-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAre some COVID-19 vaccines better than others regarding the short-term side effects?
Jsou některé vakcíny COVID-19 lepší než jiné, pokud jde o krátkodobé nežádoucí účinky?
Zvládnutí pandemie se dosahuje především očkováním proti COVID-19. Ačkoliv se ve světě používají různé vakcíny proti COVID-19, o jejich bezpečnosti a vedlejších účincích je známo jen málo. Cílem tohoto výzkumu je proto zjistit krátkodobé vedlejší účinky různých vakcín proti COVID-19 používaných v Iráku. Dále bylo cílem prozkoumat souvislost mezi pociťovanými nežádoucími účinky a značkou podané vakcíny. Současná studie hodnotila krátkodobé vedlejší účinky vakcín Pfizer, Sinopharm a AstraZeneca u pracovníků ve zdravotnictví v Iráku. Ve studii byl použit dotazník, který se skládal ze speciálních částí pro sběr demografických údajů, značky obdržené vakcíny covid-19, krátkodobých nežádoucích účinků a ochoty obdržet třetí posilovací dávku. Pokud jde o vedlejší účinky po očkování, studované vakcíny COVID-19 vykazovaly srovnatelnou škálu vedlejších účinků, jako jsou bolesti hlavy, horečka, bolesti svalů, bolesti kloubů, malátnost, citlivost, zarudnutí a také bolest v místě očkování. Vakcína Pfizer však vykazovala vyšší výskyt bolesti a citlivosti v místě vpichu a horečky ve srovnání s vakcínami AstraZeneca a Sinopharm. Na druhou stranu vakcína Sinopharm byla ve srovnání s vakcínami Pfizer a AstraZeneca spojena s vyšším výskytem bolestí hlavy, svalů, kloubů a malátnosti. Souhrnně lze říci, že krátkodobé nežádoucí účinky všech tří vakcín byly srovnatelné, nicméně vakcína AstraZeneca byla spojena s nižším rizikem výskytu nežádoucích účinků.
Klíčová slova:
nežádoucí účinky – COVID-19 – vakcína – Pfizer – Sinopharm – AstraZeneca – Sputnik V
Authors: Radhwan Al-Zidan; Omeed Darweesh; Muhanad Salah; Pshtiwan Bebane; Hemn Ahmed; Ghayth Abdulrazzaq; Sadeel Shanshal; Nohad Alomari
Published in the journal: Čes. slov. Farm., 2023; 72, 45-54
Category: Původní práce
doi: https://doi.org/https://doi.org/10.5817/CSF2023-1-45Summary
Controlling the pandemic is primarily achieved through vaccination against COVID-19. Although various COVID-19 vaccines are used worldwide, little is known about their safety and side effects. As a result, the objectives of this research are to identify the shortterm side effects of the different COVID-19 vaccines used in Iraq. Furthermore, exploring the association between experienced side effects and the brand of vaccine received. The current study evaluated the shortterm side effects of Pfizer, Sinopharm and AstraZeneca vaccines among healthcare workers in Iraq. The study used a questionnaire that consisted of dedicated sections to collect demographic data, the brand of COVID-19 vaccine received, the short-term side effects, and the willingness to receive a third booster dose. Regarding the post-vaccination side effects, the studied COVID-19 vaccines showed a comparable range of side effects, such as headaches, fever, muscle pain, joint pain, malaise, tenderness, redness, as well as pain at the site of vaccination. However, the Pfizer vaccine showed a higher incidence of pain and tenderness at the site of injection and fever compared to AstraZeneca and Sinopharm, respectively. On the other hand, the Sinopharm vaccine was associated with a higher occurrence of headaches, muscle pain, joint pain, and malaise in comparison to the Pfizer and AstraZeneca vaccines, respectively. In summary, the short-term side effects of the three vaccines were comparable; however, the AstraZeneca vaccine was associated with a lower risk of side effects.
Keywords:
COVID-19 – vaccine – side effect – Pfizer – Sinopharm – AstraZeneca – Sputnik V
Introduction
Since its debut in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, which causes coronavirus illness (COVID-19), has affected people worldwide1). People who are infected may just have minor symptoms, such as fever, cough, myalgia, malaise, chest pain, and loss of smell and taste2, 3). However, in some cases, patients may suffer from pneumonia, multiple organ failure, and, on extreme occasions, death4, 5). SARS-CoV-2 is spreading swiftly because of its ongoing mutations and quickly dispersing infection6). Even though various medications and herbal medicines have been utilized to treat COVID-19, they play a supportive role in treating SARS-CoV-2 patients, hence why a safe and effective vaccination is essential to effectively combat the COVID-19 pandemic7–11).
It was advised that the history of the pandemic should be separated between pre-vaccination and post-vaccination periods because the launch of the COVID-19 vaccine deployment in December 2020 marked a turning point in the fight against this pandemic12). Vaccination hesitancy is a significant public health problem that is stoked by myths and false information regarding the efficacy and safety of vaccines13). The perception of the vaccine’s adverse reactions, vaccination attitudes, perceived disease vulnerability, social consequences, faith in the medical profession, and improved vaccine information are only a few variables that might affect vaccine acceptance14). Inactivated vaccines (Sinopharm, Sinovac, and COVAXIN) are among the COVID-19 vaccines now widely available for users worldwide15). Other types of vaccines include mRNA vaccines (Pfizer BioNTech and Moderna) and viral vector vaccines (AstraZeneca, Sputnik V, and Johnson & Johnson), which do not use messenger RNA (mRNA) to aid in the body’s development of antiviral defenses. The Johnson & Johnson vaccine’s creators modified an adenovirus, a typical virus that produces cold or flu-like symptoms, by introducing the gene for coronavirus’ distinctive spike protein. Nevertheless, the adenovirus was altered so that it may enter cells but cannot replicate or cause infections16). All parties involved in the procedure, including the person receiving the vaccine, care providers, and healthcare professionals, must be aware of any potential negative effects of immunization17).
The COVID-19 vaccination has been associated with mild to severe adverse effects, with the intensity of these symptoms varying depending on the kind of COVID-19 vaccine administered18). Fever, headaches, localized pain at the injection site, and muscular aches were among the most common mild adverse effects that were observed with various types of COVID-19 vaccinations19). However, several studies have reported unique adverse effects of individual vaccines18, 20–22). Governments throughout the globe have worked extensively to put into place successful mass immunization programs since the discovery and availability of COVID-19 vaccines23).
According to a study on the side effects of the COVID-19 vaccine in the Iraqi population, most postvaccination adverse effects were mild to moderate24). Coronavirus immunization was also shown to be well-tolerated and safe. However, the fear of the newly introduced vaccines was not uncommon even in medical society.
Furthermore, only a few studies have been conducted to further understand the COVID-19 vaccine’s side effects by collecting information on and evaluating self-reported side effects across various demographic and medical variables1, 25–28). To assess the short-term side effects of the COVID-19 vaccines provided by the Ministry of Health in Iraq, the main goal of this study was to survey 712 members of the early-vaccinated Iraqi population about their experiences with COVID-19 vaccinations. The purpose of this survey was to ascertain how frequently side effects are experienced. Furthermore, the association of experienced side effects and the brand of the received vaccine was studied.
Experimental part
Study Design
This cross-sectional, survey-based study was conducted from February 2022 to August 2022. Informed consent was acquired from all the participants. Research Ethics approval was obtained from the Research Ethics Committee, College of Pharmacy, Al-Kitab University, Ministry of Higher Education and Scientific Research (approval ID: UoK5/49). Participants’ data were collected anonymously, without any personal identification. Google Forms tools were used to create an online survey to collect the data from the healthcare workers included in the study. As well as being distributed via healthcare institutions’ administrative managers, the survey was also distributed through social networking platforms such as Facebook, WhatsApp, and Viber as well as via e-mail invitations.
The self-administered survey consisted of several mandatory sections. The first part of the survey collected demographic information about the participants, such as age, gender, and occupation. The second part of the study examined whether healthcare workers took the vaccine. Following the first and second doses of the vaccine, participants were asked to select any symptoms they had experienced. The following section contained several questions about the participants, such as the type of vaccination they had received, whether they had been previously infected with COVID-19, were willing to accept the third dose of the vaccine, and their medical history.
The inclusion criteria
The participant must be a healthcare worker. The age limit for participation in this survey was 18 and above. Participants were eligible to be included if they had received one or two doses of either Pfizer, Sinopharm, AstraZeneca, or Sputnik V vaccine. Incomplete responses were excluded.
Sampling Technique
At the study site, approximately 30,000 people are employed in hospitals and other healthcare institutions. According to the Raosoft online sample size calculator (http://www.raosoft.com/samplesize.html), 380 people represent a minimum representative sample size with a 5% margin of error, 99% confidence interval, and 50% response distribution. However, the study enrolled 712 participants, which exceeds the required sample size by approximately two-fold. Statistical analysis was strengthened by increasing the study sample.
Data Analysis
Data were presented as frequency or percentage and analysed using IBM SPSS 28. Chi-squared test or Fisher’s exact test was used to determine the differences between the frequencies of the groups. P < 0.05 was considered statistically significant.
Results
The number of complete responses obtained from the participating healthcare providers was 733. However, 21 participants were excluded from the study because they were not vaccinated, leaving 712 participants for the final analysis.
Descriptive statistics for vaccinated participants (N = 712)
The age range for vaccinated participants was 20–70 years (mean 34.05 ± SD 11.35). Of the 712 vaccinated participants, 295 (41%) were females, and more than one-third (35%) were pharmacists. The majority of the participants (66%) have received the Pfizer vaccine. Almost half of the reviewed healthcare providers (46.6%) never had a positive test for COVID-19, and one-third (34%) had a positive before the first dose. Only 14.3% of the participants had a chronic disease, and about a similar percentage (15.3) admitted to using medications. A small minority of the participants (6%) had an allergic reaction to COVID-19 vaccines, and an even smaller percentage (5%) had an allergic reaction to other vaccines. Most of the participating healthcare providers (85%) have been recommending the COVID-19 vaccines to other people, and about two-thirds of them had to deal directly with COVID-19 patients. Table 1 summarizes these results.
Tab. 1. Descriptive statistics of the vaccinated participants (n = 712) 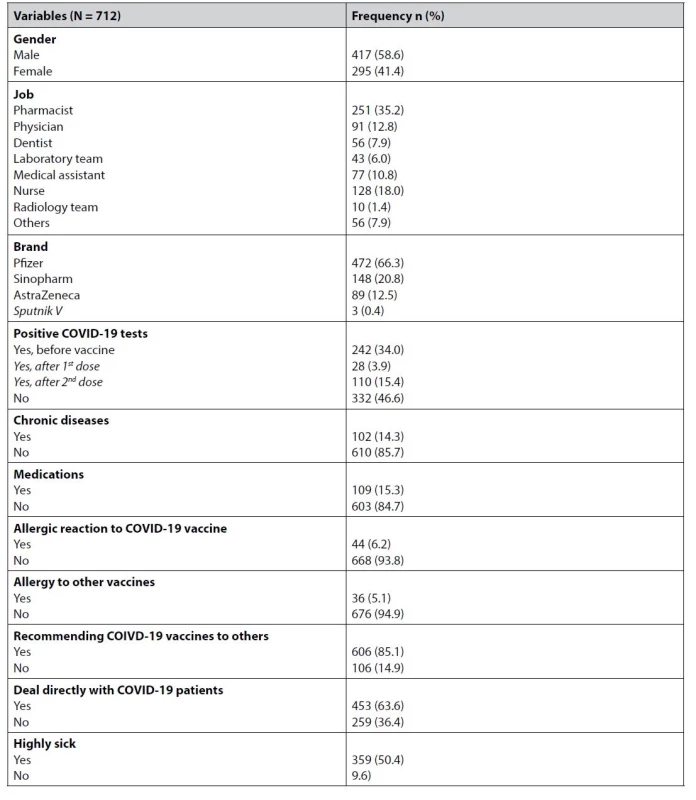
Descriptive statistics for vaccinated 2nd dose participants (N = 678)
Out of the 712 participating healthcare providers who received the first dose of the vaccine, 678 (95.2%) had the second dose, and 34 (4.8%) did not receive the second dose. About three-quarters of those who had the second dose expressed their willingness to take an additional third dose if necessary. The gender and job distribution of these participants are presented in Table 2.
Tab. 2. Gender and job distribution of the second dose participants 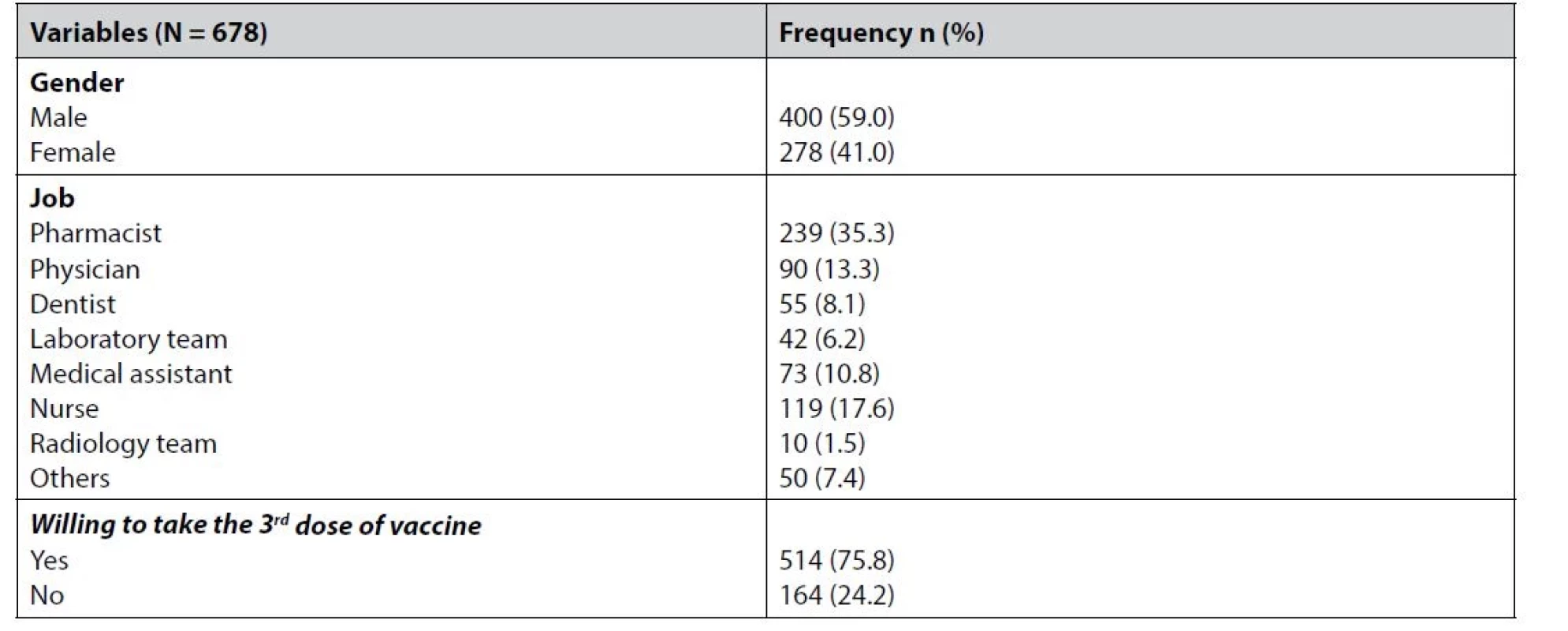
The vast majority of healthcare providers included in this survey (67%) stated that the pain at the injection site was the major complaint following the first dose of COVID-19 vaccination. Muscle pain, headache and fever were also remarkably experienced with percentages of 37.5% and 36% and 30% respectively. Diarrhea and allergy were the least reported symptoms following the first dose of the vaccine, being reported by less than 2% of the participants. The profile of adverse effects has not changed following the second dose of the COVID-19 vaccination with pain at the injection site coming first in 57% of the participants, followed by headache in 29% and muscle pain in 28% of the participants. Feeling of being sick and nausea both occurred in less than 1% of the healthcare providers following the second dose. The analysis of the data revealed a significant correlation between COVID-19 vaccination and 11 of the symptoms following the first and second doses of the vaccine. The frequency of the symptoms and the associations are presented in Table 3.
Tab. 3. Frequency and percentages of the symptoms following first and second doses of the vaccines and the associations between them 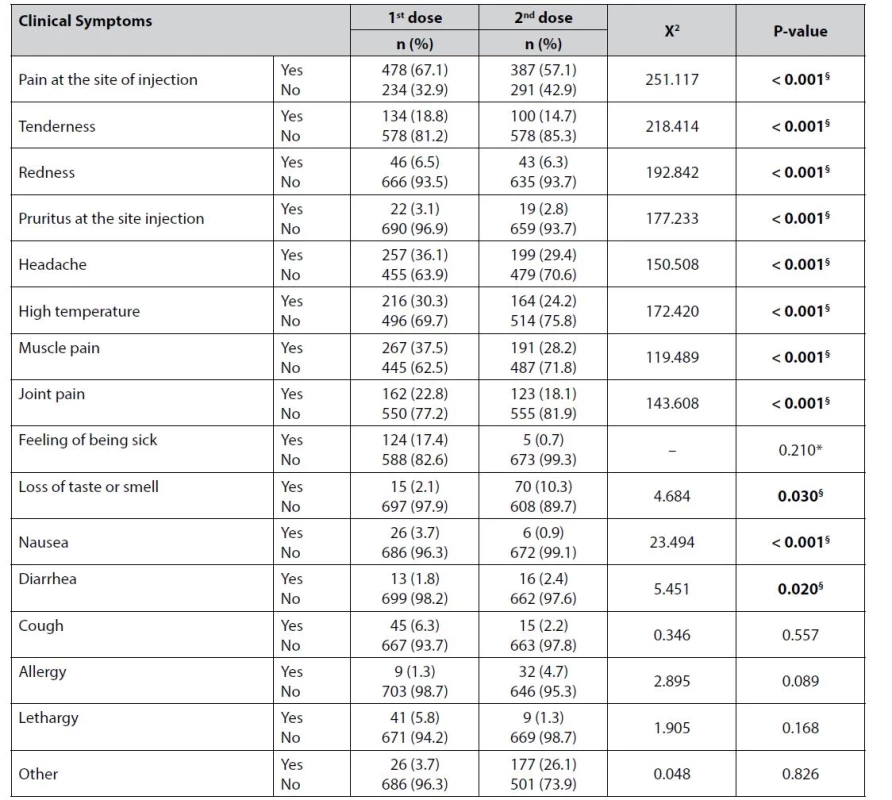
§ indicates a significant result, * Fisher’s exact test The symptoms started within 24 hours of the first dose in 455 (63.9%) participants, while the symptoms took two days to appear in 99 (13.9%) participants. One hundred and twenty participating healthcare providers acknowledged they had no symptoms at all following the first dose. When asked about the duration of the symptoms following the first dose, 201 (28.2%) participants reported that symptoms lasted for two days and for one day only in 199 (27.9%) participants. The symptoms associated with the first dose lasted for three days in 107 (15%) participants. The number of participants reporting no side effects increased to 197 (29.1%) following the second dose. For those who developed symptoms, this occurred after one day in 365 (53.8%) participants and after two days in 81 (11.9%) participants. Only 19 healthcare providers (2.8%) developed symptoms three days after the second dose. The symptoms following the second dose lasted for one day in 181 (26.7%) participants and two days in 158 (23.3%) participants. Second-dose symptoms disappeared on the same day in 200 (29.5%) participants. On the other hand, table 4 shows that gender had a significant effect on the frequency of symptoms following vaccination in seven of the studied symptoms.
Tab. 4. Association between gender and symptoms development after COVID-19 vaccination 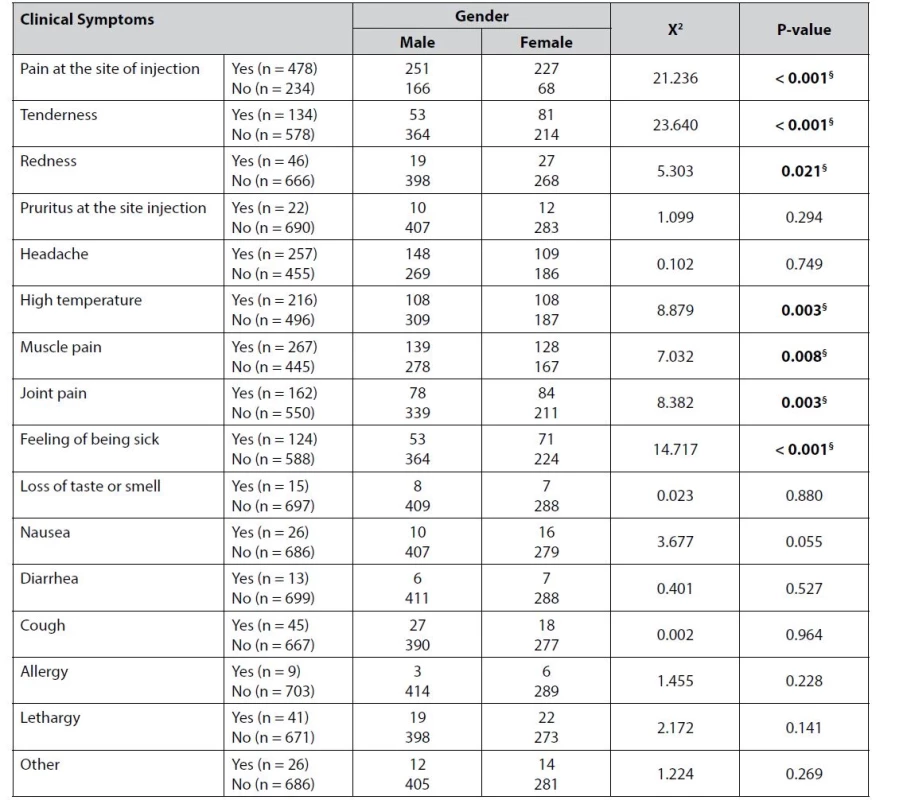
§ indicates a significant result Whereas statistically significant associations were observed for almost half of the side effects reported by the participants following the first dose of different brands of COVID-19 vaccine (Table 5).
Tab. 5. Association between the brand of the vaccine and the symptoms after vaccination 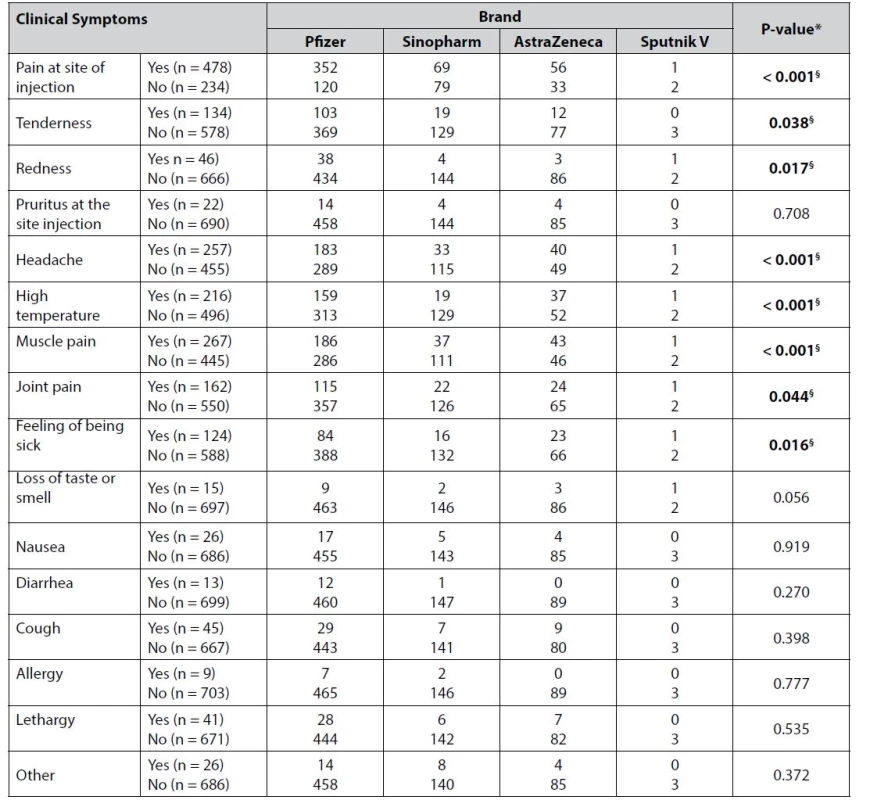
§ indicates significant result, * Fisher’s exact test Interestingly, significant associations were found between willingness to take the third dose of vaccine in terms of gender, job, and dealing directly with COVID-19 patients, as shown in Table 6.
Tab. 6. Association between gender, job, and contact level of the healthcare providers with patients willing to take the third dose of the vaccine 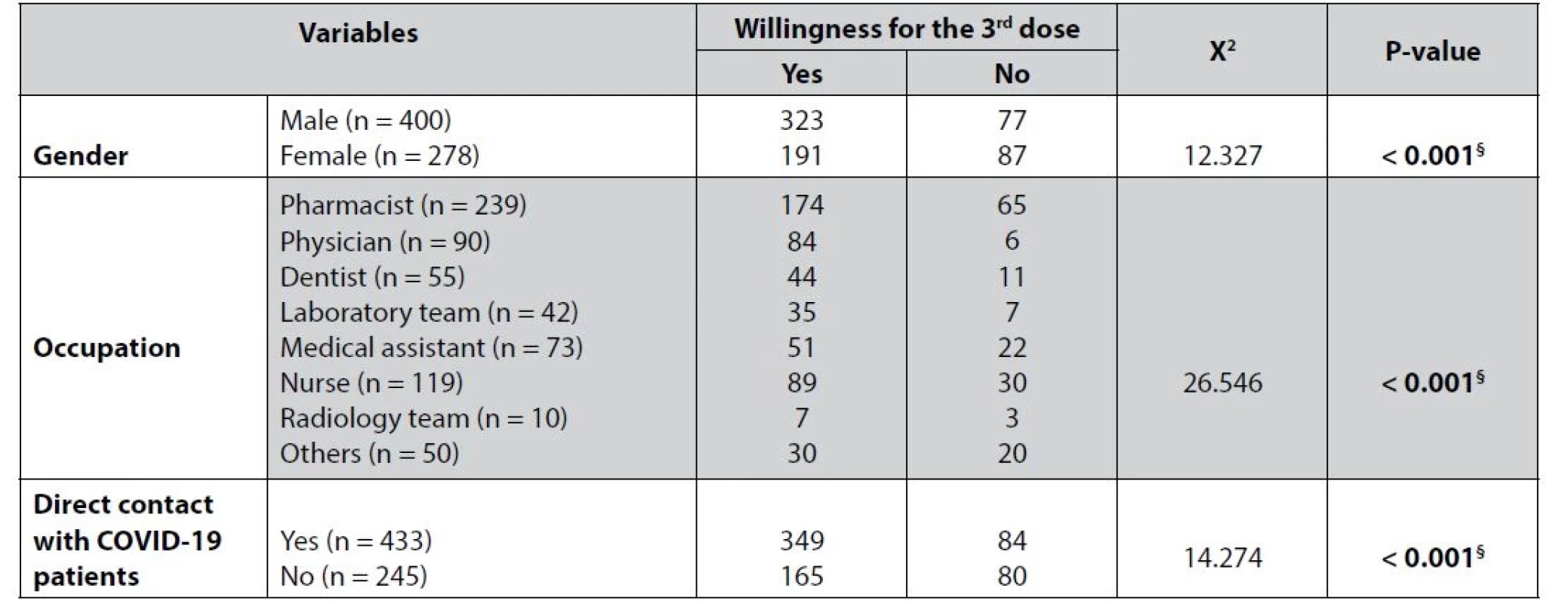
§ indicates significant result Discussion
Many types of vaccines have been developed during the past two years to eradicate COVID-19, saving millions of people’s lives annually. An effective and safe vaccine without severe side effects remains the option to be widely used in people to prevent the spread of the virus29). Knowledge of what happens after vaccination is still limited among the general population. Describing what to expect after the first and second doses of vaccination will improve public education about coronavirus vaccines and help to lower apprehension about vaccination30–32).
Vaccines may cause side effects, but in most cases, their effects are minor and will disappear within a few days33). A flu vaccination, for example, may lead to soreness, redness, pain, swelling, fever, muscle aches, joint pain, chills, and headaches34). There are also possible side effects associated with Hepatitis A vaccination, including soreness, redness, fever, headache, fatigue, and loss of appetite35). Vaccinations against polio, hepatitis B, or Hib (Haemophilus influenza type B) can lead to sore spots accompanied by redness, swelling, and pain33).
In our study, Google Forms tools were used to create an online survey. From 733 complete responses obtained from the participating healthcare providers, 712 were vaccinated, and only 21 participants were not vaccinated and excluded from the study. This indicated that the healthcare providers were more concerned about their health and feared another attack of COVID-19 infection. In this study, it was demonstrated that the most common short-term COVID-19 post-vaccination side effects were pain at the site of injection, tenderness, redness, pruritus at the site of injection, headache, high temperature, and muscle and joint pain. As shown in Table.3, we observed that the percentage of most of these post-vaccination side effects was significantly higher after the first dose of these COVID-19 vaccines (Pfizer, Sinopharm, and AstraZeneca) than after the second dose. Sputnik vaccine was included in order to determine its ratio among vaccinated healthcare workers. Thus, this study was unable to identify Sputnik vaccine side effects in detail.
According to the participants, most of them suffered from post-vaccine side effects, which indicated that their immune systems did what they were supposed to do36). Following the first dose of the vaccine, 67% of them experienced pain at the injection site, followed by muscle pain (37.5%), headache (36%), and fever (30%). These results are comparable to those shown in recently published studies36–41). Interestingly, most of the study participants reported more post-vaccine side effects (ex: pain at the injection site, tenderness, redness, pruritus, headaches, high-temperature muscle, and joint pain) after the first dose of the main three types of vaccines than after the second dose. Furthermore, the post-vaccination side effects (loss of taste, smell, diarrhoea, and/or allergy) were more common after the second dose than after the first, as shown in Table 3, which is consistent with the findings of a previous study42).
The results of this study showed that symptoms started after one day of the first dose in 455 participants (63.9%). These results agree with previous research showing that most post-vaccine side effects begin during the first 24 h following vaccination43). Moreover, following the first dose of the three different types of COVID-19 vaccine, the symptoms lasted for one day in 199 participants (27%), two days in 201 participants (28%), and three days in 107 participants (15%). The symptoms following the second dose lasted for one day in 181 participants (26.7%) and for two days in 158 participants (23.3%). Second-dose symptoms disappeared on the same day in 200 participants (29.5%). The study conducted by Menni C. et al. reported that most post-vaccine side effects persist for one, two, and three days after the first and second doses of different COVID-19 vaccines43).
Interestingly, Table 4 shows that gender affected the frequency of symptoms following vaccination in seven of the symptoms studied. Women, for example, had a higher incidence of headaches, fever, muscle pain, joint pain, malaise, tenderness and redness at the injection site. On the other hand, males were only more sensitive to pain at the vaccination site. A previous study discovered that women experienced more side effects than men, which may have contributed to men having higher testosterone levels42). Each vaccine’s nature and mechanism of action were linked to the severity and number of post-vaccine side effects36).
Furthermore, regarding the type of post-vaccination side effects, the studied COVID-19 vaccines showed a similar range of side effects such as headache, fever, muscle pain, joint pain, malaise, tenderness, redness, as well as pain at the site of vaccination. However, on the one hand, the Pfizer vaccine showed a higher incidence of pain and tenderness at the site of injection, and fever in comparison to AstraZeneca and Sinopharm, respectively. On the other hand, the Sinopharm vaccine was associated with a higher occurrence of headaches, muscle pain, joint pain, and malaise compared to the Pfizer and AstraZeneca vaccines, respectively, as shown in Table 5. Hence, the AstraZeneca vaccine was associated with the least occurrence of side effects. This finding is consistent with the conclusions of an observational study conducted in the UAE44).
Finally, the willingness to take the third dose of the vaccine among the healthcare providers in this study was significantly high, 72.19%, as shown in Table 6. The healthcare workers were more careful about being vaccinated due to the nature of their work and their usual exposure to COVID-19 patients to protect themselves from the risk of infection29).
Author Contributions
Study design: Nohad AlOmari, Omeed Darweesh
Data collection: Omeed Darweesh, Pshtiwan Bebane, Hemen Ahmed, Radhwan Al-Zidan
Data analysis and visualization: Sadeel Shanshal, Radhwan Al-Zidan, Omeed Darweesh, Ghayth Abdulrazzaq
Writing original draft: Radhwan Al-Zidan, Omeed Darweesh, Muhanad Salah, Pshtiwan Bebane, Hemen Ahmed, Ghayth Abdulrazzaq, Sadeel Shanshal, Nohad AlOmari
Manuscript editing and approval: Radhwan Al-Zidan, Omeed Darweesh, Muhanad Salah, Pshtiwan Bebane, Hemen Ahmed, Ghayth Abdulrazzaq, Sadeel Shanshal, Nohad AlOmari
Ethical Approval
This study has been approved by Ethics Committee of the Faculty of Pharmacy/Al-Kitab University/Ministry of Higher Education and Scientific Research (6/49 in 17/10/2022).
Participant Consent for Publication
All participants gave informed consent.
Conflict of interest: none.
Received November 20, 2022 / Accepted January 7, 2023
R. Al-Zidan • G. Abdulrazzaq • S. Shanshal
College of Pharmacy, University of Mosul, Iraq
Dr. Omeed Darweesh
College of Pharmacy, Al-Kitab University, Kirkuk, Iraq
Department of Molecular and Cell Biology, University of Leicester,
Leicester, LE1 7RH, UK; https://orcid.org/0000-0002-6369-296X
e-mail: od54@uoalkitab.edu.iq
M. Salah
College of Pharmacy, Hawler Medical University, Erbil, Iraq
Department of Clinical Pharmacy and Pharmacy Management
Faculty of Pharmacy
Tishk International University, Erbil, Iraq
P. Bebane
College of Education, Charmo University, Chamchamal, Iraq
H. Ahmed
Department of Pharmacy, Kirkuk Health Directorate, Ministry of Health, Kirkuk, Iraq
N. AlOmari
Pharmacy Department, AlNoor University College, Mosul, Nineveh, Iraq
e-mail: nohad.alomari@alnoor.edu.iq
Zdroje
1. Alagoz O., Sethi A. K., Patterson B. W., Churpek M., Alhanaee G., Scaria E., Safdar N. The impact of vaccination to control COVID-19 burden in the United States: A simulation modeling approach. PLoS One 2021; 16, e0254456. https://doi.org/10.1371/journal.pone.0254456
2. Alhazmi A., Alamer E., Daws D., Hakami M., Darraj M., Abdelwahab S., Maghfuri A., Algaissi A. Evaluation of Side Effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines 2021; 9, 674. https://doi.org/10.3390/ vaccines9060674
3. Alkotaji M., Al-Zidan R. N. Indomethacin: Can It Counteract Bradykinin Effects in COVID-19 Patients? Curr. Pharmacol. Rep. 2021; 7, 102–106. https://doi.org/10.1007/ s40495-021-00257-6
4. Almufty H. B., Mohammed S. A., Abdullah A. M., Merza M. A. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab. Syndr. 2021; 15, 102207. https://doi.org/10.1016/j.dsx.2021.102207
5. Al-Qazaz H. K., Al-Obaidy L. M., Attash H. M. COVID-19 vaccination, do women suffer from more side effects than men? A retrospective cross-sectional study. Pharm. Pract. 2022; 20, 2678–2678. https://doi.org/10.18549/ PharmPract.2022.2.2678
6. Alsaaty M. H., Alyouzbaki A. Z., Younis W. T. The impact of smoking on severity and outcome in patients with covid-19 infection in Mosul city. Mil. Med. Sci. Lett. 2022; 91, 98–104. https://doi.org/10.31482/mmsl.2021.044
7. Althanoon Z. A. Evaluation of the therapeutic effects of combined hydroxychloroquine and azithromycine in patients with covid-19. MMSL 2022; 91, 216–223. https:// doi.org/10.31482/mmsl.2021.045
8. Alzarea A. I., Khan Y. H., Alatawi A. D., Alanazi A. S., Alzarea S. I., Butt M. H., Almalki Z. S., Alahmari A. K., Mallhi T. H. Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real - World Estimation of Safety Profile. Vaccines 2022; 10, 924. https://doi.org/10.3390/vaccines10060924
9. Al-Zidan R. N. Potential Drug-Drug and Drug-Disease interactions of selected experimental therapies used in treating COVID-19 patients. J. Drug Deliv. Ther. 2020; 10, 219–230. https://doi.org/10.22270/jddt.v10i6.4383
10. Attia S., Howaldt H.-P. Impact of COVID-19 on the Dental Community: Part I before Vaccine (BV). J. Clin. Med. 2021; 10, 288. https://doi.org/10.3390/jcm10020288
11. Baden L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S. A., Rouphael N., Creech C. B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B. S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021; 384, 403–416. https://doi.org/10.1056/NEJMoa2035389
12. Callaway E. The race for coronavirus vaccines: a graphical guide. Nature 2020; 580, 576–577. https://doi. org/10.1038/d41586-020-01221-y
13. Cuschieri S., Borg M., Agius S., Souness J., Brincat A., Grech V. Adverse reactions to Pfizer-BioNTech vaccination of healthcare workers at Malta’s state hospital. Int. J. Clin. Pract. 2021; 75, e14605. https://doi.org/10.1111/ ijcp.14605
14. Darweesh O., Abdulrazzaq G. M., Al-Zidan R. N., Bebane P., Merkhan M., Aldabbagh R., AlOmari N. Evaluation of the Pharmacologic Treatment of COVID-19 Pandemic in Iraq. Curr. Pharmacol. Rep. 2021; 7, 171–178. https://doi.org/10.1007/s40495-021-00262-9
15. Elgendy M. O., Abdelrahim M. E. A. Public awareness about coronavirus vaccine, vaccine acceptance, and hesitancy. J. Med. Virol. 2021; 93, 6535–6543. https://doi. org/10.1002/jmv.27199
16. Elgendy M. O., El-Gendy A. O., Alzarea A. I., Mahmoud S., Alqahtani S. S., Fahmy A. M., El-Seedi H. R., Sayed A. M., Alatawi A. D., Abdelrahim M. E. A., Alanazi A. S. SARS-CoV-2 Post Vaccinated Adverse Effects and Efficacy in the Egyptian Population. Vaccines 2022; 10, 18. https://doi.org/10.3390/vaccines10010018
17. El-Shitany N. A., Harakeh S., Badr-Eldin S. M., Bagher A. M., Eid B., Almukadi H., Alghamdi B. S., Alahmadi A. A., Hassan N. A., Sindi N., Alghamdi S. A., Almohaimeed H. M., Mohammedsaleh Z. M., Al-Shaikh T. M., Almuhayawi M. S., Ali S. S., El-Hamamsy M. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2021; 14, 1389–1401. https://doi.org/10.2147/IJGM.S310497
18. El-Sokkary R. H., El Seifi O. S., Hassan H. M., Mortada E. M., Hashem M. K., Gadelrab M. R. M. A., Tash R. M. E. Predictors of COVID-19 vaccine hesitancy among Egyptian healthcare workers: a cross-sectional study. BMC Infect. Dis. 2021; 21, 762. https://doi.org/10.1186/ s12879-021-06392-1
19. Galindo R., Chow H., Rongkavilit C. COVID-19 in Children: Clinical Manifestations and Pharmacologic Interventions Including Vaccine Trials. Pediatr. Clin. North Am. 2021; 68, 961–976. https://doi.org/10.1016/j. pcl.2021.05.004
20. Ganesan S., Al Ketbi L. M .B., Al Kaabi N., Al Mansoori M., Al Maskari N. N., Al Shamsi M. S., Alderei A. S., El Eissaee H. N., Al Ketbi R. M., Al Shamsi N. S., Saleh K. M., Al Blooshi A. F., Cantarutti F. M., Warren K., Ahamed F., Zaher W. Vaccine Side Effects Following COVID-19 Vaccination Among the Residents of the UAE – An Observational Study. Front. Public Health 2022; 10, 876336. https://doi.org/10.3389/fpubh.2022.876336
21. Gorial F. I., Abdulrahman Younis A., Alkazzaz A., Arif Maroof A. M., Qaradaghi T. A., Mahmood C. H., ALosami M. H., Yasiry D. S., Ihsan Awadh N., Younis M. M., Mahdi F. J., Mihan S. W., Jassim N. A. Covid-19 among a sample of iraqi patients with rheumatic diseases: a multicenter study. MMSL 2022; 91, 89–97. https://doi. org/10.31482/mmsl.2021.038
22. Hatmal M. M., Al-Hatamleh M. A. I., Olaimat A. N., Hatmal M., Alhaj-Qasem D. M., Olaimat T. M., Mohamud R. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. Vaccines 2021; 9, 556. https://doi. org/10.3390/vaccines9060556
23. Hause A. M. Safety Monitoring of an Additional Dose of COVID-19 Vaccine — United States, August 12–September 19, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021; 70. https://doi.org/10.15585/mmwr.mm7039e4
24. Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2021; 15(5), 102207.
25. Hervé C., Laupèze B., Del Giudice G., Didierlaurent A .M., Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. Npj Vaccines 2019; 4, 1–11. https:// doi.org/10.1038/s41541-019-0132-6
26. Im J. H., Kim E., Lee E., Seo Y., Lee Y., Jang Y., Yu S., Maeng Y., Park Soyeon, Park Seohee, Kim J., Lee J. - S., Baek J. H. Adverse Events with the Pfizer-BioNTech COVID-19 Vaccine among Korean Healthcare Workers. Yonsei Med. J. 2021; 62, 1162–1168. https://doi. org/10.3349/ymj.2021.62.12.1162
27. Inactivated Influenza Vaccine Information Statement | CDC [www Document], 2022. URL https://www.cdc.gov/ vaccines/hcp/vis/vis-statements/flu.html (12.23.22).
28. Jackson L. A., Anderson E. J., Rouphael N. G., Roberts P. C., Makhene M., Coler R. N., McCullough M. P., Chappell J. D., Denison M. R., Stevens L. J., Pruijssers A. J., McDermott A., Flach B., Doria-Rose N. A., Corbett K. S., Morabito K. M., O’Dell S., Schmidt S. D., Swanson P. A., Padilla M., Mascola J. R., Neuzil K. M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J. E., Graham B. S., Beigel J. H. An mRNA Vaccine against SARSCoV - 2 — Preliminary Report. N. Engl. J. Med. 2020; 383, 1920–1931. https://doi.org/10.1056/NEJMoa2022483
29. Jarrett C., Wilson R., O’Leary M., Eckersberger E., Larson H. J., SAGE Working Group on Vaccine Hesitancy. Strategies for addressing vaccine hesitancy – A systematic review. Vaccine 2015; 33, 4180–4190. https://doi. org/10.1016/j.vaccine.2015.04.040
30. Kadali R. A. K., Janagama R., Peruru S., Malayala S. V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int. J. Infect. Dis. 2021; 106, 376–381. https://doi.org/10.1016/j.ijid.2021.04.047
31. Li P., Fu J.-B., Li K.-F., Liu J.-N., Wang H.-L., Liu L.-J., Chen Y., Zhang Y.-L., Liu S.-L., Tang A., Tong Z.-D., Yan J.-B. Transmission of COVID-19 in the terminal stages of the incubation period: A familial cluster. Int. J. Infect. Dis. 2020; 96, 452–453. https://doi.org/10.1016/j. ijid.2020.03.027
32. Marzo R. R., Sami W., Alam Md. Z., Acharya S., Jermsittiparsert K., Songwathana K., Pham N. T., Respati T., Faller E. M., Baldonad, A. M., Aung Y., Borkar S. M., Essar M. Y., Shrestha S., Yi S. Hesitancy in COVID-19 vaccine uptake and its associated factors among the general adult population: a cross-sectional study in six Southeast Asian countries. Trop. Med. Health 2022; 50, 4. https:// doi.org/10.1186/s41182-021-00393-1
33. Menn C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C. H., Nguyen L. H., Drew D. A., Merino J., Hu C., Selvachandran S., Antonelli M., Murray B., Canas L. S., Molteni E., Graham M. S., Modat M., Joshi A. D., Mangino M., Hammers A., Goodman A. L., Chan A. T., Wolf J., Steves C. J., Valdes A. M., Ourselin S., Spector T. D. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021; 21, 939–949. https://doi. org/10.1016/S1473-3099(21)00224-3
34. Mohammed R. A., Garout R. M., Wahid S., Ayub F., Firas ZinAlddin L. M., Sultan I. A Survey on the Side Effects of Pfizer/BioNTech COVID-19 Vaccine Among Vaccinated Adults in Saudi Arabia. Cureus 2021; 13, e19222. https://doi.org/10.7759/cureus.19222
35. Possible Side effects from Vaccines | CDC [www Document], 2022. URL https://www.cdc.gov/vaccines/vacgen/ side-effects.htm (12.23.22).
36. Riad A., Hocková B., Kantorová L., Slávik R., Spurná L., Stebel A., Havriľak M., Klugar M. Side Effects of mRNA-Based COVID-19 Vaccine: Nationwide Phase IV Study among Healthcare Workers in Slovakia. Pharm. Basel Switz. 2021a; 14, 873. https://doi.org/10.3390/ ph14090873
37. Riad A., Pokorná A., Attia S., Klugarová J., Koščík M., Klugar M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021b; 10, 1428. https://doi.org/10.3390/ jcm10071428
38. Smith L. E., Amlôt R., Weinman J., Yiend J., Rubin G. J. A systematic review of factors affecting vaccine uptake in young children. Vaccine 2017; 35, 6059–6069. https:// doi.org/10.1016/j.vaccine.2017.09.046
39. Solomon Y., Eshete T., Mekasha B., Assefa W. COVID-19 Vaccine: Side Effects After the First Dose of the Oxford AstraZeneca Vaccine Among Health Professionals in Low-Income Country: Ethiopia. J. Multidiscip. Healthc. 2021; 14, 2577–2585. https://doi.org/10.2147/JMDH. S331140
40. Tahir A. I., Ramadhan D. S., Piro S. S., Abdullah R. Y., Taha A. A., Radha R. H. COVID-19 vaccine acceptance, hesitancy and refusal among Iraqi Kurdish population. Int. J. Health Sci. 2022; 16, 10–16.
41. Teh H. S., Woon Y. L., Leong C. T., Hing N. Y. L., Mien T. Y. S., Roope L. S. J., Clarke P. M., Lim L.-L., Buckell J. Malaysian public preferences and decision making for COVID-19 vaccination: A discrete choice experiment. Lancet Reg. Health – West. Pac. 2022; 27. https://doi. org/10.1016/j.lanwpc.2022.100534
42. Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li, Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents 2020; 55, 105955. https://doi. org/10.1016/j.ijantimicag.2020.105955
43. Zhan Y., Zeng G., Pan H., Li C., Hu Yaling, Chu K., Han W., Chen Z., Tang R., Yin W., Chen X., Hu Yuansheng, Liu X., Jiang C., Li J., Yang M., Song Y., Wang X., Gao Q., Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo - controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021; 21, 181–192. https://doi.org/10.1016/S1473 - 3099(20)30843-4
44. Zhou G., Chen S., Chen Z. Advances in COVID-19: the virus, the pathogenesis, and evidence-based control and therapeutic strategies. Front. Med. 2020; 14, 117–125. https://doi.org/10.1007/s11684-020-0773-x
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2023 Číslo 1- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Rozhodovanie o vstupe a konkurencia na trhu lekární: Prípadová štúdia voľnopredajných liekov a deregulácie vlastníctva
- Dětská obezita: příčiny, důsledky a prevence
- Příspěvek k pojmu polypragmazie II. Preskripce a užívání léčiv
- Nové trendy v terapii pokročilých štádií parkinsonovej choroby
- Jsou některé vakcíny COVID-19 lepší než jiné, pokud jde o krátkodobé nežádoucí účinky?
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Nové trendy v terapii pokročilých štádií parkinsonovej choroby
- Dětská obezita: příčiny, důsledky a prevence
- Příspěvek k pojmu polypragmazie II. Preskripce a užívání léčiv
- Rozhodovanie o vstupe a konkurencia na trhu lekární: Prípadová štúdia voľnopredajných liekov a deregulácie vlastníctva
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



