-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaWhy Are tRNAs Overproduced in the Absence of Maf1, a Negative Regulator of RNAP III, Not Fully Functional?
article has not abstract
Published in the journal: . PLoS Genet 11(12): e32767. doi:10.1371/journal.pgen.1005743
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1005743Summary
article has not abstract
tRNA biosynthesis in the eukaryotic cell is a multistep pathway, involving transcription, 5' and 3' end maturation, intron removal, and numerous modifications of nucleotides. Most of the genes coding for each of the elements essential for tRNA biosynthetic activities were primarily identified by genetic selection in yeast [1]. In these studies, the parental strain contained a tRNA gene that had been converted to a nonsense suppressor. Given the appropriate genetic background, phenotypic loss of suppression was used to select mutants producing non-functional tRNA. Among other proteins controlling tRNA biosynthesis, this approach led to identification of Maf1, a global repressor of tRNA transcription that is activated in response to stress. The maf1-1 mutant was originally selected in a genetic screen for decreased efficiency of tRNA suppressor SUP11 (tRNA Tyr/UAA) in budding yeast, Saccharomyces cerevisiae [2]. The role of Maf1 has been suggested by tRNA accumulation in maf1∆ cells, observed regardless of the repressive growth conditions [3]. An analogous decrease of tRNA-suppressor (tRNA Ser/UCA) activity was detected for the maf1∆ mutant in Schizosaccharomyces pombe [4]. Interestingly, the effect of Maf1 on the efficiency of tRNA-mediated suppression is contrary to that expected. Although one would assume that increased cellular tRNA levels should improve the efficiency of tRNA-mediated nonsense suppression, data show the opposite is true.
Despite nearly two decades since the original discovery, the mechanism by which tRNA accumulation in the maf1∆ mutant leads to the antisuppressor phenotype is still not understood. The simplest hypothesis is that tRNAs transcribed in maf1∆ cells are incompletely processed, hypomodified, or fail to be appropriately delivered to ribosomes. It is worth noting that both primary transcripts and end-processed, intron-containing tRNA precursors were abnormally abundant in the absence of Maf1, and the nuclear export machinery was overloaded [5]. It was, however, unknown which processes in the tRNA maturation pathway were saturated by the increased amounts of primary transcripts in cells lacking Maf1.
The current study by Arimbasseri and colleagues [4] solves a long-term conundrum: why tRNAs overproduced in the absence of Maf1 are not fully functional. Their elegant work makes a convincing case for the saturation of the dimethyltransferase Trm1 playing a crucial role in the mechanism by which Maf1 affects tRNA suppression. By using tRNA-HydroSeq technique to examine tRNA modification levels in S. pombe on the global scale, the authors have shown that Trm1 substrates are not fully modified even in wild type cells. Further decrease of Trm1-mediated G26 dimethylation on certain tRNAs was detected in the maf1∆ mutant. Consequently, hypomodification of G26 due to limited Trm1 reduces the activity of tRNA-suppressor-Ser/UCA and accounts for antisuppression. This hypothesis was validated by genetic complementation of the antisuppressor phenotype of the maf1∆ mutant in fission yeast by overproduced Trm1 [4]. Moreover, treatment with rapamycin or overexpression of Maf1 reduced tRNA transcription with increase in the m22G26 content of tRNA-suppressor-Ser/UCA and its specific activity for suppression. Additionally, RNA polymerase III (RNAP III) mutations associated with hypomyelinating leukodystrophy decreased tRNA transcription, increased m22G26 efficiency, and reversed antisuppression. Taken together, these results demonstrate that increases or decreases in global RNAP III activity lead to inverse changes in the efficiency of m22G26 modification of specific tRNAs. Thus, a previously unknown link connecting RNAP III activity and m22G26 efficiency is due to a limiting amount of Trm1 (Fig 1). This link has been conserved through evolution, since the authors showed that the increase of m22G26 content in specific tRNAs in response to starvation was detected in the human embryonic kidney. Moreover, increased production of Trm1 in S. cerevisiae maf1∆ cells led to reversal of the antisuppression phenotype, as was also observed in fission yeast. In both these cases, however, reversal of antisuppression by overproduced Trm1 is incomplete, suggesting that other factors involved in tRNA maturation might also be saturated in the context of increased tRNA synthesis in maf1Δ [4].
Fig. 1. A novel link between RNAP III transcription and tRNA modification. 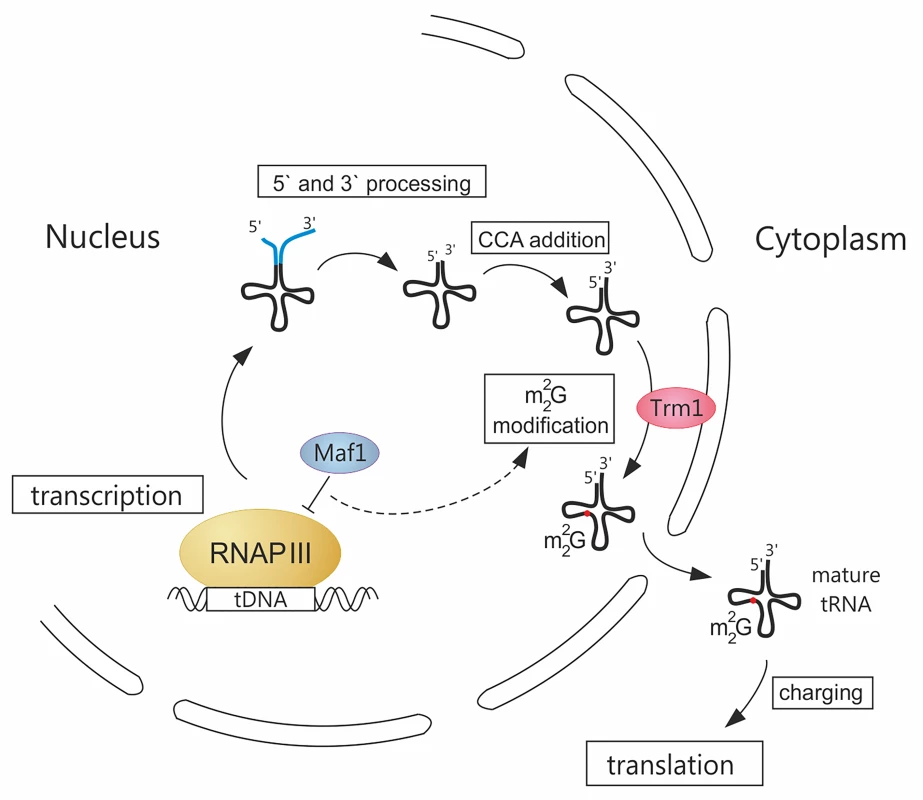
Transcription of tRNA precursors by RNA polymerase III (RNAP III) in the nucleus is controlled by the general repressor Maf1. Initial 5' and 3' end maturation including addition of CCA sequence, takes place in the nucleus. Following m22G26 modification by Trm1 methyltransferase tethered to the inner nuclear membrane, tRNAs are exported to the cytoplasm, charged, and directed to translation machinery. A previously unknown link connecting RNAP III activity and m22G26 efficiency, due to a limiting amount of Trm1, is designated by a dashed line. Splicing of intron-containing tRNA precursors and other modifications that can be added in each step of tRNA biosynthesis are not presented on the scheme. Why might tRNA modification by Trm1 be limited? A simple explanation could be that the level of Trm1 protein is regulated by the growth conditions that affect tRNA transcription. This was, however, excluded experimentally, suggesting that Trm1 activity may be controlled by a posttranslational mechanism. Another interesting possibility is that Trm1 modification might be limited by tRNA retention time in the nucleus. In S. cerevisiae, Trm1 is tethered to the inner nuclear membrane via a specific amino acid sequence tract [6]. Although nuclear residence may limit the time during which a nascent pre-tRNA transcript might have access to acquire the m22G26 modification, retrograde RNA transport should theoretically allow iterative access to Trm1 [7]. Therefore, the mechanism by which cells maintain Trm1 activity in a functionally limiting amount is unclear.
G26 resides at the junction between the D-stem and the anticodon stem, and its N2-dimethylation, which interferes with normal Watson-Crick base pairing, may contribute to prevention of tRNA misfolding. It is also notable that treatment of cells with 5-flurouracil (5FU), which is incorporated into RNA, sensitizes S. cerevisiae to loss of genes that encode tRNA modification enzymes whose nucleoside targets localize at or near the stems junction and include gene-encoding Trm1 [8]. These observations, together with evidence that m22G26 can stabilize correctly folded anticodon stems [9], suggest that m22G26 may enhance tRNA-specific activity by improving the fit in the ribosome.
Although such a speculative role of Trm1 is beyond the scope of the current study, there are examples of tRNA modification enzymes that affect posttranscriptional regulatory mechanisms. Trm9, a methyltransferase that catalyzes modification of wobble bases in the tRNA anticodon, enhances the translation of the class of transcripts overrepresented with specific arginine and glutamic acid codons, which encode key damage response proteins [10]. Next, modification of tRNA-Lys/UUU by an elongator is essential for efficient translation of stress mRNAs [11]. Finally, loss of tRNA anticodon wobble uridine modification slows translation at cognate codons, leading to widespread protein aggregation [12]. Because of its hierarchical substrate preference, convincingly documented by Arimbasseri and colleagues, Trm1 may also contribute to maintaining proteome integrity.
Zdroje
1. Hopper AK, Martin NC (1992) Processing of yeast cytoplasmic and mitochondrial precursor tRNA In: Jones EW, Pringle JR, Broach JR (eds) The Molecular and Cellular Biology of the Yeast Saccharomyces. CSHL, Cold Spring Harbor NY pp 99–141
2. Boguta M, Czerska K, Zoladek T (1997) Mutation in a new gene MAF1 affects tRNA suppressor efficiency in Saccharomyces cerevisiae. Gene 185 : 291–296. 9055829
3. Pluta K, Lefebvre O, Martin NC, Smagowicz WJ, Stanford DR, Ellis SR, Hopper AK, Sentenac A, Boguta M (2001) Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevisiae. Mol Cell Biol 21 : 5031–5040. 11438659
4. Arimbasseri AG, Blewett1 NH, Iben JR, Lamichhane TN, Cherkasova V, Hafner M, Maraia RJ (2015) RNA Polymerase III output is functionally linked to tRNA dimethyl-G26 modification. PLoS Genet 11(12): e1005671. doi: 10.1371/journal.pgen.1005671
5. Karkusiewicz I, Turowski TW, Graczyk D, Towpik J, Dhungel N, Hopper AK, Boguta M (2011) Maf1 protein, repressor of RNA polymerase III, indirectly affects tRNA processing. J Biol Chem 286 : 39478–39488. doi: 10.1074/jbc.M111.253310 21940626
6. Diaz-Munoz G, Harchar TA, Lai TP, Shen KF, Hopper AK (2014) Requirement of the spindle pole body for targeting and/or tethering proteins to the inner nuclear membrane. Nucleus 5 : 352–366. doi: 10.4161/nucl.29793 25482124
7. Kramer EB, Hopper AK (2013) Retrograde transfer RNA nuclear import provides a new level of tRNA quality control in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 110 : 21042–7. doi: 10.1073/pnas.1316579110 24297920
8. Gustavsson M, Ronne H (2008) Evidence that tRNA modifying enzymes are important in vivo targets for 5-fluorouracil in yeast. RNA 14 : 666–674. doi: 10.1261/rna.966208 18314501
9. Steinberg S, Cedergren R (1995) A correlation between N2-dimethylguanosine presence and alternate tRNA conformers. RNA 1 : 886–891. 8548653
10. Begley U, Dyavaiah M, Patil A, Rooney JP, DiRenzo D, Young CM, Conklin DS, Zitomer RS, Begley TJ (2007) Trm9-catalyzed tRNA modifications link translation to the DNA damage response. Mol Cell 28 : 860–870 18082610
11. Fernandez-Vazgues J, Vargas-Pérez I, Sansó M, Buhne K, Carmona M, Paulo E, Hermand D, Rodríguez-Gabriel M, Ayté J, Leidel S, Hidalgo E (2013) Modification of tRNA(Lys) UUU by elongator is essential for efficient translation of stress mRNAs. PLoS Genet 9(7):e1003647. doi: 10.1371/journal.pgen.100364 23874237
12. Nediakova DD, Leidel SA (2015) Modifications Maintains Proteome Integrity. Cell 161 : 1606–1618. doi: 10.1016/j.cell.2015.05.022 26052047
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 12
-
Všechny články tohoto čísla
- Data Sharing Policy: In Pursuit of Functional Utility
- Catching a (Double-Strand) Break: The Rad51 and Dmc1 Strand Exchange Proteins Can Co-occupy Both Ends of a Meiotic DNA Double-Strand Break
- Why Are tRNAs Overproduced in the Absence of Maf1, a Negative Regulator of RNAP III, Not Fully Functional?
- "Women Who Don't Give a Crap"
- A Point Mutation in Suppressor of Cytokine Signalling 2 () Increases the Susceptibility to Inflammation of the Mammary Gland while Associated with Higher Body Weight and Size and Higher Milk Production in a Sheep Model
- Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s
- A Simple Model-Based Approach to Inferring and Visualizing Cancer Mutation Signatures
- An Empirical Bayes Mixture Model for Effect Size Distributions in Genome-Wide Association Studies
- Mouse Y-Encoded Transcription Factor Is Essential for Sperm Formation and Function in Assisted Fertilization
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- "Women Who Don't Give a Crap"
- A Point Mutation in Suppressor of Cytokine Signalling 2 () Increases the Susceptibility to Inflammation of the Mammary Gland while Associated with Higher Body Weight and Size and Higher Milk Production in a Sheep Model
- Data Sharing Policy: In Pursuit of Functional Utility
- Catching a (Double-Strand) Break: The Rad51 and Dmc1 Strand Exchange Proteins Can Co-occupy Both Ends of a Meiotic DNA Double-Strand Break
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



