a valid diagnostic tool in a particular patient, or when alternative diagnostics may be preferable.
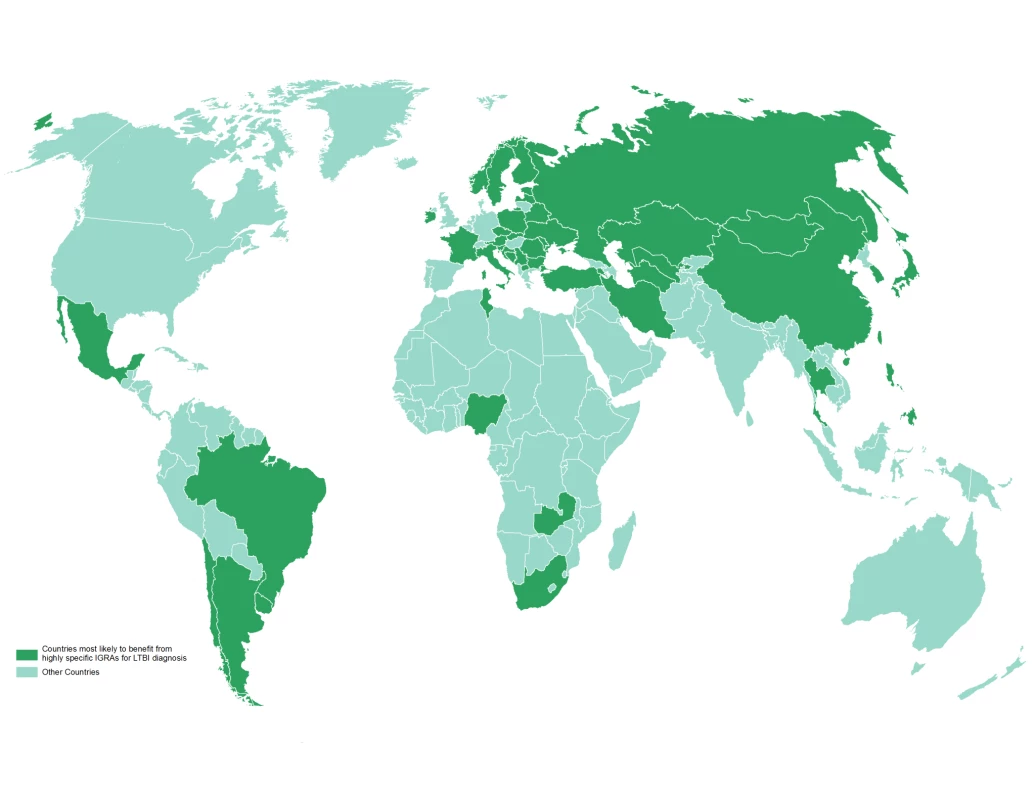
Newly available interferon-gamma release assays (IGRAs) are more specific than TST because they are not affected by previous BCG vaccination [19],[22]. Recent meta-analyses show that the specificity of IGRA is high in all populations, and will be of greatest utility in BCG-vaccinated populations [19],[23]. For example, TST may be less specific for LTBI in Japan [24],[25] (Figure 1) or other countries that revaccinate with BCG or have recently ceased revaccination programs (Table 1); in these settings, IGRAs may be more specific than the TST. Conversely, the TST should not suffer from non-specificity in India, for example, where BCG is given once at birth, as has been borne out by research studies [26]–[28]. One of the applications of this database is its ability to identify populations where repeated BCG immunizations were administered or where BCG was administered after infancy, as these populations are most likely to benefit from the use of highly specific IGRAs for the diagnosis of LTBI (Figure 3 and Box 1). Table 3 lists the 22 countries identified by the WHO as representing 80% of the global TB burden, and their respective BCG policies [29]. The six bolded countries have either recommended booster BCGs in the past or currently doing so, while the remaining 16 countries have not recommended booster BCG vaccinations.
Box 1. Case Study Using the BCG Atlas.
-
A 30-year-old man, born in the Slovak Republic
-
Recently arrived in Canada, as a new immigrant
-
TST-positive (10 mm), and unremarkable chest X-ray
-
No known contact with an active TB case
-
No documentation of BCG vaccination status, but has a vague memory of receiving more than one BCG shot
From the BCG Atlas:
-
Slovak Republic: Universal BCG vaccinations at birth and BCG boosters at ages 7 and 14 until 2001
→ This individual probably received multiple BCG vaccinations post-infancy, which may seriously compromise specificity of the TST; clinician decides to order an IGRA, and based on a negative IGRA and other clinical factors, clinician decides against recommending isoniazid preventative therapy (IPT).
Tab. 3. 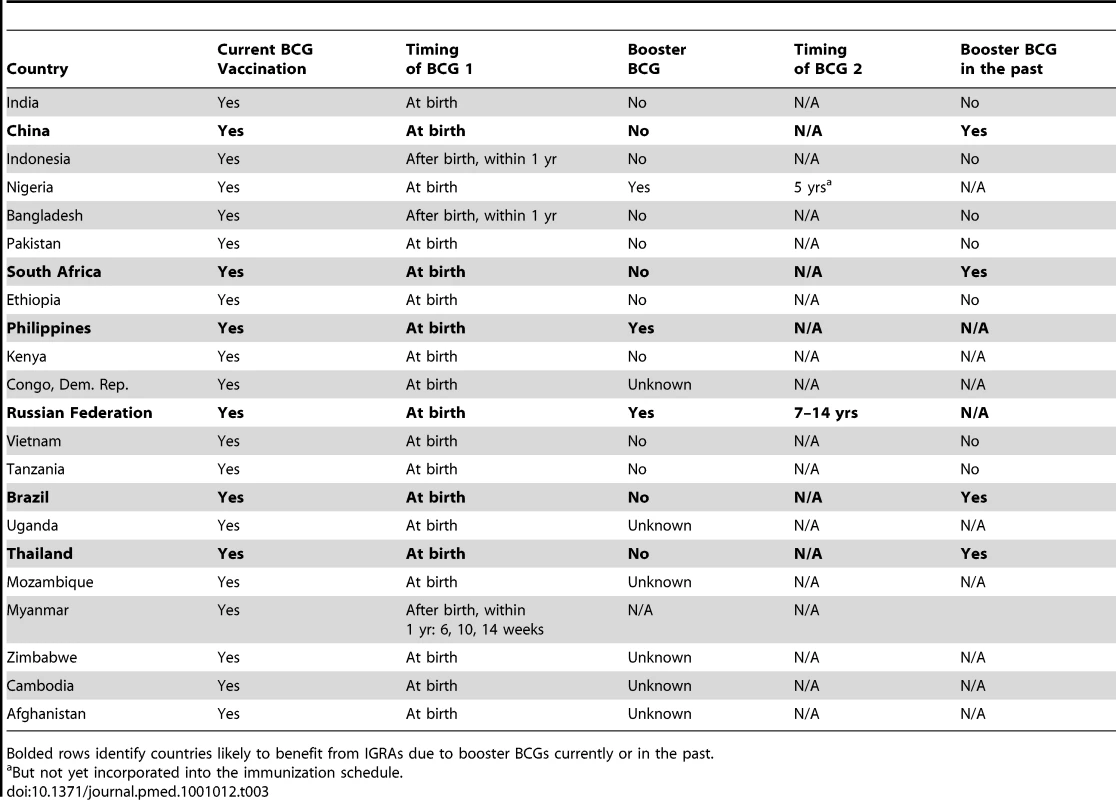
But not yet incorporated into the immunization schedule. Along with novel approved diagnostics such as the IGRAs, there exist other novel diagnostics in research and development stages that use antigens that vary across BCG strains. These include, for example, the MPB64 patch test and serological tests for the diagnosis of TB [30]–[32]. The BCG policies and practices of a particular country may influence the use and utility of these tests in the future.
Implications for Immunization Strategies
Recently there has been renewed interest in developing novel vaccines for TB. According to the Global Plan to Stop TB, 2006–2015, “effective TB vaccines will be an essential component of any strategy to eliminate tuberculosis (TB) by 2050” [33]. In 2009, at least six different vaccine candidates completed Phase I clinical trials, and three are currently in Phase II [18]. Novel vaccine candidates include both live and sub-unit vaccines. Many employ a heterologous “prime-boost” strategy that complements the existing immune response to BCG. Either the existing BCG or a new recombinant BCG is administered first, and then the new vaccine serves as a “booster”. Different vaccines are being developed that could be administered in infants and young children pre-exposure, and others as adjuvants to chemotherapy post-exposure. Given that novel vaccines may work to complement the existing BCG, it may be relevant to know what previous BCG vaccination individuals have had, how many and at what ages prior to administering novel “booster vaccines”. Similarly, we may be concerned that antigens from the primary vaccination with BCG may affect the booster vaccine. Therefore, in countries where revaccination with BCG was practiced, we might expect higher rates of Koch response, or delayed hypersensitivity response.
In 2007, the WHO revised its policy on BCG vaccination of children with HIV, making HIV infection in infants a full contra-indication for BCG vaccination, even in settings highly endemic for TB [34]. In 2008, the IUATLD BCG Working Group published a consensus statement supporting the revised WHO BCG vaccination policy, but recommended that current universal BCG immunization of infants continue in countries highly endemic for TB until countries have programs in place for implementing selective deferral of BCG vaccination in infants exposed to HIV [35]. As countries respond to these new global recommendations, changes in vaccination policies because of the HIV epidemic should be captured in future updates of the Atlas.
Conclusions
Despite nearly a century of use, the BCG vaccine continues to be controversial, and policies and practices vary widely across the world. Many countries have experienced major changes in regards to revaccination over the past 20 years. The BCG World Atlas: A Database of Global BCG Vaccination Policy and Practices is an interactive Web site that attempts to provide the clinician, researcher, and pubic health practitioner alike with resources and information necessary to interpret current and novel TB diagnostics and conduct fruitful research on novel vaccines. Most critically, this is a useful resource for the TB community and is publicly available free of charge through an easy-to-use Web site.
Zdroje
1. World Health Organization [WHO]
2010
WHO global tuberculosis control: WHO report 2010
Geneva
World Health Organization
2. Centers for Disease Control and Prevention [CDC]
2009
Reported tuberculosis in the United States, 2008
Atlanta
CDC
3. CDC
2010
Reported tuberculosis in the United States 2009
4. EllisEDawsonKGallantVScholtenD
2009
Tuberculosis in Canada: 2008 pre-release
Ottawa
Public Health Agency of Canada
5. CalmetteA
1931
Preventive vaccination against tuberculosis with
BCG.
Proc R Soc Med
24
1481
1490
6. GreenwoodM
1928
Professor Calmette's statistical study of B.C.G.
vaccination.
Br Med J
1
793
795
7. ColditzGABrewerTFBerkeyCSWilsonMEBurdickE
1994
Efficacy of BCG vaccine in the prevention of tuberculosis.
Meta-analysis of the published literature.
JAMA
271
698
702
8. BrewerTFWilsonMENardellEA
1995
BCG immunization: review of past experience, current use, and
future prospects.
Curr Clin Top Infect Dis
15
253
270
9. BehrMAWilsonMAGillWPSalamonHSchoolnikGK
1999
Comparative genomics of BCG vaccines by whole-genome DNA
microarray.
Science
284
1520
1523
10. BehrMA
2002
BCG–different strains, different vaccines?
Lancet Infect Dis
2
86
92
11. BrewerTFColditzGA
1995
Relationship between bacille Calmette-Guerin (BCG) strains and
the efficacy of BCG vaccine in the prevention of
tuberculosis.
Clin Infect Dis
20
126
135
12. RitzNCurtisN
2009
Mapping the global use of different BCG vaccine
strains.
Tuberculosis (Edinb)
89
248
251
13. WHO
2007
WHO Statistical Information System (WHOSIS)
Geneva
WHO
14. KochR
1891
Weitere Mitteilungen uber ein Heilmittel gegen
Tuberculose.
Dtsch Med Wschr
17
101
102
15. WallisRPaiMMenziesDDohertyTWalzlG
2010
Biomarkers and diagnostics for tuberculosis: progress, needs, and
translation into practice. Lancet
375
1920
1937
16. EwerKDeeksJAlvarezLBryantGWallerS
2003
Comparison of T-cell-based assay with tuberculin skin test for
diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis
outbreak.
Lancet
361
1168
1173
17. MazurekGHLoBuePADaleyCLBernardoJLardizabalAA
2001
Comparison of a whole-blood interferon gamma assay with
tuberculin skin testing for detecting latent Mycobacterium tuberculosis
infection.
JAMA
286
1740
1747
18. Stop TB Partnership Working Group on New TB Vaccines
2009
Tuberculosis vaccine candidates - 2009
19. PaiMZwerlingAMenziesD
2008
Systematic review: T-cell-based assays for the diagnosis of
latent tuberculosis infection: an update.
Ann Intern Med
149
177
184
20. PaiMMinionJSohnHZwerlingAPerkinsMD
2009
Novel and improved technologies for tuberculosis diagnosis:
progress and challenges.
Clin Chest Med
30
701
716, viii
21. FarhatMGreenawayCPaiMMenziesD
2006
False-positive tuberculin skin tests: what is the absolute effect
of BCG and non-tuberculous mycobacteria?
Int J Tuberc Lung Dis
10
1192
1204
22. MenziesDPaiMComstockG
2007
Meta-analysis: new tests for the diagnosis of latent tuberculosis
infection: areas of uncertainty and recommendations for
research.
Ann Intern Med
146
340
354
23. DielRGolettiDFerraraGBothamleyGCirilloD
2011
Interferon-{gamma} release assays for the diagnosis of latent M.
tuberculosis infection: A systematic review and
meta-analysis.
Eur Respir J
37
88
99
24. HiguchiKHaradaNMoriTSekiyaY
2007
Use of QuantiFERON-TB Gold to investigate tuberculosis contacts
in a high school.
Respirology
12
88
92
25. HaradaNNakajimaYHiguchiKSekiyaYRothelJ
2006
Screening for tuberculosis infection using whole-blood
interferon-gamma and Mantoux testing among Japanese healthcare
workers.
Infect Control Hosp Epidemiol
27
442
448
26. PaiMGokhaleKJoshiRDograSKalantriSP
2005
Mycobacterium tuberculosis infection in health care workers in
rural India: comparison of a whole-blood, interferon-g assay with tuberculin
skin testing.
JAMA
293
2746
2755
27. PaiMJoshiRDograSMendirattaDKNarangP
2006
Serial testing of health care workers for tuberculosis using
interferon-gamma assay.
Am J Respir Crit Care Med
174
349
355
28. PaiMJoshiRDograSZwerlingAAGajalakshmiD
2009
T-cell assay conversions and reversions among household contacts
of tuberculosis patients in rural India.
Int J Tuberc Lung Dis
13
84
92
29. WHO
2009
WHO global TB report
Geneva
World Health Organization
30. NakamuraRMVelmonteMAKawajiriKAngCFFriasRA
1998
MPB64 mycobacterial antigen: a new skin-test reagent through
patch method for rapid diagnosis of active tuberculosis.
Int J Tuberc Lung Dis
2
541
546
31. SteingartKRHenryMLaalSHopewellPCRamsayA
2007
A systematic review of commercial serological antibody detection
tests for the diagnosis of extrapulmonary tuberculosis.
Thorax
62
911
918
32. SteingartKRHenryMLaalSHopewellPCRamsayA
2007
Commercial serological antibody detection tests for the diagnosis
of pulmonary tuberculosis: a systematic review.
PLoS Med
4
e202
doi:10.1371/journal.pmed.0040202
33. Stop TB Partnership
2006
The Global Plan to Stop TB, 2006–2015. Actions for life:
towards a world free of tuberculosis.
Int J Tuberc Lung Dis
10
240
241
34. WHO
2007
Revised BCG vaccination guidelines for infants at risk for HIV
infection.
Wkly Epidemiol Rec
82
193
35. International Union Against Tuberculosis and Lung Disease
[IUATLD]
2008
Consensus IUATLD statement on the revised world health organization
recommendations regarding BCG vaccination in HIV-infected infants
Paris
IUATLD
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Léčba bolesti u seniorů
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Strengthening the Reporting of Genetic Risk Prediction Studies: The GRIPS Statement
- Towards Open and Equitable Access to Research and Knowledge for Development
- HIV-1 Drug Resistance Emergence among Breastfeeding Infants Born to HIV-Infected Mothers during a Single-Arm Trial of Triple-Antiretroviral Prophylaxis for Prevention of Mother-To-Child Transmission: A Secondary Analysis
- The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices
- Promotional Tone in Reviews of Menopausal Hormone Therapy After the Women's Health Initiative: An Analysis of Published Articles
- How Can Institutional Review Boards Best Interpret Preclinical Data?
- Equity Must Accompany Economic Growth for Good Health
- Predicting Harms and Benefits in Translational Trials: Ethics, Evidence, and Uncertainty
- Effectiveness of the Standard WHO Recommended Retreatment Regimen (Category II) for Tuberculosis in Kampala, Uganda: A Prospective Cohort Study
- Scaling Up Diarrhea Prevention and Treatment Interventions: A Lives Saved Tool Analysis
- Triple-Antiretroviral Prophylaxis to Prevent Mother-To-Child HIV Transmission through Breastfeeding—The Kisumu Breastfeeding Study, Kenya: A Clinical Trial
- Is Economic Growth Associated with Reduction in Child Undernutrition in India?
- Mutations in Complement Regulatory Proteins Predispose to Preeclampsia: A Genetic Analysis of the PROMISSE Cohort
- A Randomized Controlled Trial Comparing the Effects of Counseling and Alarm Device on HAART Adherence and Virologic Outcomes
- Effectiveness and Cost Effectiveness of Expanding Harm Reduction and Antiretroviral Therapy in a Mixed HIV Epidemic: A Modeling Analysis for Ukraine
- On the Path to Global Open Access: A Few More Miles to Go
- The Challenge of Discharging Research Ethics Duties in Resource-Constrained Settings
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices
- How Can Institutional Review Boards Best Interpret Preclinical Data?
- The Challenge of Discharging Research Ethics Duties in Resource-Constrained Settings
- HIV-1 Drug Resistance Emergence among Breastfeeding Infants Born to HIV-Infected Mothers during a Single-Arm Trial of Triple-Antiretroviral Prophylaxis for Prevention of Mother-To-Child Transmission: A Secondary Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání