-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDevelopment of the composition of intramammary combined preparation based on silver citrate for veterinary use
Vývoj složení kombinovaného intramamárního přípravku na bázi citrátu stříbra pro veterinární použití
Cílem studie bylo vyvinout nový intramamární kombinovaný přípravek pro veterinární užití založený na citrátu stříbrném, argininu a dexpanthenolu pro léčbu a profylaxi subklinické mastitidy u skotu. Při vytváření kombinovaných léků pro veterinární užití musí být věnována pozornost faktorům ovlivňujícím jejich stabilitu v dané kombinaci, celkovému složení přípravku a technologii přípravy. V této studii byla provedena kvalitativní a kvantitativní kontrola modelových směsí účinných látek, stejně jako kombinovaného přípravku na bázi citrátu stříbrného. Byly studovány následující parametry definující stabilitu intramamarního přípravku: čirost, pH, kvantitativní obsah účinné složky. Na základě experimentálních studií byly pro nový přípravek stanoveny optimální limity pH v rozmezí od 6,4 do 6,7, při kterých jsou roztoky založené na citrátu stříbrném stabilní. Z tohoto důvodu byly studie stabilizace přípravku provedeny při daných hodnotách pH. Stabilitu přípravku se podařilo dosáhnout použitím povidonu, který vykazuje stabilizační vlastnosti. Bylo stanoveno množství argininu, které poskytuje optimální hladinu pH pro zvolenou kombinaci účinných složek. Závěrem lze říci, že bylo vyvinuto optimální složení intramamarního přípravku pro použití v oblasti veterinárního lékařství adjustovaného v 10 ml ampulích s dobou použitelnosti 6 měsíců.
Klíčová slova:
citrát stříbra • arginin • dexpanthenol • intramamární léková forma • stabilita
Authors: Zhanna Polova; Lyudmyla Almakayeva; Tetiana Nehoda
Published in the journal: Čes. slov. Farm., 2017; 66, 227-232
Category: Původní práce
Summary
The goal of the present study was to develop a new intramammary combination preparation for veterinary use based on silver citrate, arginine and dexpanthenol for the treatment and prophylaxis of subclinical mastitis in cattle. When creating combined medicines for veterinary use, attention must be paid to the factors that influence their stability in a combined presence, and the composition of drugs, as well as the technology for their preparation should be determined. In the current study, a qualitative and quantitative control of model mixtures of active ingredients, as well as of a silver citrate-based combined preparation was carried out. The following parameters that define intramammary drug stability were studied: clarity, pH, and the quantitative content of the active ingredient. Based on the experimental studies, optimal pH limits for a new preparation were determined ranging from 6.4 to 6.7. Considering the instability of solutions based on silver citrate, studies on the stabilization of the preparation were conducted at the obtained pH values. As the result, we used povidone, which possessed the stabilizing properties. The amount of arginine that provides the optimum pH level for the selected combination of active ingredients was also determined. In conclusion, the optimal composition of an intramammary preparation for veterinary medicine in 10 ml ampoules with a shelf life of 6 months was developed.
Key words:
silver citrate • arginine • dexpanthenol • intramammary dosage form • stabilityIntroduction
Bovine mastitis is an important animal production disease that affects the dairy industry globally1). The dairy industry has undergone substantial structural changes as intensive farming has developed during recent decades. Nevertheless, mastitis continues to be the most common production disease of dairy cows not only in the African states with developing economics, but also in Europe2). This problem had also affected Ukraine. Mastitis is an inflammation of the mammary gland which develops as a consequence of mechanical, thermal, chemical, or biological factors. This is the most prevalent disease in cows and one of the most complicated problems in veterinary medicine. This disease should be also considered as an economic factor which decreases the efficiency of dairying and as a social problem (consumption of milk and dairy products obtained from cows with mastitis creates a hazard for human health, particularly in children)3, 4).
Etiological agents of mastitis in cows are predominantly antibiotic-resistant bacteria, so the use of antibiotics in the treatment of animals would not always lead to a positive result5). Timely diagnostics and investigation of the cause of the spread of mastitis in the herd plays special significance in the treatment of cows with subclinical forms of mastitis. The development of effective methods of treatment, prophylaxis, as well as performing adequate measures would prevent complications of inflammatory processes in the udder and their transition into more complicated forms6).
Despite the advances of modern science and practice in the diagnostics, treatment, and prophylaxis of subclinical mastitis, pharmaceutical development of veterinary anti-mastitis preparations without using antibiotics, remains a challenge.
The antimicrobial activity of the silver ions was first identified in the 19th century, and colloidal silver was accepted by the US Food and Drug Administration (FDA) as an effective agent for wound management in the 1920s. However, after the introduction of penicillin in the 1940s, antibiotics had become the standard treatment for bacterial infections and the use of silver was diminished7, 8). Nowadays silver is a well-known disinfectant that is widely used in the treatment of clinical disease. Silver ions may be a potentially beneficial treatment against bovine mastitis, particularly in cases induced by S. aureus9).
Silver citrate has a wide spectrum of antimicrobial activity against gram-positive and gram-negative, aerobic and anaerobic, spore-forming and asporogenous bacteria, has a well-pronounced anti-inflammatory effect, and stimulates reparative processes in the wound and skin10, 11).
Arginine is an aliphatic amino acid, which is considered to be conditionally indispensable. To date, it has been proven that L-arginine has a bactericidal effect and affects the process of normal healing of various wounds. This is due to the exceptional role of L-arginine as the only endogenous source of nitric oxide (NO), one of the most important signalling molecules of all body tissues. The transformation of L-arginine in the body occurs with the participation of two basic enzymes: NO-synthase, which leads to the formation of NO, and arginase, which involves the formation of L-proline, a substrate for the synthesis of collagen in tissues, necessary for normal regeneration of body tissues12, 13).
Dexpanthenol (vitamin B5) is converted into the body into pantothenic acid which is an integral part of coenzyme A (CoA), and participates in the processes of acetylation, carbohydrates and fats metabolism, in the synthesis of acetylcholine, corticosteroids, porphyrins, stimulates the restoration of skin and mucous membranes, normalizes cellular metabolism, accelerates mitosis and increases the strength of collagen fibres. Dexpanthenol-containing preparations have a regenerative, metabolic and a weak anti-inflammatory effect14).
Combined drugs are fixed combinations of active substances. The pharmacological effects of these active substances mutually complement each other; the spectrum of their synergistic effect will potentially lead to a wide range of indications for use15).
The aim of the current study was to develop a combined veterinary preparation for intramammary use containing silver citrate, dexpantenol and arginine for the treatment and prophylaxis of subclinical mastitis in cattle.
Experimental part
Materials
As the study objects, batches of preparation with different formulations were poured into glass ampoules made of Soda Lime Silica glass (Shandong Pharmaceutical Glass Co., Ltd., China) to a volume of 10 ml under the aseptic conditions of the scientific research laboratory of parenteral an oral liquid medicinal products of the National University of Pharmacy (Kharkiv, Ukraine). The following active substances were used: silver citrate (manufacturer: “Nanomaterials and nanotechnologies”, LLC, Kyiv, Ukraine); D-Pantenol USP (BASF, Germany); L-arginine hydrochloride (Tianjin Tiyanyan Pharmaceutical Co. Ltd., China); excipients: stabilizer: Povidone 8000 (Zhangzhou HuaFu Chemical Co., Ltd., China); solvent: water for injections.
Production of combined veterinary preparation for intramammary use
Preparation of a silver citrate-based solution for intramammary administration was performed in a laboratory reactor with a built-in homogenizer (manufacturer: Ukraine). Silver citrate was loaded into the reactor, and then the water with the temperature of 20 ± 5 °С was added. Then the following substances were loaded stepwise within 10–20 min of mixing: arginine, dexpanthenol, povidone. The obtained solution was diluted to the required volume with water for injections. The solution was then stirred within 10–15 min. Percentage (w/w) of the excipients of model solution mixtures are summarized in Table 1.
Tab. 1. The composition of model solution mixtures 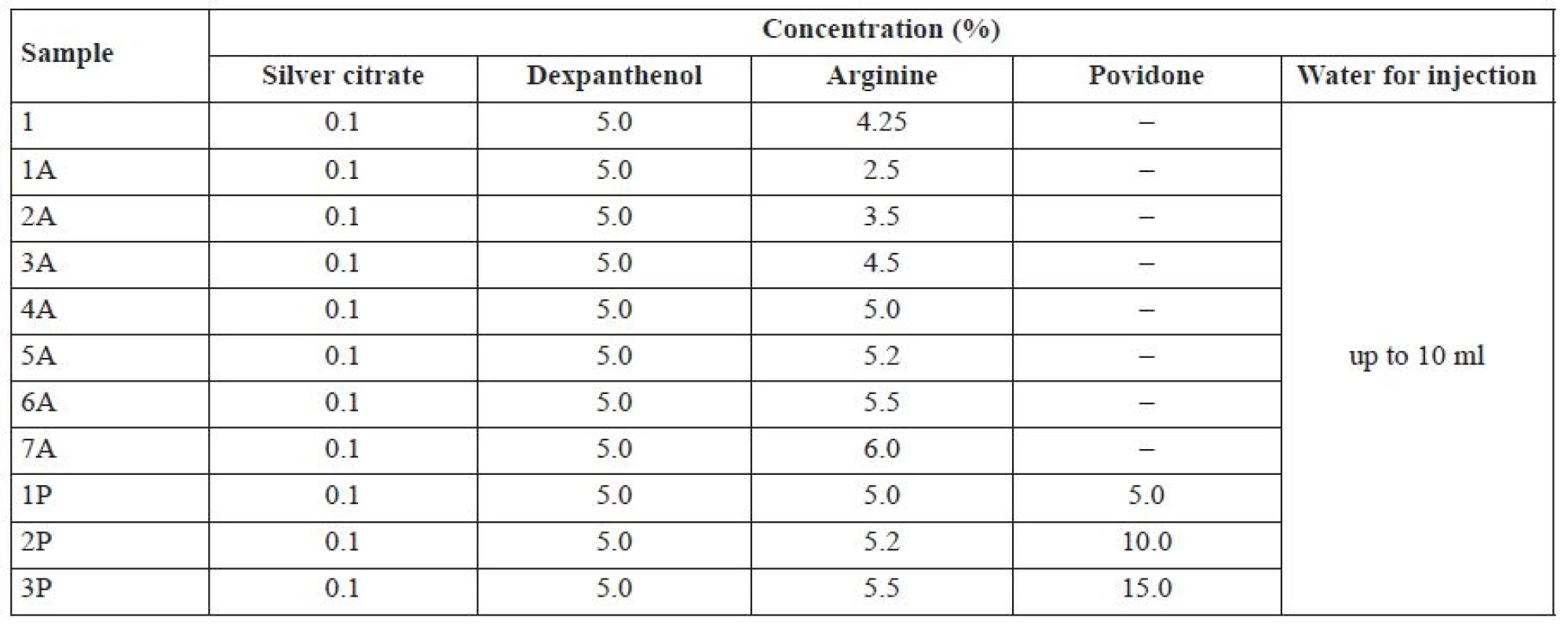
Consequently, a sample was collected from each prepared solution in order to verify the quantitative content of active pharmaceutical ingredients and pH value according to the developed project of analytical documentation for the veterinary preparation. Then the solution was filtered using an Ultipor N 66 filter (Pall, Germany). Filtered solution was evaluated for the degree of clarity. Then filling and sealing of ampoules were performed using a laboratory filling and sealing machine Cadmach KAF 08 (Cadmach, India).
Methods
The obtained samples were studied according to the following parameters: appearance, degree of clarity, quantitative content of active substances, and pH according to the methods specified in the European Pharmacopoeia16).
Quantitative assay of silver ions in the veterinary preparation was performed by the thiocyanometric method. Quantitative assay of arginine and dexpanthenol was performed by the method of liquid chromathography Eur. Ph. 8th ed. «2.2.29. Liquid chromatography».
Potentiometric determination of pH of the samples was performed according to Eur. Ph. 8th ed., 2.2.3 «Potentiometric determination of pH». The pH measurements were performed using a pH-meter «Seven Easy pH» with a set of electrodes manufactured by «Mettler Toledo» (China). All measurements were performed in the range of temperatures from 20 °С to 25 °С. The pH-meter was calibrated using reference buffer solutions.
The clarity of preparation was determined according to the method Eur. Ph. 8th ed. «2.2.1. Clarity and degree of opalescence of liquids».
Results and discussion
In developing combined intramammary medicines for veterinary use, a special attention was paid to the factors that affect stability of active ingredients when they were combined together. Such factors as physical and chemical properties of the active ingredients and excipients, their quantitative amounts, as well as possible chemical interactions between them, were also considered.
In our experimental study, physicochemical properties of solutions containing silver citrate, arginine, and dexpanthenol in the form of a new antimicrobial composition for veterinary use were studied. The mode of behaviour of these substances in solutions was subsequently taken into account in the development of the combined preparation. Based on our previous findings, 1% solution of silver citrate was determined to be stable in solutions with a pH below 7.0. Stability of silver citrate depends on the pH of the used medium, temperature, and presence of air oxygen17). The preferred pH of the medium for arginine was 6.0–7.018).
Dexpanthenol belongs to the group of organic alcohols and the pH of its 5% solution ranges from 9.0 to 10.5, but it is also stable in slightly acidic solutions. Therefore, it can be assumed that the new veterinary preparation can include all these active pharmaceutical ingredients if neutral or slightly acidic pH level is maintained. Thus, the optimal pH level for this combination of substances ranges between 6.4 and 6.7. In our opinion, such pH level corresponds with the pH of milk from healthy cows and would be optimal for intramammary administration19).
Arginine is resistant to temperature effects, while the solution of silver citrate is not recommended to be heated to a temperature above 50 °C, and dexpanthenol can undergo destructive changes under the influence of temperature to form an impurity of dexpanthenol-3-aminopropanol. It is possible to reduce the formation of impurities by producing the preparation under aseptic conditions (excluding thermal sterilization), using a set of excipients of various chemical nature that will ensure the stability of the solution of the active substances, as well as to provide the appropriate storage conditions for the preparation at a temperature not higher than 25 °C.
Firstly, the pH of solutions of each ingredient were measured and their appearance was evaluated (Table 2).
Tab. 2. Particular quality parameters of silver citrate, arginine, and dexpanthenol solutions, respectively 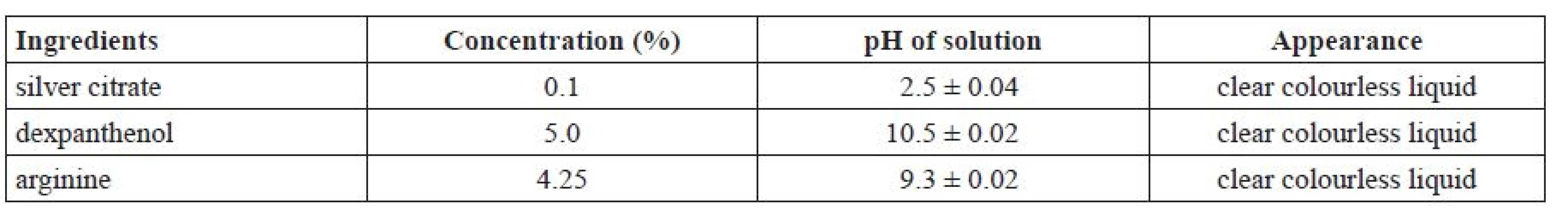
Р ± 95%, n = 5 Both solutions of dexpanthenol and arginine, respectively, exhibited alkaline pH values, and the pH of a 0.1% solution of silver citrate was in the acidic range. In addition, the solutions of all ingredients are clear and colourless liquids.
In order to confirm the compatibility of silver citrate, dexpanthenol and arginine co-presence in solution, model mixtures of these substances were prepared at the selected concentrations (Table 3). The quality parameters of sample 1 were evaluated under different temperature conditions.
Tab. 3. The study of particular quality parameters of sample 1 under different temperature conditions 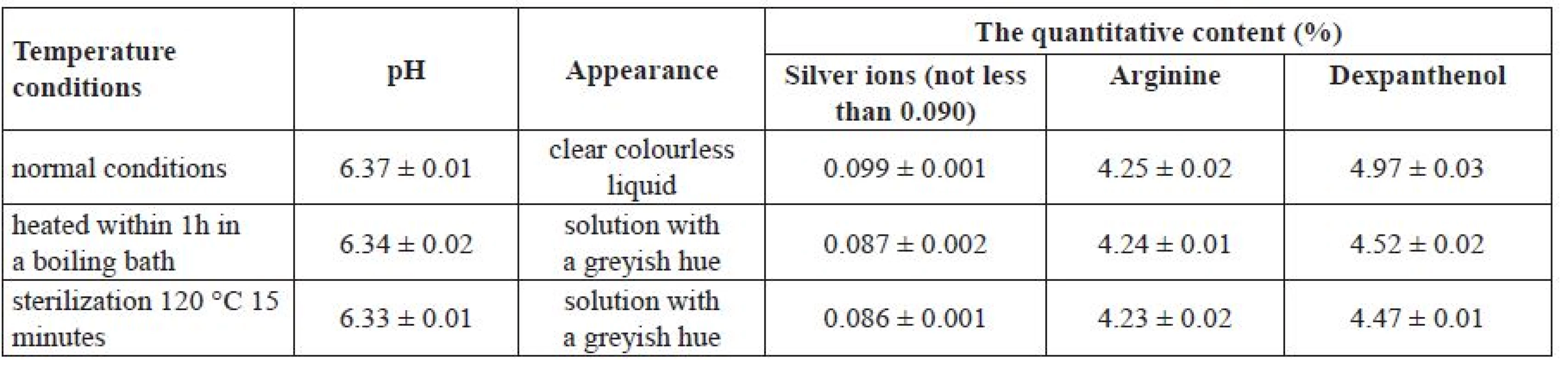
Р ± 95%, n = 5 Based on the data from Table 3, there was no interaction between the substances under normal conditions. When the mixture was heated in a water-bath within 1 hour and after the sterilization under 120 °C for 15 minutes, a colour change of the solution was observed, greyish colour appeared, and a suspension was formed. The quantitative content of silver ions and dexpanthenol was below the defined limit under these conditions. This can be explained by the instability of a 0.1% solution of silver citrate and dexpanthenol when they are combined with arginine under the influence of high temperature. Therefore, the purpose of our further research was to select the optimal pH for the above-mentioned combination of active ingredients and the conditions for carrying out the technological process (excluding thermal sterilization) with the use of a set of auxiliary substances of different chemical nature and functions.
Based on the experimental studies, the optimal pH for a combined preparation based on silver citrate was determined in the range from 6.0 to 7.0. Therefore, arginine (also the active substance of this preparation) in various concentrations was used in order to reduce the pH of a 0.1% solution of silver citrate.
Quality parameters of samples 1A–7A with different content of arginine were studied and presented in Table 4.
Tab. 4. Dependence of the quality of the solution on the basis of silver citrate on the arginine concentration 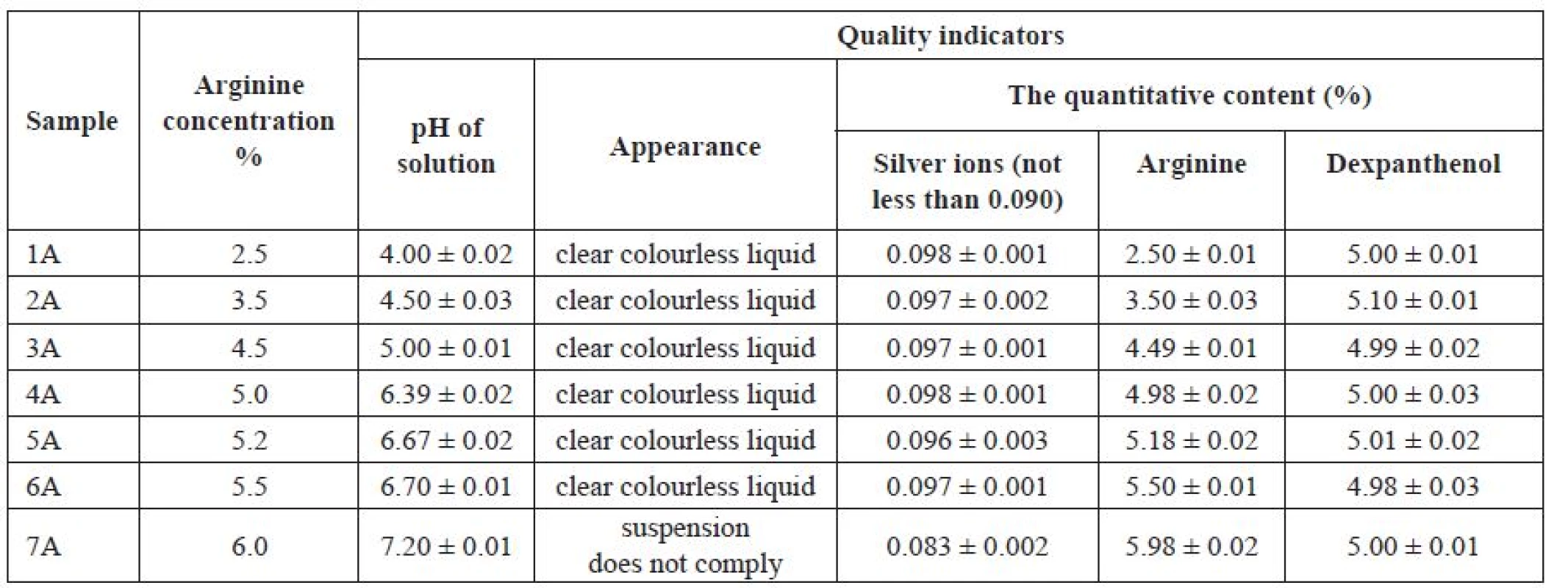
Р ± 95%, n = 5 As a result of the conducted studies it was found that the sample 7A with pH 7.20 did not comply with the requirements of the analytical project documentation for the preparation. In terms of the “Appearance” parameter and the quantitative content of silver ions, this sample was below the defined limit.
The use of solutions with a pH below 5.0 for the drug development is not recommended because this range significantly differs from the pH of milk and can cause undesirable reactions following intramammary administration. For this reason, samples 1A–3A were excluded. From tested samples, only samples 4A, 5A, 6A were considered as optimal due to the pH ranging between 6.4–6.7.
Thus, the model solutions 4A–6A were filtered through the membrane filters Ultipor N 66 into ampoules under the aseptic conditions for analysis and stability monitoring. The results of the studies are presented in Table 5.
Tab. 5. The influence of duration of storage on stability of experimental samples 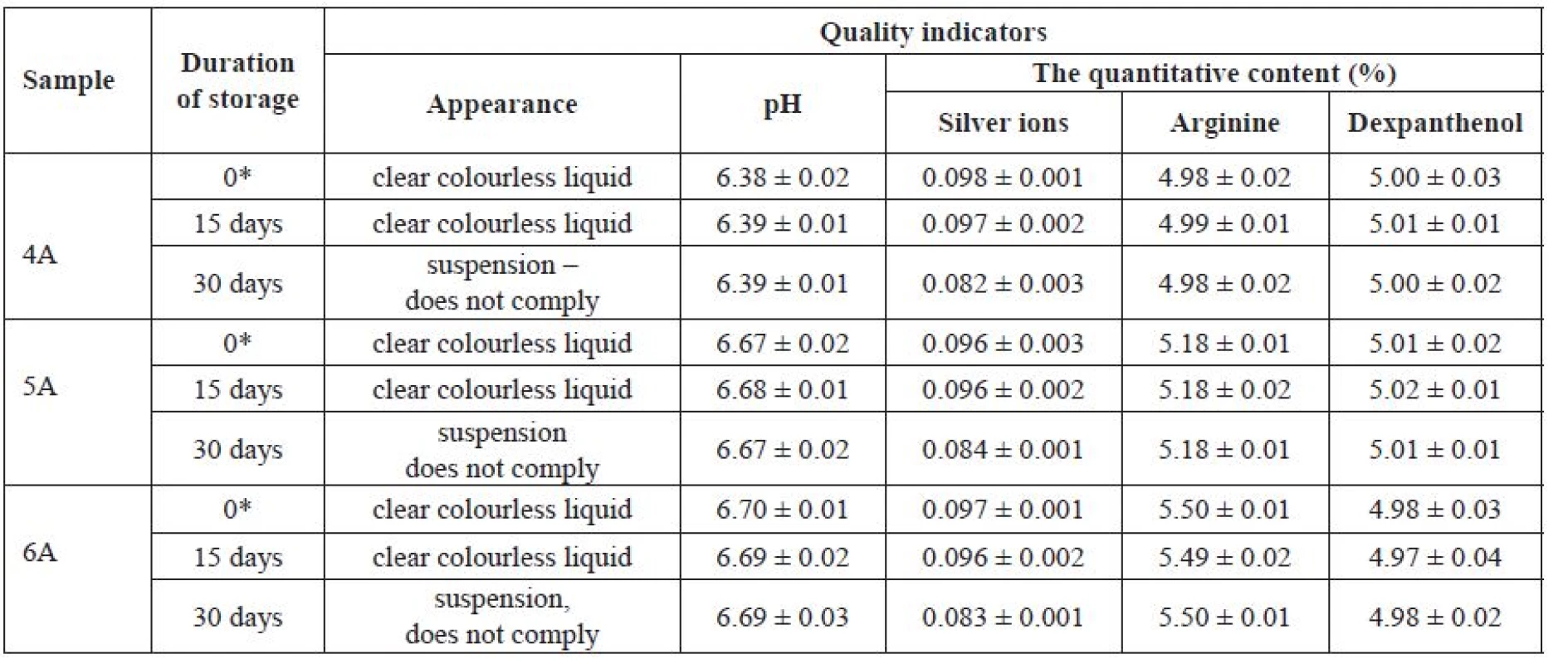
Р ± 95%, n = 5 * initial data The use of arginine in the preparation of silver citrate-based solutions with a pH of 6.4–6.7 made it possible to obtain solutions that were stable for at least 15 days. After 30 days, creation of a suspension with a slightly greyish colour and a significantly lower content of silver ions was observed.
Thus, maintaining a certain pH level of the solution was not effective enough to prevent the instability of silver citrate in the medicinal form for 30 days, so the purpose of further studies was to select excipients to increase the stability of the solution and, consequently, increase the shelf-life of the drug.
A wide range of compounds such as sodium dodecyl sulfate, polyvinyl alcohol, povidone, starch, gelatine and other low-molecular weight and polymer products of natural and artificial origin are used as stabilizing agents (stabilizers) maintaining the stability and high bactericidal activity of the silver citrate solution20, 21).
Considering the instability of solutions based on silver citrate, studies on the stabilization of the preparation were conducted at the obtained pH values. In this case, povidone exhibiting stabilizing properties in solutions was used (samples 1P, 2P, 3P). The results of studies for the selection of the optimal quantities of povidone are presented in Table 6.
Tab. 6. Dependence of the quality parameters of solutions on the amount of povidone during storage 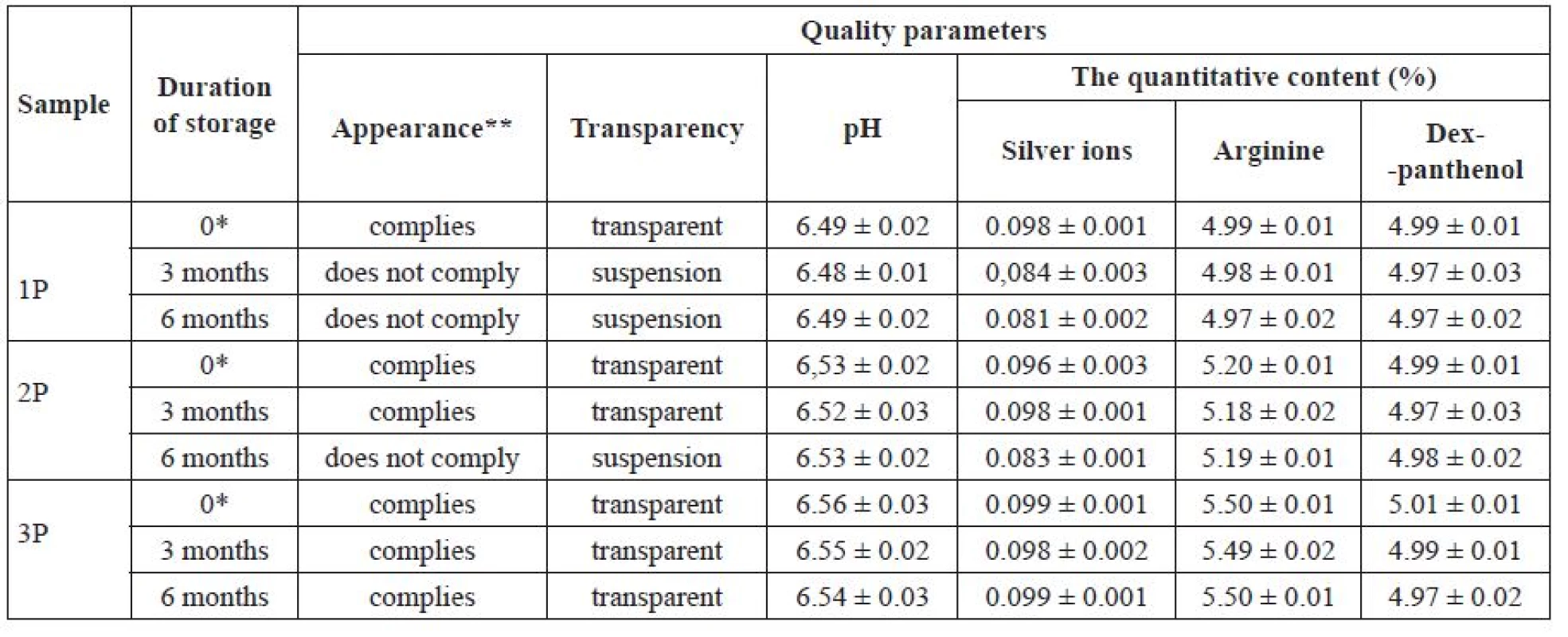
Р ± 95%, n = 5 * initial data, ** required clear liquid of light yellow colour It can be seen from the data in Table 6 that the use of povidone at a concentration of 15% (sample 3P) ensured a possibility of obtaining transparent colourless solutions with a pH of 6.5, with an acceptable level of a quantitative content of active substances. Solutions prepared with a 5% and a 10% povidone content (samples 1P and 2P) had a greyish colour and did not meet the requirements for the clarity and quantitative content of silver ions, the suspension appearing in sample 1P after 3 months and in sample 2P after 6 months of storage.
It can be concluded from the obtained results that povidone in an amount of 15% ensured the stability of the above-mentioned composition of the combined medicinal product for veterinary medicine during the storage for 6 months in ampoules. The optimal formulation for intramammary application was developed and allowed us to propose the following antimicrobial combination medicines for veterinary use, with a shelf life of 6 months (sample 3P).
Conclusions
In this experimental study, the properties of the active ingredient solutions (silver citrate, arginine and dexpanthenol, respectively) were investigated. Possible causes of their instability in the case of their co-presence in solution were determined. Theoretical and experimental studies of their compatibility in the intramammary dosage form for veterinary use was carried out. The amount of arginine providing the optimal pH level for the selected combination of active ingredients was revealed. The optimal composition had the following formulation (in % w/v): silver citrate – 0.1; dexpanthenol – 5; arginine – 5.5; povidone – 15.0; water for injection – up to 10 ml. Thus, the optimal composition of an intramammary preparation for veterinary medicine in 10 ml ampoules, with a shelf life of 6 months was developed.
Acknowledgement
The work was carried out as a part of research work: “Marketing, pharmacoeconomic and technological research on the development of medicines” (State registration number in Ukraine 0115U001530).
Conflicts of interest: none.
Received July 27, 2017
Accepted October 18, 2017
Assoc. Prof. Zhanna Polova, Ph.D.
T. Nehoda
O. O. Bogomolets National Medical University Department of Pharmaceutical and Industrial Technology of Medicines
22 Pushkinskaya Street, 01004 Kiev, Ukraine
е-mail: zpolova@ukr.net
L. Almakayeva
National University of Pharmacy Kharkiv, Ukraine
Head, Laboratory of Parenteral and Oral Liquid Medicines
Zdroje
1. Motaung T. E., Petrovski K. R., Petzer I. M., Thekisoe O., Tsilo T. J. Importance of bovine mastitis in Africa. Anim. Health Res. Rev. 2017; 18(1), 58–69. doi: 10.1017/S1466252317000032
2. Hiitiö H., Vakkamäki J., Simojoki H., Autio T., Junnila J., Pelkonen S., Pyörälä S. Prevalence of subclinical mastitis in Finnish dairy cows: changes during recent decades and impact of cow and herd factors. Acta Vet. Scand. 2017; 59(1), 22. doi: 10.1186/s13028-017-0288-x
3. Kosenko M. V., Serhiyechko O. I., Kovalchuk L. M. Changes in the cellular composition of milk during subclinical mastitis in cows. Bulletin of Lviv. State. Acad. Vet. Medicine 2002; 4(5), 247–248.
4. Vasil M., Pecka-Kiełb E., Elečko J., Zachwieja A., Zawadzki W., Zigo F., Illek J., Farkaová Z. Effects of udder infections with Staphylococcus xylosus and Staphylococcus warneri on the composition and physicochemical changes in cows milk. Pol. J. Vet. Sci. 2016; 19(4), 841–848. doi: 10.1515/pjvs-2016-0105
5. WHO (2014). Antimicrobial resistance: global report on surveillance. Geneva World Health Organisation. Dostupné z: http://www.who.int/drugresistance/global_action_plan/en/
6. Harutyа G., Lototsky. Efficiency of different methods of treatment of cows suffering from subclinical mastitis. Vet. Med. of Ukraine 2004; 11, 31–33.
7. Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J. Antimicrob. Chemother. 2007; 59, 587–590. doi: 10.1093/jac/dkm006
8. Klasen H. J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000; 26(2), 131–138.
9. Seol J. W., Kang S. J., Park S. Y. Silver ion treatment of primary cultured bovine mammary gland epithelial cell (BMEC) damage from Staphylococcus aureus-derived alpha-toxin. Vet. Res. Commun. 2010; 34(1), 33–42. doi: 10.1007/s11259-009-9330-4
10. Polova Z. Investigation of antimicrobial activity of silver and copper citrate for development pharmaceuticals preparations. Actual questions of pharmaceutical and medical science and practice 2016; 1(20), 71–74.
11. Djokić.S. Synthesis and antimicrobial activity of silver citrate complexes. Bioinorg. Chem. Appl. 2008. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2590638/
12. Wu T., Wang C., Ding L., Shen Y., Cui H., Wang M., Wang H. Arginine relieves the inflammatory response and enhances the casein expression in bovine mammary epithelial cells induced by lipopolysaccharide. Mediators Inflamm. 2016. https://www.hindawi.com/journals/mi/2016/9618795/.
13. Satriano J. Arginine pathways and the inflammatory response: interregulation of nitric oxide and polyamines: review article. Amino Acids 2004; 26(4), 321–329.
14. Proksch E., de Bony R., Trapp S., Boudon S. Topical use of dexpanthenol: a 70th anniversary article. J. Dermatolog. Treat. 2017; 1–8.
15. Kramer A., Assadian O., Koburger-Janssen T. Antimicrobial efficacy of the combination of chlorhexidine digluconate and dexpanthenol. GMS Hyg. Infect. Control. 2011; 11.
16. European pharmacopoeia 8th ed. – Strasbourg: European Directorate for the Quality of Medicines & Health Care, 2013.
17. Amadeus P. Z. Stevenson, Duani Blanco Bea, Sergi Civit, Sonia Antoranz Contera, Alberto Iglesias Cerveto, Sonia Trigueros. Three strategies to stabilise nearly monodispersed silver nanoparticles in aqueous solution. Nanoscale Res. Lett. 2012; 7(1): 151.
18. Almakayeva L. G. Development of the composition and standardization of the technology of parental and liquid oral medicinal products on the basis of amino acids. State enterprise “State scientific center for medicinal products” Kharkiv, 2008.
19. The chemistry of milk. Dairy processing handbook. http://dairyprocessinghandbook.com/chapter/chemistry-milk.
20. Vijay Kumar Thakur, Manju Kumari Thakur. Нandbook of polymers for pharmaceutical technologies. In: Volum 1, Structure and Chemistry. New Jersey: 2015.
21. Veeran Gowda Kadajj, Guru V. Betageri. Water soluble polymers for pharmaceutical applications. Polymers 2011; 3, 1972–2009; doi: 10.3390/polym3041972
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2017 Číslo 5- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Active substances from marine organisms in clinical trials and practice
- Herbs for increasing breast-milk production
- Fatty acid composition of lipids of Iris sibirica
- Development of the composition of intramammary combined preparation based on silver citrate for veterinary use
- Idiopathic thrombocytopenia refractery to therapy of cyclosporine A in clinical practice – case report
- 36. technologické dni* Nové trendy v oblasti výskumu a vývoja liekov Inovácie v oblasti zdravotníckych pomôcok - Štrbské Pleso, 25.–27. október 2017
- 60 rokov Katedry farmaceutickej chémie FaF UK v Bratislave
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Herbs for increasing breast-milk production
- Idiopathic thrombocytopenia refractery to therapy of cyclosporine A in clinical practice – case report
- 36. technologické dni* Nové trendy v oblasti výskumu a vývoja liekov Inovácie v oblasti zdravotníckych pomôcok - Štrbské Pleso, 25.–27. október 2017
- Active substances from marine organisms in clinical trials and practice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



