-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Persisting Burden of Intracerebral Haemorrhage: Can Effective Treatments Be Found?
article has not abstract
Published in the journal: . PLoS Med 7(10): e32767. doi:10.1371/journal.pmed.1000353
Category: Research in Translation
doi: https://doi.org/10.1371/journal.pmed.1000353Summary
article has not abstract
Summary
Intracerebral haemorrhage (ICH) accounts for ∼10% and ∼20% of strokes in high and low-middle income countries, respectively, but ICH incidence and case fatality do not appear to be declining. Evidence supports organised stroke unit care and secondary prevention with blood pressure lowering after ICH. Ongoing randomised controlled trials of treatments that are either intended to limit early ICH growth, reduce perihaematomal oedema, or modify other key pathophysiological mechanisms underlying deterioration after acute ICH, offer hope for future improvements in outcome.
Introduction
Spontaneous intracerebral haemorrhage (ICH) that is apparently unrelated to trauma or an underlying vascular, neoplastic, or coagulopathic cause has incurred the same global burden over the past quarter of a century [1],[2]. During the last decade, spontaneous ICH accounted for ∼10% of strokes in high income countries and ∼20% of strokes in low and middle income countries, where the one month case fatalities were 25%–35% and 30%–48%, respectively [3].
The incidence of ICH is higher in Asians [2], and the major risk factors for spontaneous ICH without an identified cause (so-called primary ICH) are male gender, systemic arterial hypertension, excessive alcohol consumption, increasing age, smoking, and diabetes mellitus [4]. However, over the past quarter of a century, the incidence of primary ICH associated with pre-stroke hypertension seems to have declined, whereas there seems to have been an increase associated with antithrombotic use and presumed cerebral amyloid angiopathy in those aged ≥75 years [1]. Whilst primary prevention with antihypertensive medication is probably the most effective strategy to reduce the burden of ICH, could the management of ICH influence outcome?
The outcome after primary ICH seems to be worse than after a bleed secondary to an arteriovenous malformation [5], which justifies thorough investigation for all patients (Table 1). However, there is a shortage of evidence and lack of consensus about who, when, and how to further investigate for a cause underlying ICH [6]. There appears to be a modest association between ICH deep in the brain and hypertension, and between ICH in the lobes of the brain and cerebral amyloid angiopathy [7],[8], but these associations by no means rule out the need for further investigation of patients who are likely to survive and benefit from the identification of a treatable underlying cause (Table 1) [9].
Tab. 1. Investigations into Common Causes of Secondary Intracerebral Haemorrhage (ICH). 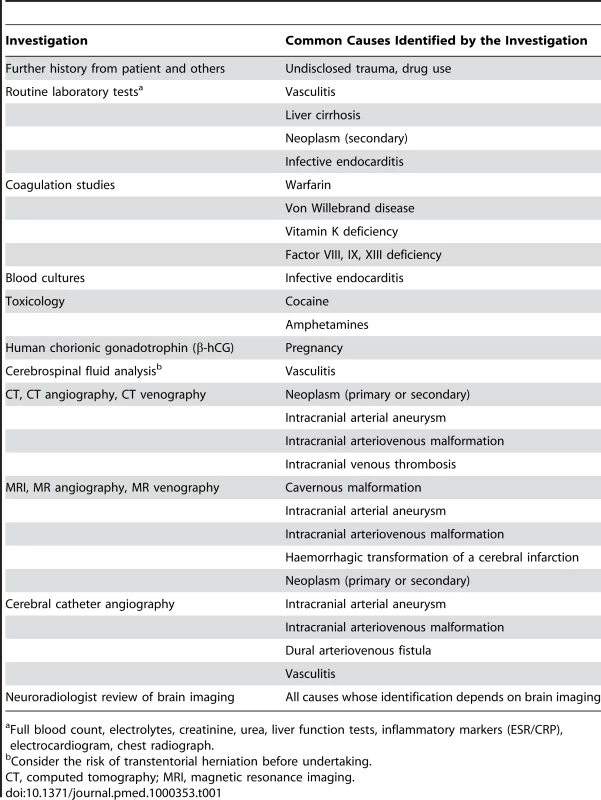
Full blood count, electrolytes, creatinine, urea, liver function tests, inflammatory markers (ESR/CRP), electrocardiogram, chest radiograph. Apart from identifying and treating underlying causes of ICH, this review focuses on other strategies to improve outcome, bearing in mind the pathophysiological mechanisms underlying clinical deterioration after ICH. We go on to address the treatments for primary ICH that are supported by randomised controlled trials (RCTs) and those that are not, and discuss which interventions appear to be the most promising in ongoing and future RCTs.
Pathophysiology of Acute ICH
In humans, known pathophysiological mechanisms underlying further clinical deterioration soon after ICH include hydrocephalus, intraventricular extension of ICH, and recurrent ICH [10]; pathological and radiological studies have illuminated additional mechanisms (Figure 1). Human studies performing brain computed tomography within two time windows after ICH onset have documented haematoma expansion (Figure 2)—either due to growth of the original haemorrhage or re-bleeding [11]–[21]—that is associated with poor outcome [12],[16],[18],[22]. Imaging studies have demonstrated peri-haematomal hypoperfusion within the first week of ICH onset [23],[24], but not an “ischaemic penumbra” [25],[26]. However, there is evidence of a compensatory reduction in the metabolic rate, or a “metabolic penumbra”, around ICH [25],, as well as peri-haematomal hyperglycolysis (possibly due to inflammation, excitotoxicity, spreading depression, or seizures) [27]. Perihaematomal oedema appears to be vasogenic (plasma-derived) [28], its volume may increase within 24 hours of ICH onset and peak within 14 days [29]–[31], and it may be caused or exacerbated by thrombin and activated platelets [32],[33].
Fig. 1. Selected pathophysiological mechanisms that have been identified in humans after acute, spontaneous intracerebral haemorrhage. 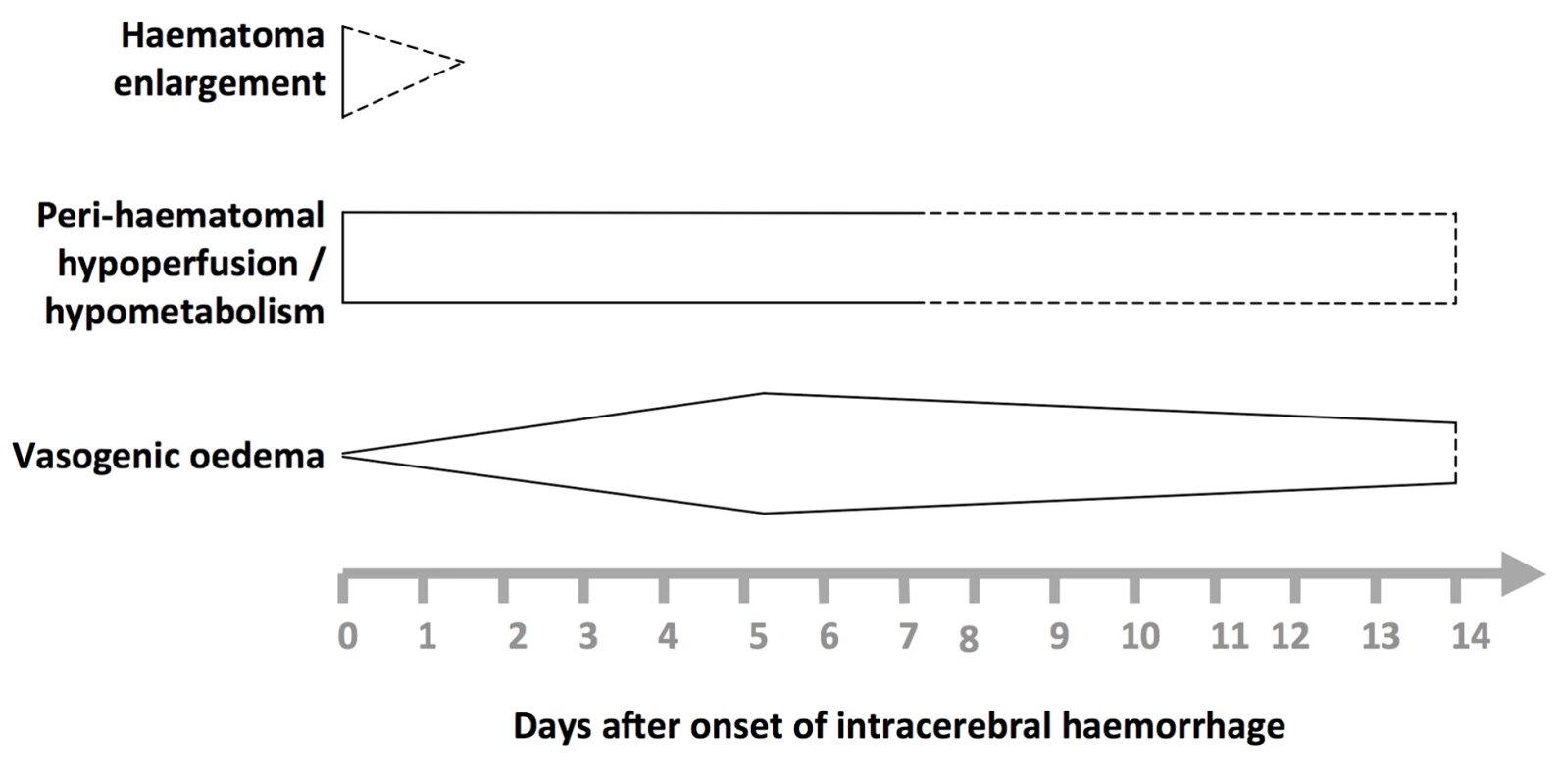
Shapes are approximate illustrations of when pathophysiological mechanisms are at their peak and their known durations. Uncertainties about the duration and intensity of mechanisms are indicated by dashed lines. Fig. 2. Summary of selected radiological studies of spontaneous intracerebral haemorrhage growth. 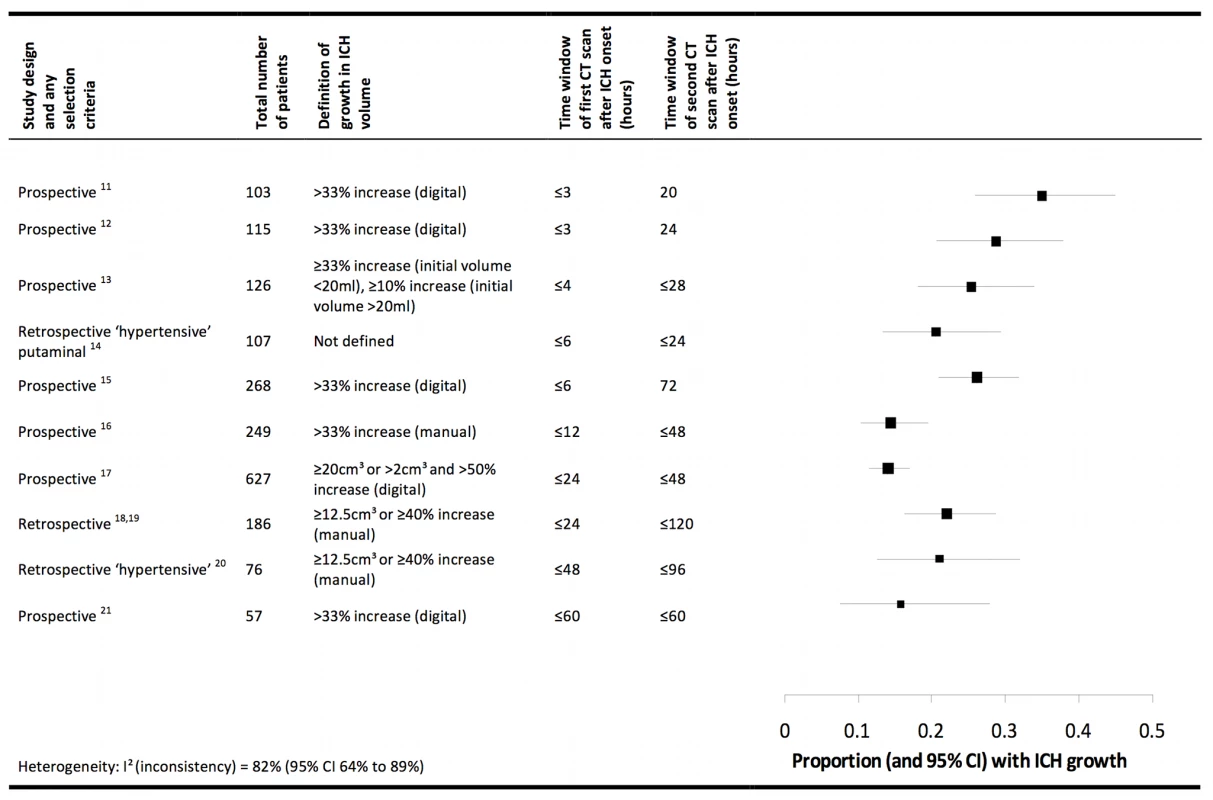
Studies are organised in ascending order of the duration of the time window of the first computed tomogram. Manual calculations of haematoma volume used the ABC/2 method. We excluded data on patients taking anticoagulant drugs in these studies, and excluded studies from which data could not be extracted [77],[78], studies that incorporated patients already included in the summary above [79],[80], or studies in which interventions may have influenced haematoma growth [58],[81]. Animal models support the contributions to peri-haematomal oedema made by clot retraction, hydrostatic pressure, enhanced thrombin production, increased blood–brain barrier permeability, and products of erythrocyte lysis (such as haeme oxygenase-mediated liberation of iron from haeme rings) [32],[34]–[37]. ICH in animal models seems to trigger humoral and cellular inflammatory responses: the consequent migration and recruitment of neutrophils and activation of native microglia results in oxidative stress and neuronal necrosis [38],[39], cytokines such as tumour necrosis factor α and interleukin 1-β lead to apoptosis [40],[41], complement activation causes erythrocyte lysis [42], and matrix metalloproteinases may result in oedema, necrosis, and blood–brain barrier disruption [43].
A better understanding of the pathophysiology of ICH could emerge if the decline in human autopsy rates reverses [44], and if animal models of ICH better represent human ICH [45]. Rodent models of ICH involve either stereotactic intraparenchymal infusion of autologous whole blood (which may cause simultaneous intraventricular or subarachnoid haemorrhage [46]), or injection of proteolytic bacterial collagenase (which incites a vigorous immune response in excess of that seen in humans [46]), after which very few animals die, which is quite unlike spontaneous ICH in humans [2].
Treatments Shown to Be Beneficial in Humans, Either in a Meta-Analysis of RCTs or in a Single Large RCT
A Cochrane meta-analysis of 31 RCTs involving 6,936 participants showed that organised inpatient care in stroke units benefits patients with stroke (whether ischaemic or due to ICH) by reducing the odds of death or dependency by 18% [47]. Large observational studies corroborate these findings in patients with ICH [48],[49]. Which aspects of organized stroke care, either individually or together, improve outcome in patients with ICH remain to be determined. One small, non-randomised, observational analysis, which was adjusted for some of the known influences on ICH prognosis, found survival after ICH to be better when managed in neuro-intensive care units compared to general intensive care units [50]: RCTs of some of the interventions used in the “black box” of neuro-intensive care (such as acute blood pressure lowering) are underway (see below). Furthermore, the benefits of standard care can be inferred from the effects on clinical outcome of do-not-resuscitate orders in a case-mix adjusted multi-hospital observational study [51], and withdrawal of care in a multivariable analysis at a single hospital [52].
A Cochrane meta-analysis of ten RCTs involving 2,059 participants found a reduction in death or dependence from the neurosurgical evacuation of spontaneous supratentorial ICH (odds ratio [OR] 0.71, 95% confidence interval [CI] 0.58 to 0.88) [53]. However, most of the RCTs included in this meta-analysis were of modest quality, their methods differed, and the largest RCT (Surgical Trial in Intracerebral Hemorrhage [STICH]) found no difference between early surgery or initial conservative management [54]. A sub-group with lobar ICH within 1 cm of the cortical surface appeared to benefit from surgery in STICH, so the STICH II RCT (ISRCTN 22153967) is evaluating early ICH evacuation in this sub-group of patients.
Secondary prevention with anti-hypertensive drugs reduced the risk of vascular events after stroke in the PROGRESS RCT; amongst the subset of 611 participants with ICH, the risk of subsequent stroke was halved by a perindopril-based blood pressure lowering regimen [55].
Treatments Neither Shown to be Beneficial to Humans in a Meta-Analysis of RCTs, Nor in a Single Large RCT
Haemostatic drugs are a biologically plausible intervention to improve outcome after ICH by limiting the early growth of spontaneous ICH (Figure 2). Despite the ability of recombinant activated factor VII (rFVIIa) to curtail early haematoma growth by 4–6 ml, a Cochrane meta-analysis of four RCTs involving 1,305 participants found that this surrogate outcome did not translate into any net clinical benefit (risk ratio of death or dependence [modified Rankin Scale score 4 to 6] at 90 days = 0.91 [95% CI 0.72 to 1.15]). This reduction in ICH growth may have been too small to improve clinical outcome, its benefit may have been offset by the thrombo-embolic adverse effects of rFVIIa, or the RCTs might have been unable to detect a small benefit of rFVIIa because of insufficient precision or some methodological weaknesses [56]. Other haemostatic drugs including antifibrinolytic agents seem to be worth testing in future RCTs.
Similarly, early blood pressure lowering might improve outcome after ICH by limiting the early growth of spontaneous ICH, but there has been a shortage of evidence supporting this intervention, unsurprisingly leading to differences between ICH guidelines [9],[57]. The Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT) randomized 404 patients presenting within 6 hours of onset to a systolic blood pressure target of ≤140 mmHg achieved within 1 hour and continued for 7 days, versus the American Heart Association guideline's target [9],[58]. There was a non-significant reduction in ICH growth by 1–2 ml, and no effect on clinical outcome, but the safety data are encouraging for the large, ongoing Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT-2; ISRCTN 73916115).
Attenuating peri-haematomal oedema might improve outcome after ICH (Figure 1), but meta-analyses have demonstrated neither benefit nor harm from dexamethasone (five RCTs involving 206 participants) [59], glycerol (two RCTs involving 224 participants) [60], and mannitol (two RCTs involving 149 participants) [61]. Neuroprotection, too, has caused neither benefit nor harm after acute ICH with the anti-oxidant free-radical scavenger NXY-059 (one RCT involving 607 participants) [62] or the glycine antagonist gavestinel (one RCT involving 571 participants) [63].
Future Directions
Attenuation of Haematoma Growth
The major treatment target of ongoing RCTs is haematoma growth, because ICH size and growth are determinants of outcome [12],. So far, the attenuation of early ICH growth seen with the haemostatic agent rFVIIa [56] or intensive acute blood pressure lowering [58],[64] has not improved clinical outcome in the RCTs performed. However, this biologically plausible mechanism for improving outcome is worthy of further research in RCTs that are large enough to detect small clinical benefits, such as the ongoing RCTs of acute blood pressure lowering (including INTERACT-2, the Efficacy of Nitric Oxide in Stroke Trial [ENOS; ISRCTN 99414122], Antihypertensive Treatment of Acute Cerebral Hemorrhage II [ATACH-II; ISRCTN R01-NS044976], and the Scandinavian Candesartan Acute Stroke Trial [SCAST; ISRCTN 13643354]). RCTs of alternative approaches to improve outcome after primary ICH by limiting haematoma expansion include rFVIIa in sub-groups of patients whose haematomas are more likely to grow (The Spot Sign for Predicting and Treating ICH Growth Study [STOP-IT; NCT00810888]), or testing the effectiveness of antifibrinolytic drugs such as the lysine analogue tranexamic acid, which seems to have attenuated ICH growth in two non-randomised studies [65],[66].
The greater risk of haematoma expansion and death after ICH associated with warfarin [67] and the contemporary increase in the incidence of primary ICH associated with all antithrombotic drugs [1] make RCTs of the management of antithrombotic-associated ICH a priority. Whilst stopping warfarin after ICH is common sense, and intravenous vitamin K administration is standard practice, there is a shortage of evidence about how else to treat anticoagulant-associated ICH [68], so RCTs comparing prothrombin complex concentrate with fresh frozen plasma in this context are ongoing (International Normalized Ratio (INR) Normalization in Coumadin Associated Intracerebral Haemorrhage [INCH; NCT00928915] and Efficacy and Safety of BERIPLEX P/N Compared with Plasma in Patients with Acute Major Bleeding Caused by Anticoagulant Therapy [NCT00708435]), as are studies of rFVIIa. The finding that mortality is higher for patients who were on antiplatelet agents at the time of ICH compared to those who were not [69] has led to an ongoing RCT of platelet transfusion to limit ICH growth and improve outcome after ICH associated with antiplatelet drugs (Platelet Transfusion in Cerebral Haemorrhage [PATCH; http://www.strokecenter.org/trials/TrialDetail.aspx?tid=730).
Other Approaches
Firstly, targeting other potentially treatable determinants of poor outcome after ICH may be fruitful. Intraventricular extension of ICH is one such mechanism [10], and there are two RCTs of ventricular drainage combined with intraventricular recombinant tissue plasminogen activator (Dutch Intraventricular Thrombolysis after Cerebral Haemorrhage Study [DITCH; ISRCTN 19105863] and Clot Lysis: Evaluation Acceleration of Resolution of IVH [CLEAR-IVH; NCT00650858]).
Secondly, just as the PATCH RCT is a response to the apparent rise in incidence of antiplatelet-associated ICH, the apparent rise in the incidence of lobar ICH that may be caused by cerebral amyloid angiopathy merits consideration of treatments that might reduce amyloid deposition [1]. Tramiprosate is a synthetic compound that competes with glycosaminoglycans for binding to β-amyloid peptide, reducing amyloid fibril formation and deposition, and demonstrated a good safety profile in a phase II study [70]. Amyloid-depleting agents, which have shown remarkable effects in Alzheimer's disease [71], are an alternative approach and may be preferable to amyloid-β immunisation, which can induce an immune-mediated encephalomyelitis [72].
Lastly, treatments that have proven beneficial in animal models might translate from the bench to the bedside, although there are concerns about the rodent ICH models used and the methodological quality of animal experiments [45],[46],[73]. One such example is deferoxamine (an iron-chelating agent that crosses the blood–brain barrier, and has been associated with a reduction in brain oedema, neurological deficits, and biochemical markers of oxidative damage in animals) [74],[75], which has led to the Dose Finding and Safety study of Deferoxamine in Patients with Brain Hemorrhage (DFO in ICH; NCT00598572).
Conclusions
The incidence and risk of dying from ICH seem not to have changed in recent decades, whilst the incidence of ischaemic stroke has declined [2],[3]. In contrast to the advances in the treatment of ischaemic stroke, stroke unit care and secondary prevention with blood pressure reduction are the only interventions for patients with stroke due to ICH that are based on robust evidence [47],[55]. However, insights gleaned from radiological and pathological investigations of the cause and pathophysiology of ICH, and the relentless pursuit of potential treatments in ongoing RCTs, are all cause for optimism [76].
Five Key Papers in the Field
-
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, et al. (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9(2): 167–176.
-
Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, et al. (2006) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66(8): 1175–1181.
-
Stroke Unit Trialists' Collaboration (2007) Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev (4): CD000197.
-
Chapman N, Huxley R, Anderson C, Bousser MG, Chalmers J, et al. (2004) Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke 35(1): 116–121.
-
NINDS ICH Workshop Participants (2005) Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke 36(3): e23–e41.
Zdroje
1. LovelockCE
MolyneuxAJ
RothwellPM
2007 Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol 6 487 493
2. van AschCJ
LuitseMJ
RinkelGJ
van der TweelI
AlgraA
2010 Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9 167 176
3. FeiginVL
LawesCM
BennettDA
Barker-ColloSL
ParagV
2009 Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8 355 369
4. AriesenMJ
ClausSP
RinkelGJ
AlgraA
2003 Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 34 2060 2065
5. van BeijnumJ
LovelockCE
CordonnierC
RothwellPM
KlijnCJ
2009 Outcome after spontaneous and arteriovenous malformation-related intracerebral haemorrhage: population-based studies. Brain 132 537 543
6. CordonnierC
KlijnCJ
van BeijnumJ
Al-Shahi SalmanR
2010 Radiological investigation of spontaneous intracerebral hemorrhage: systematic review and trinational survey. Stroke 41 685 690
7. RitterMA
DrosteDW
HegedusK
SzepesiR
NabaviDG
2005 Role of cerebral amyloid angiopathy in intracerebral hemorrhage in hypertensive patients. Neurology 64 1233 1237
8. JacksonCA
SudlowCL
2006 Is hypertension a more frequent risk factor for deep than for lobar supratentorial intracerebral haemorrhage? J Neurol Neurosurg Psychiatry 77 1244 1252
9. BroderickJ
ConnollyS
FeldmannE
HanleyD
KaseC
2007 Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 38 2001 2023
10. QureshiAI
MendelowAD
HanleyDF
2009 Intracerebral haemorrhage. Lancet 373 1632 1644
11. BrottT
BroderickJ
KothariR
BarsanW
TomsickT
1997 Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 28 1 5
12. DavisSM
BroderickJ
HennericiM
BrunNC
DiringerMN
2006 Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66 1175 1181
13. JiN
LuJJ
ZhaoYL
WangS
ZhaoJZ
2009 Imaging and clinical prognostic indicators for early hematoma enlargement after spontaneous intracerebral hemorrhage. Neurol Res 31 362 366
14. FujitsuK
MuramotoM
IkedaY
InadaY
KimI
1990 Indications for surgical treatment of putaminal hemorrhage. Comparative study based on serial CT and time-course analysis. J Neurosurg 73 518 525
15. SansingLH
MesseSR
CucchiaraBL
CohenSN
LydenPD
2009 Prior antiplatelet use does not affect hemorrhage growth or outcome after ICH. Neurology 72 1397 1402
16. LeiraR
DavalosA
SilvaY
Gil-PeraltaA
TejadaJ
2004 Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 63 461 467
17. FujiiY
TakeuchiS
SasakiO
MinakawaT
TanakaR
1998 Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke 29 1160 1166
18. KazuiS
NaritomiH
YamamotoH
SawadaT
YamaguchiT
1996 Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 27 1783 1787
19. KazuiS
MinematsuK
YamamotoH
SawadaT
YamaguchiT
1997 Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke 28 2370 2375
20. OhwakiK
YanoE
NagashimaH
HirataM
NakagomiT
2004 Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke 35 1364 1367
21. FlibotteJJ
HaganN
O'DonnellJ
GreenbergSM
RosandJ
2004 Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 63 1059 1064
22. HemphillJCIII
BonovichDC
BesmertisL
ManleyGT
JohnstonSC
2001 The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32 891 897
23. RosandJ
EskeyC
ChangY
GonzalezRG
GreenbergSM
2002 Dynamic single-section CT demonstrates reduced cerebral blood flow in acute intracerebral hemorrhage. Cerebrovasc Dis 14 214 220
24. PascualAM
Lopez-MutJV
BenllochV
ChamarroR
SolerJ
2007 Perfusion-weighted magnetic resonance imaging in acute intracerebral hemorrhage at baseline and during the 1st and 2nd week: a longitudinal study. Cerebrovasc Dis 23 6 13
25. ZazuliaAR
DiringerMN
VideenTO
AdamsRE
YundtK
2001 Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab 21 804 810
26. HerwehC
JuttlerE
SchellingerPD
KlotzE
JenetzkyE
2007 Evidence against a perihemorrhagic penumbra provided by perfusion computed tomography. Stroke 38 2941 2947
27. ZazuliaAR
VideenTO
PowersWJ
2009 Transient focal increase in perihematomal glucose metabolism after acute human intracerebral hemorrhage. Stroke 40 1638 1643
28. ButcherKS
BairdT
MacGregorL
DesmondP
TressB
2004 Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke 35 1879 1885
29. ZazuliaAR
DiringerMN
DerdeynCP
PowersWJ
1999 Progression of mass effect after intracerebral hemorrhage. Stroke 30 1167 1173
30. GebelJMJr
JauchEC
BrottTG
KhouryJ
SauerbeckL
2002 Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke 33 2631 2635
31. InajiM
TomitaH
ToneO
TamakiM
SuzukiR
2003 Chronological changes of perihematomal edema of human intracerebral hematoma. Acta Neurochir Suppl 86 445 8
32. GebelJM
BrottTG
SilaCA
TomsickTA
JauchE
2000 Decreased perihematomal edema in thrombolysis-related intracerebral hemorrhage compared with spontaneous intracerebral hemorrhage. Stroke 31 596 600
33. SansingLH
KaznatcheevaEA
PerkinsCJ
KomaroffE
GutmanFB
2003 Edema after intracerebral hemorrhage: correlations with coagulation parameters and treatment. J Neurosurg 98 985 992
34. WagnerKR
XiG
HuaY
KleinholzM
de Court
1996 Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke 27 490 497
35. LeeKR
KawaiN
KimS
SagherO
HoffJT
1997 Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg 86 272 278
36. HuangFP
XiG
KeepRF
HuaY
NemoianuA
2002 Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg 96 287 293
37. LevineJM
SniderR
FinkelsteinD
GurolME
ChanderrajR
2007 Early edema in warfarin-related intracerebral hemorrhage. Neurocrit Care 7 58 63
38. WeissSJ
1989 Tissue destruction by neutrophils. N Engl J Med 320 365 376
39. PowerC
HenryS
Del BigioMR
LarsenPH
CorbettD
2003 Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol 53 731 742
40. MayneM
NiW
YanHJ
XueM
JohnstonJB
2001 Antisense oligodeoxynucleotide inhibition of tumor necrosis factor-alpha expression is neuroprotective after intracerebral hemorrhage. Stroke 32 240 248
41. HolminS
MathiesenT
2000 Intracerebral administration of interleukin-1beta and induction of inflammation, apoptosis, and vasogenic edema. J Neurosurg 92 108 120
42. XiG
HuaY
KeepRF
YoungerJG
HoffJT
2001 Systemic complement depletion diminishes perihematomal brain edema in rats. Stroke 32 162 167
43. WangJ
TsirkaSE
2005 Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain 128 1622 1633
44. AyoubT
ChowJ
2008 The conventional autopsy in modern medicine. J R Soc Med 101 177 181
45. JamesML
WarnerDS
LaskowitzDT
2008 Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care 9 139 152
46. AndaluzN
ZuccarelloM
WagnerKR
2002 Experimental animal models of intracerebral hemorrhage. Neurosurg Clin N Am 13 385 393
47. Stroke Unit Trialists' Collaboration 2007 Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev CD000197
48. CandeliseL
GattinoniM
BersanoA
MicieliG
SterziR
2007 Stroke-unit care for acute stroke patients: an observational follow-up study. Lancet 369 299 305
49. TerentA
AsplundK
FarahmandB
HenrikssonKM
NorrvingB
2009 Stroke unit care revisited: who benefits the most? A cohort study of 105,043 patients in Riks-Stroke, the Swedish Stroke Register. J Neurol Neurosurg Psychiatry 80 881 887
50. DiringerMN
EdwardsDF
2001 Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med 29 635 640
51. HemphillJCIII
NewmanJ
ZhaoS
JohnstonSC
2004 Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke 35 1130 1134
52. BeckerKJ
BaxterAB
CohenWA
BybeeHM
TirschwellDL
2001 Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology 56 766 772
53. PrasadK
MendelowAD
GregsonB
2008 Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database Syst Rev CD000200
54. MendelowAD
GregsonBA
FernandesHM
MurrayGD
TeasdaleGM
2005 Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 365 387 397
55. ChapmanN
HuxleyR
AndersonC
BousserMG
ChalmersJ
2004 Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke 35 116 121
56. Al-Shahi SalmanR
2009 Haemostatic drug therapies for acute spontaneous intracerebral haemorrhage. Cochrane Database Syst Rev CD005951
57. SteinerT
KasteM
ForstingM
MendelowD
KwiecinskiH
2006 Recommendations for the management of intracranial haemorrhage - part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 22 294 316
58. AndersonCS
HuangY
WangJG
ArimaH
NealB
2008 Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 7 391 399
59. FeiginVL
AndersonN
RinkelGJ
AlgraA
vanGJ
2005 Corticosteroids for aneurysmal subarachnoid haemorrhage and primary intracerebral haemorrhage. Cochrane Database Syst Rev CD004583
60. RighettiE
CelaniMG
CantisaniT
SterziR
BoysenG
2004 Glycerol for acute stroke. Cochrane Database Syst Rev CD000096
61. BereczkiD
FeketeI
PradoGF
LiuM
2007 Mannitol for acute stroke. Cochrane Database Syst Rev CD001153
62. LydenPD
ShuaibA
LeesKR
DavalosA
DavisSM
2007 Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke 38 2262 2269
63. HaleyECJr
ThompsonJL
LevinB
DavisS
LeesKR
2005 Gavestinel does not improve outcome after acute intracerebral hemorrhage: an analysis from the GAIN International and GAIN Americas studies. Stroke 36 1006 1010
64. GeeganageC
BathPM
2008 Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev CD000039
65. SorimachiT
FujiiY
MoritaK
TanakaR
2005 Rapid administration of antifibrinolytics and strict blood pressure control for intracerebral hemorrhage. Neurosurgery 57 837 844
66. OjacastroMF
TabuenaMP
DulosID
TabuenaRP
2008 Efficacy of tranexamic acid in reducing hematoma volume in patients with hypertensive intracerebral hemorrhage. Int J Stroke 3 197 198 (abstract)
67. CucchiaraB
MesseS
SansingL
KasnerS
LydenP
2008 Hematoma growth in oral anticoagulant related intracerebral hemorrhage. Stroke 39 2993 2996
68. AguilarMI
HartRG
KaseCS
FreemanWD
HoebenBJ
2007 Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 82 82 92
69. ThompsonBB
BejotY
CasoV
CastilloJ
ChristensenH
2010 Prior antiplatelet therapy and outcome following intracerebral hemorrhage. A systematic review. Neurology Epub ahead of print. doi:WNL.0b013e3181f735e5v1
70. GreenbergSM
RosandJ
SchneiderAT
CreedPL
GandySE
2006 A phase 2 study of tramiprosate for cerebral amyloid angiopathy. Alzheimer Dis Assoc Disord 20 269 274
71. KolstoeSE
RidhaBH
BellottiV
WangN
RobinsonCV
2009 Molecular dissection of Alzheimer's disease neuropathology by depletion of serum amyloid P component. Proc Natl Acad Sci U S A 106 7619 7623
72. OrgogozoJM
GilmanS
DartiguesJF
LaurentB
PuelM
2003 Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 61 46 54
73. FrantziasJ
SenaES
MacleodMR
Al-Shahi SalmanR
2010 Treatment of intracerebral hemorrhage in animal models: Meta-analysis Ann Neurol In press
74. NakamuraT
KeepRF
HuaY
SchallertT
HoffJT
2004 Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg 100 672 678
75. GuY
HuaY
KeepRF
MorgensternLB
XiG
2009 Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke 40 2241 2243
76. NINDS ICH Workshop Participants 2005 Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke 36 e23 e41
77. HeroldS
vonKR
JaegerC
1982 Follow-up of spontaneous intracerebral haemorrhage by computed tomography. J Neurol 228 267 276
78. KelleyRE
BergerJR
ScheinbergP
StokesN
1982 Active bleeding in hypertensive intracerebral hemorrhage: computed tomography. Neurology 32 852 856
79. SilvaY
LeiraR
TejadaJ
LainezJM
CastilloJ
2005 Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke 36 86 91
80. JauchEC
LindsellCJ
AdeoyeO
KhouryJ
BarsanW
2006 Lack of evidence for an association between hemodynamic variables and hematoma growth in spontaneous intracerebral hemorrhage. Stroke 37 2061 2065
81. QureshiAI
PaleschYY
MartinR
NovitzkeJ
Cruz-FloresS
2010 Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol 67 570 576
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 10- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children Under Five: The Pneumococcal Global Serotype Project
- The Persisting Burden of Intracerebral Haemorrhage: Can Effective Treatments Be Found?
- Conflicts of Interest at Medical Journals: The Influence of Industry-Supported Randomised Trials on Journal Impact Factors and Revenue – Cohort Study
- An Urgent Need to Restrict Access to Pesticides Based on Human Lethality
- A Field Training Guide for Human Subjects Research Ethics
- Oral Ondansetron Administration in Emergency Departments to Children with Gastroenteritis: An Economic Analysis
- Being More Realistic about the Public Health Impact of Genomic Medicine
- Epigenetic Epidemiology of Common Complex Disease: Prospects for Prediction, Prevention, and Treatment
- Acute Human Lethal Toxicity of Agricultural Pesticides: A Prospective Cohort Study
- Information from Pharmaceutical Companies and the Quality, Quantity, and Cost of Physicians' Prescribing: A Systematic Review
- Increased Responsibility and Transparency in an Era of Increased Visibility
- Editors, Publishers, Impact Factors, and Reprint Income
- Reflections on Pandemic (H1N1) 2009 and the International Response
- Prenatal Treatment for Serious Neurological Sequelae of Congenital Toxoplasmosis: An Observational Prospective Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Epigenetic Epidemiology of Common Complex Disease: Prospects for Prediction, Prevention, and Treatment
- Editors, Publishers, Impact Factors, and Reprint Income
- Oral Ondansetron Administration in Emergency Departments to Children with Gastroenteritis: An Economic Analysis
- Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children Under Five: The Pneumococcal Global Serotype Project
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



