-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUse of plant extracts as an efficient alternative therapy of respiratory tract infections*
Použití rostlinných extraktů jako efektivní alternativní terapie zánětů cest dýchacích
Léčivé rostliny jsou s výhodou používány v terapii onemocnění cest dýchacích. Katary horních cest dýchacích patří mezi nemoci spojené se sezónním snížením imunity, proto je často používáno rostlinných drog s nespecifickým imunomodulačním účinkem. Mezi takové rostliny patří např. echinacea (Echinacea purpurea) a americký ženšen (Panax quinquefolius). Ve spojení s antibakteriálně a antisepticky účinnými léčivými rostlinami, jako jsou tymián (Thymus vulgaris) a pelargonium (Pelargonium sidoides) mohou představovat účinnou pomoc při onemocněních cest dýchacích, zkracovat trvání nemoci a snižovat nutnost antibiotické terapie. Prezentovaný text shrnuje základní informace o těchto rostlinách, jejich obsahových látkách, mechanismech účinku a klinických zkouškách potvrzujících efekt a monitorujících případné nežádoucí účinky.
Klíčová slova:
Echinacea purpurea • Panax quinquefolius • Pelargonium sidoides • Thymus vulgaris • katary horních cest dýchacích • imunita
Authors: Karel Šmejkal; Veronika Rjašková
Authors place of work: University of Veterinary and Pharmaceutical Sciences Brno, Faculty of Pharmacy, Department of Natural Drugs
Published in the journal: Čes. slov. Farm., 2016; 65, 139-160
Category: Přehledy a odborná sdělení
Summary
Medicinal plants are advantageously used in the treatment of respiratory tract diseases. Upper respiratory tract catarrh is one of the diseases associated with seasonal weakening of immunity, and therefore, plant drugs with a non-specific immunomodulation effect are often used. Such plants include, but are not limited to, Echinacea (Echinacea purpurea) and American ginseng (Panax quinquefolius). In combination with medicinal plants having antibacterial and antiseptic effects, such as thyme (Thymus vulgaris) and pelargonium (Pelargonium sidoides), they can constitute efficient help in the treatment of respiratory tract diseases, shorten the duration of the disease and reduce the need of antibiotic therapy. The text presented summarizes the basic information about these plants, their ingredients, mechanisms of action and clinical tests confirming their effect and monitoring eventual adverse effects.
Key words:
Echinacea purpurea • Panax quinquefolius • Pelargonium sidoides • Thymus vulgaris • upper respiratory tract catarrh • immunityIntroduction
Medicinal plants are commonly used for the therapy of various diseases. People have been appealing to nature for curing diseases since ancient times and the use of medicinal plants has been somewhat instinctive and based on experience. Over the course of history, medical procedures including medicinal plants have been created, developing from empirical procedure, representing in fact the only possible treatment of individual diseases before the discovery of chemical medicinal products. The first documented reference to plant preparations and their use is a record on Sumerian clay tablets from the period of 4000 B.C. Another valuable source of information about the use of plants was Ancient China, India and the medicine of Ancient Greece and Rome. This mainly includes the works of the physician Hippocrates (Corpus Hippocraticum, 5th century B.C.) and Dioscorides’ De Materia Medica (1st century B.C.) where the author describes over 600 plant species and their medicinal use. Later studies showed that traditional European “materia medica” had been based on Dioscorides’ tradition that lasted for more than 19 centuries with only slight variations. The continuous development of science and engineering was associated with the development of synthetic medicinal products and plant therapy was pushed aside. However, plants have recently come into the centre of interest as a potential source of new medicinal products. Mainly in connection with the possibility to obtain scientific confirmation of the therapeutic value of medicinal plant products, their popularity is increasing and they are often used as an effective alternative to standard therapy.
Medicinal plants, plant extracts or, as the case may be, isolated substances can be successfully combined to achieve a proper therapeutic effect and to reduce the occurrence of eventual adverse effects. There are a number of diseases for which such a combination is of great advantage, as it covers a number of symptoms. A typical example can be the use of plant preparations in the therapy of colds, viral infections and slight bacterial diseases associated with coughs, colds and high temperature (URT catarrh = upper respiratory tract catarrh). Bronchitis (actually tracheobronchitis) is a form of inflammatory damage of the respiratory tract, in particular, at child age. It is caused by viruses in about 50–90% (adenoviruses, echoviruses, rhinoviruses); other causal agents mainly include Mycoplasma pneumoniae and Chlamydia pneumoniae. The percentage of C. pneumoniae in the formation of bronchitis, including acute exacerbation, ranges from 5 to 10% in the case of children. Haemophilus influenzae, Streptococcus pyogenes (β-haemolytic streptococcus), Staphyloccocus aureus and Moraxella catarhalis are proven in a smaller extent, and bacterial infection often sets on viral infection, thus causing exacerbation of diseases. Bronchitis complications include otitis, sinusitis and bronchopneumonia1, 2).
A combination of medicinal plants with an antimicrobial and antivirotic effect with an expectorant effect and effect on immune system function enhancement can thus be highly beneficial for the patient and enhance his recovery1, 2). There are a lot of plants affecting immune system which can be used for upper respiratory tract catarrh therapy. Their ingredients are often known and their activity has been thoroughly examined both in vitro and in vivo3–6). Extracts of some of these plants have also been subjected to clinical testing confirming their action and monitoring their safety. The following literature summary deals with some selected plants used in the therapy of upper respiratory tract catarrh, ingredients known for their action or considered active, action mechanism or other clinical studies.
Echinacea purpurea
Echinacea purpurea (L.) Moench (Asteraceae) (Purple coneflower) is an important plant, both ornamental and medicinal. Products from the purple coneflower are among the most often used and best sold not only in the USA, but also in European countries (Fig. 1). The plant comes from the Midwest of the United States of America and was brought to Europe as an ornamental plant (it tolerates dry, sunny and very heavy ground), later started to be grown as a medicinal one7).
Fig. 1 E. purpurea (by Ed (Photography)) 
E. purpurea is a relatively common representative of the Asteraceae family. It is perennial, growing to the height of approximately 60 cm, with a ligneous rhizome8).
There are three Echinacea species usually used for therapeutic purposes – E. purpurea, E. angustifolia and E. pallida. Both aerial (flowers) and underground (root) parts are used for therapeutic purposes. The European Medicines agency (EMA) evaluated E. purpurea9), E. angustifolia10) and E. pallida11) roots in area of traditional use as herbal products for supportive treatment of common cold, next evaluated material was E. purpurae herba, with well-established use status as herbal medicinal product for the short-term prevention and treatment of common cold12). Traditional use is based on the knowledge of North American Indians who used it to treat various infections and coughs associated with cold, as well as against snakebites. Indians from the Great Plains used it for curing toothache, coughs, colds and sore throats. Choctaw Indians used the blossom for curing coughs and gastrointestinal diseases. Delaware Indians used an infusion from the root for gonorrhoea therapy13).
Another, rather more up-to-date use consists in the stimulation of the immune system in the therapy of respiratory infections and inflammatory diseases, as well as malign tumours. Echinacea stimulates the immune system by affecting non-specific cellular and humoral immune response and affects the complement system8, 13).
Ingredients
Ingredients of E. purpurea are relatively well explored, as well as their distribution in various parts of the plant14). The root of E. purpurea is rich in alcamides, caffeic acid derivates, polysaccharides and glycoproteins. We also find oil and a small amount of polyynes, as well as traces of pyrrolizidine alkaloids. The entire complex of ingredients with significantly varying bio-availability needs to be considered bioactive, because alcamides, for instance, have been evaluated as bio-available, whereas availability of the caffeic acid derivates is described as possible insufficient for a clinical effect7, 15). The main representatives of Echinacea compounds are displayed in Figure 2.
Fig. 2. Main Echinacea compounds 
Phenolic compounds
There have been a number of phenolic compounds isolated from various parts of Echinacea. The major portion is formed by hydroxycinnamic acid derivates, such as cichoric acid. The cichoric acid is a dicaffeoylquinic acid. Its content in Echinacea roots can exceed 2%16). Echinacea further contains other, relatively common caffeic acid derivates, such as chlorogenic acid (5-caffeoylquinic) or cynarine (1,3-dicaffeoylquinic acid) that is, however, described only for the root of E. angustifolia. Other phenolic compounds of Echinacea also include phenylpropanoid glycosides, the major ones of which include acteoside (verbascoside) and echinacoside7).
Alcamides
More than 15 alcamide derivates can be isolated from Echinacea ethanolic extract. These substances are currently considered the main lipophilic bioactive compounds of Echinacea17). As far as their structure is concerned, these substances belong to the isobutylamides of fatty acids (unsaturated fatty acids and acetylenes). Isobutylamides from the roots of E. purpurea mainly contain 2,4-dien, E. angustifolia mostly 2-monoene unit. Content of alcamides in analyzed and used material varies based on type and also based on organ. Commercial samples of E. purpurea root can contain 0.1–1.2% of alcamides, the content of alcamides in E. purpurea root, however, often reaches about 0.01–0.04%. E. pallida more or less does not contain alcamides. Alcamides have been identified in both the above-ground part and in the roots, whereas the content in the roots is higher14).
Polysaccharides
Polysaccharides are, along with glycoproteins, another group of ingredients. PS1 polysaccharides (methylglucuronoarabinoxylan 35 kD), PS2 (acidic rhamnoarabinogalactan 450 kD) and xyloglucan (79 kD) were obtained from the above-ground part of E. purpurea. Juice obtained by pressing the above-ground parts of E. purpurea contains a mixture of heterogeneous polysaccharides (< 10 kD), inulin fraction (6 kd) and strongly acidic branched polysaccharide of arabinogalactan type (70 kD). Another study describes isolation of galactan-protein containing 83% of polysaccharide (galacto-arabinan), uronic acid and protein derived mainly from serine, alanine and hydroxyproline. The polysaccharide content can again be affected by the pressing technique; higher humidity during the drying process reduces the content in the case of both E. angustifolia and E. pallida14).
Action mechanism
Echinacea is known and explored the best in the area of immune system modulation. There are a number of in vitro studies describing stimulation of various cell types of the immune system, such as macrophages, various monocytes and natural killer cells. It is more difficult to transfer such effects to humans. The problem with identifying active substances and action mechanisms consists in the complexity of the compound of the ingredients of Echinacea, as well as in method of extract preparation and last but not least in the testing model applied. Immunity is a highly complicated and balanced system of interacting elements. Development of inflammatory reaction appears to be the centre of symptoms of infectious rhinosinusitis (common cold) and pharyngitis (sore and inflamed throat) when Echinacea is primarily used. Inflammation is represented here by tissue swelling associated with vasodilatation and leakage of liquid from capillary tubes and tissue infiltration by leucocytes. These phenomena are primarily initiated by inflammation mediators secreted by macrophages: Il-1, Il-6, Il-8, Il-12, TNF-α and leucotrienes, as well as ROS and NOS. C-reactive protein also appears in pathogenesis of upper respiratory tract infection. Echinacea ingredients activate macrophages and affect in vitro cytokine secretion; however, the mechanism of their in vivo action is not entirely clear. The “non-specific immunity stimulation” appears with Echinacea, as with other natural products. As massive and wide stimulation of immune processes may result in symptomatic expression of inflammation, selective activation of mechanisms of defence against viruses associated with inflammatory process moderation is theoretically ideal. Successful immunomodulation by Echinacea should result in moderation of the severity and duration of symptoms and also in higher elimination of inciting causes (viruses, bacteria). Such a product with a multiphase effect is identified as an adaptogen by some authors18).
As mentioned above, there are a number of studies trying to explain the immunomodulation effects of complex extracts from Echinacea, whether in vitro or using animal models, and they have been repeatedly summarized. For illustration, we have mentioned an interesting study from the year 2000, conducted on mouse macrophages and ex vivo on human macrophages19). The viability of human peripheral polymorphonuclears was measured with the interference from various products obtained from Echinacea, and anti-inflammatory and anti-oxidation activity was also measured. Measurement was expanded with extract digestion simulation (incubation of tested material with gastric juice). Different extract concentrations provided the dose-dependent effects – higher secretion of TNF-α was observed the most after 30 hours, whereas higher production of other cytokines was observed after several days. What was interesting were the different results with extracts prepared from various powder drugs and with extracts standardized to the content of phenolic acids and echinacoside or fresh juice obtained by pressing, which did not provide immunostimulation, but rather showed anti-inflammatory and anti-oxidation effects. Another interesting study showed that cells infected by virus after exposure to action from Echinacea extract to a greater extent present virus antigen for immunocompetent cells20).
Only little understanding is present concerning results obtained from testing of lipophilic or hydrophilic Echinacea extracts, because both water-soluble and ethanol - and chloroform-soluble extracts have proven stimulation of phagocytic functions21–23). So the question is, what substances and to what extent are actually responsible for the effect. Fraction of extracts rich in glycoproteins or polysaccharides (hydrophilic) stimulated production of cytokines and stimulated activity of macrophages; however, in vivo transmission of this effect is complicated, because some authors refer to actually null absorption from the gastrointestinal tract, also referring to potentially higher activity of alkylamides present in lipophilic extracts. Alcamides appear as substances well-available after p. o. application; they are present in the blood in relevant concentrations after application. Thanks to the structural similarity to anandamide, endogenous ligand of cannabinoid receptors, they prove interaction with cannabinoid receptors of type 2, which is associated with the immune system (not with psychoactivity) and such interaction can at least partly explain the immunomodulation effect of Echinacea24, 25). As experiments have confirmed the effect of polysaccharides also on animal models, the discussion in connection with polysaccharides includes the possible interaction with immune system cells of the GIT epithelium which subsequently affects endocrine, neurological and immunological functions in a manner not fully understood.
Clinical studies, adverse effects
Clinical studies aimed at evaluating the effect of Echinacea preparations on the immune system have been conducted since the 1980s. There have been a number conducted at various quality levels and many prove that Echinacea stimulates the immune system. Studies conducted with humans have, for instance, proven higher production of inflammatory cytokines activated by macrophages and polymorphonuclears. However, it is more difficult to determine whether these effects are associated with a clinical benefit for the patient (reduced occurrence, severity and duration of disease). For instance, ROS produced by macrophages and polymorphonuclears are important for destroying the cells infected by viruses; however, oxidative processes are also associated with the damage of cells and organs. In the case of upper respiratory tract infections, we can also discuss that reduced activity of macrophages can be an advantage before stimulation, because stimulation can increase the occurrence of symptoms. However, on the other hand, stimulation of the immune system can strengthen antivirotic activity resulting in a reduction of initial replication of viruses, and therefore, in a reduction of the symptoms of infection. However, clinical comparison of Echinacea preparations with anti-inflammatory substance agents has not been conducted in a satisfactory manner to date.
Tab. 1. Summary of selected clinical studies assessing the effect of purple coneflower on upper respiratory tract (URT) catarrh 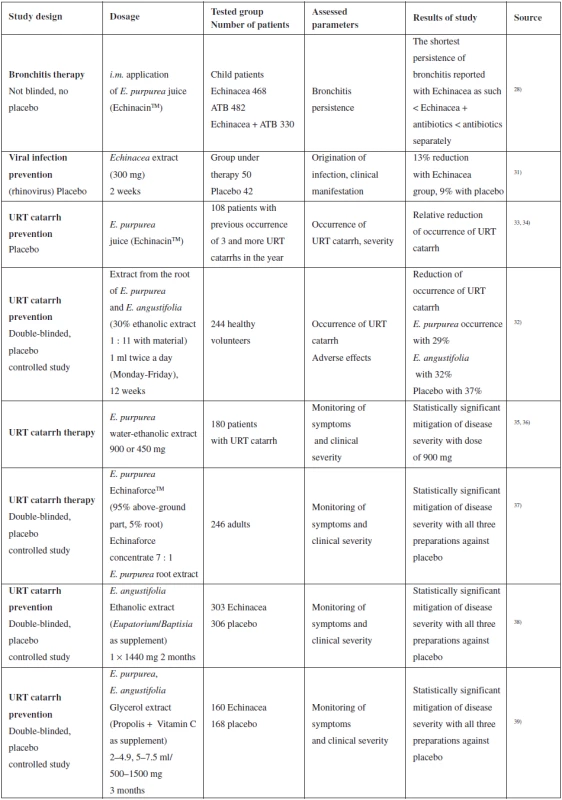
An extensive review summarizing information about the curative properties of Echinacea was published in 200318). A significant part of this overview is dedicated to clinical studies of Echinacea preparations. Other works describing meta-analysis of several clinical studies were published in 201426) and 201527). Therefore, the next part of the text states the most significant and the most recent clinical studies evaluating the use of Echinacea in the case of bronchitis, colds, prevention and therapy of upper respiratory tract infections.
An extensive retrospective study was published by Baetgen in 1984, another in 1988. They summarize intramuscular administration of E. purpurea juice (EchinacinTM) separately or in combination with antibiotics to 1280 children. The shortest persistence of bronchitis is reported with separate Echinacea < Echinacea + antibiotic < antibiotic separately. However, the study was not randomized or double-blinded and was retrospective, so its results are hard to interpret. A positive fact is that this study does not report any severe adverse effects (even after intramuscular application)28–30).
Insignificant mitigation of the risk and manifestation of rhinoviral infection with preventive administration of non-specified Echinacea extract was described in a study from the year 2000 where, however, extrapolation of the data of 50 patients under the study into a more extensive sample of the population may result in a clinically significant effect18, 31). Unfortunately, the study does not provide any supporting data related to origin, preparation or phytochemical analysis of the extract. Reduced occurrence of catarrh of the upper respiratory tract is also described in another study; however, it is complicated to interpret the results for a relatively small set of patients28).
The results of a number of studies prove that the Echinacea preparations are safe and well tolerated18). Although rare cases of allergic reaction are reported, there have not been any really severe cases or death reported. The main adverse effects reported include unpleasant flavour, nausea, vomiting, sore throat, stomach ache and diarrhoea. A convincing argument confirming the safety of using Echinacea preparations is the fact that there have been less than 100 severe adverse effects reported for an estimated 10 million users (commercially available phyto-preparation).
Use of Echinacea preparations does not even have major contraindications, only chronic and progressive diseases related to immunity, such as tuberculosis and multiple sclerosis are limiting. In this case, the risk is associated with the theoretical exacerbation of such diseases. However, more studies and evidence will be required for full confirmation of safety. Use of Echinacea preparations during pregnancy has not been sufficiently documented; only the limited study by Gallo et al. concluded that use of Echinacea during pregnancy during organogenesis is not associated with a higher risk of major malformations42).
Panax quinquefolius
The ginseng root has been used for thousands of years for its curative actions. Asian ginseng (Panax ginseng C. A. Mey.) and American ginseng (P. quinquefolius L.) (Araliaceae) are the two most well-known species used. American ginseng (Fig. 3) became highly popular in western countries when its pharmacological activity turned out to include effects similar to P. ginseng. The spectrum of use is broad, including action on the cardiovascular system, action on the CNS, anticancer actions, and last but not least, immunomodulation.
Fig. 3. P. quinquefolius (by Jacob Bigelow (1786–1879) 
P. quinquefolius is a perennial. American ginseng naturally ranges in the temperate zone of woodlands in the eastern part of North America, e.g. in the southern part of Quebec, Minnesota and in the north of Wisconsin. The greatest population of wild ginseng is found in mountain regions, on north - and east-facing slopes, in shielded and typically clay soil. The market offers three ginseng species: plantation-cultivated, wild and cultivated in a method simulating the wild. Naturally, wild ginseng is considered to have the highest quality and is the most expensive, like the P. ginseng. American ginseng is currently grown also in Asian countries, e.g. in China43, 44).
Ingredients
Growing conditions affect the quantity of the ginseng ingredients. Factors, such as soil character, fertilization, temperature, rainfall and distance between the individual plants, significantly affect the quality of the drug. Another major factor is age of the harvested material. The saponin content, which is considered the main active substance along with polysaccharides, fluctuates based on the age of the material, in the root from 2.6% of four-year-old plants to 5.89% of ten-year-old plants, and therefore, cultivation less than 4 years is considered disadvantageous for harvesting; on the other hand, the concentration will most likely not change much after year 5. The total content of saponins is also high in the floral buds (12–16%), in the leaves (10–16%) and in the fruit (10–12%).
There have been more than 80 saponins mentioned above isolated from various ginseng species. We can divide them into several sub-groups based on structure: protopanaxadiols (such as protopanaxadiols Ra1-Ra3, Rb1, Rh2, Rb3 Rc, Rd, 20(S)-Rg3, Rb2), ginsenosides (such as (Q)-R1, Rs1, Rs2), malonyl-substituted derivates [(MA)-Rb1, MA-Rb2, MA-Rc, MA-Rd, Rg3], protopanaxatriols (such as Re, Rf, Rg1, Rg2, Rh1, 20-glucopyranosyl (Glc)-Rf, r-R1, 20R-Rg2, 20R-Rh1), oleanolic acid derivates and ocotillol type derivates43, 44) The main representatives of ginseng saponin aglycones are displayed in Figure 4.
Fig. 4. Main ginseng compounds 
Besides saponins, the presence of polysaccharides and glycoproteins45) in ginseng is also described. They are described mainly in P. ginseng, their presence in P. quinquefolius is known, but the information is little46). Besides polysaccharides (GH-1) (Mr 4500) and GH-2 (Mr 5300) more than 20 panaxans have been identified (A-U, with Mr 2500–1300000). Glycopeptides P. ginseng P-21 (Mr 6000) and glycoproteins PA and PB have been isolated from P. ginseng. The presence of a significant amount of glycopeptides and polysaccharides in P. quinquefolius can be predicted from the amount of water-soluble agents obtained by extraction and a significant amount of proteins. An interesting fact is that the amount of polysaccharides in grown ginseng is not, unlike saponins, dependent on the size or age of the material. Other bioactive ingredients of American ginseng include polyacetylenes (such as panaxynol), and arginine derivates.
Use of P. quinquefolius is very popular at the present time. Similarly to P. ginseng, both the drug and extracts are used mainly as a general tonic and adaptogene for improving organism resistance to external stressing conditions, for improving physical performance, improving vitality and against “ageing symptoms”. These effects are probably mediated by action on the hypothalamus-hypophysis-adrenal gland axis or by anti-oxidation action and increased utilization of oxygen and glucose by tissues. Ginseng research is also being intensively conducted in the area of cardiovascular tract diseases, diabetes, CNS diseases and cancer43, 44).
In this summary we will focus on the action of ginseng on the immune system. The action of ginseng on the immune system is not associated only with cold-related diseases, but also with cancer. According to information obtained from literature, the immune system can be affected by at least two groups of substance: saponins and polysaccharides.
Metabolisms, action mechanism
It is well known that as ingredients, plant preparations contain a number of compounds with a β-glycosidic bond. Following oral application, such ingredients must come into contact with the stomach environment (low pH), with the small intestine environment (pH 7–8, presence of pancreatic enzymes), as well as with the colon environment (presence of bacterial colonization). All parts of the GIT may participate in the metabolism of such ingredients, and therefore, these ingredients may function as pro-drugs. Metabolism of ginseng saponins has been explored only to a certain extent and only partly on humans. As an example, we can state ginsenoside Rb2. It is known that there are differences between products obtained by application of ginsenoside Rb2 directly in the stomach of rats and products obtained in acidic conditions by hydrolase of HCl47). Ginsenoside Rb2 decomposes in the acidic environment of the stomach48, 49). The effect of human intestinal microflora on the metabolism of ginsenosides has been explored. Oligosaccharides forming a sugar component of these glycosides attached to C-3 or C-20 are step-by-step removed from terminal sugar. Protopanaxadiols (ginsenosides Rb1, Rb2, Rc, and Rd) are metabolized to the final compound K50). Compound K has also proved a number of biological effects and is considered to be an active metabolite51, 52). Besides, esterification of this metabolite by fatty acids in the liver and its partial accumulation has been identified in metabolism continuation. Esterification of bacterial metabolites of ginsenosides by fatty acids also probably potentiates their biological activity, as proven, for instance, in studies by Hasegawa and coll.53, 54). Further explanation of the effects of ginseng will, therefore, require metabolic studies evaluating both bio-availability and metabolism, as well as studies evaluating biological activity of the metabolites described.
As we can see further, the action of ginseng is complex; results vary based on the model used to conduct the experiments. Another influence is whether a compound of saponins or individual isolated substances were tested, or, as the case may be, what ginseng species the tested extract has been made from. The spectrum of ingredients present in a non-standardized extract can be highly variable, thus the effect also varies. As an example, we can state the level of glycaemia reduction after administration of the ginseng preparation55), as well as observable good repeatability of effect56).
The effects of ginseng on the immune system are summarized in detail in the survey article by Kang and Min57). There are different opinions on the mechanism of the action of ginseng on the immune system at present, but a number of studies associate their effect with influence on balance of Th1 and Th2 cells. CD4+ population of T lymphocytes can be classified at Th1 (primarily including macrophages and destruction of intracellular pathogens) and Th2 (primarily including B cells and destruction of extracellular pathogens). As implied from the action on the level of antibodies, cytokines, amount of splenocytes and CD4+ T cells, ginsenosides probably strengthen both aspects of immunity in balance. Studies have been conducted both in vitro and using animal models and on humans. Ginsenosides stimulate production of antibodies (IgG2a and IgG3) connected with Th1 and Th2 ((IgG1 and IgG2b)58–62). In rats which had been administered ginsenosides, a higher level of Th1 connected cytokines was found, such as IFN-γ, IL-2, TNF-α, and Th2 connected cytokines (Il-4, Il-5, Il-10)58, 59). Activity of CD4+ T-cells was stimulated, Th1 CD4+ produced more Il-2 and IFN-γ, which was verified at both the gene expression level and the protein level; similarly, Th2 CD4+ T cells produced more Il-4 and Il-1063, 64). The administration of ginsenosides stimulated CD69 expression of Th1 CD4+ T cells, their proliferation and increased their total share in lymphocytes63, 65). Splenocytes from rats obtained after the administration of ginsenosides reported higher production of Th1 connected Il-2 and INF-γ (at the level of both mRNA and protein), higher production of TNF-α (measured as a protein), and higher production of Th2 connected Il-4 and Il-10 (mRNA and protein)58–62, 66).
It was further proven that ginsenosides modulate activity of NK cells, stimulate Il-1β production in peritoneal macrophages, phagocytosis and Il-12 production in stimulated dendritic cells65, 67, 68). Reduction of cytokine expression via the PPAR path (peroxisome proliferator activated receptor) in dendritic cells was also observed in native cells of the immune system69) or by inhibition of transcription factor function (such as NF-κκB) or mitogen-associated kinases in macrophages70).
Polysaccharides46, 71) can also play an important role in the immunomodulation effect. Unfortunately, their role has not been explored sufficiently to date and the mechanism of their action is unclear. Ginsenan PA (P. ginseng polysaccharide) activated in vitro phagocytosis72). Acidic polysaccharides of P. ginseng affected production of cytokines in macrophages73–75). Other studies also prove that polysaccharides of P. quinquefolius increase production of cytokines including TNF, interleukins (Il-6, Il-12), IFN-γ, also GM-CSF (granulocytes-macrophages colony-stimulating factor) and NO production in vitro in macrophages76, 77). These phenomena are associated with up-regulation of surface markers in dendritic cells and up-regulation of iNOS and COX-2 in macrophages. On the other hand, some studies indicate instead a reduction in production of pro-inflammatory cytokines in macrophages isolated from rats or mice78–80). They compared, for instance, in vivo extracts of P. quinquefolius rich in polysaccharides and in saponins and the results of such comparison proved that the extract rich in polysaccharides (COLD-fX) modified immune response in rats (they monitored, for instance, the number of lymphocytes, cytokine levels, and others) with higher efficiency than extract rich in saponins and that the effect was associated with intestinal immunity81).
Use of ginseng in the area of upper respiratory tract catarrh therapy can be explained even by direct interaction of the ingredients of ginseng with bacteria and viruses. Ginseng polysaccharides reduce bacteria adhesion to cells and block initiation of bacterial infection82, 83). Ginseng polyacetylenes have a direct antimicrobial effect84). The antiviral effect of Rg1 and PPT ginsenosides is associated with protection of endothelial cells against inflammation and apoptosis induced by in vitro H5N1 virus85). Red ginseng extract increased the rate of survival of mice infected by influenza A virus (H1N1) after oral application and the results were also confirmed at the cell level86). It is possible and most likely that the antiviral activity of ginseng is related to its interaction with the immune system, but activity of T cells and NK cells, production of interferon and neutralization antibodies have been studied only to an insufficient extent87). Also, the effect of ginsenoside Re against infection C. albicans on a mouse model seemed to indicated stimulation of the immune system (effect on CD4+ T-cellar immunity) rather than on a direct antifungal effect88).
Results of some other experiments refer to the possibility of using ginseng extracts, or ginsenosides, as adjuvants in the administration of antibodies and vaccination. Results obtained in vitro or on mice89, 90) have already been further confirmed in a clinical study91). Final confirmation and full clarification of the mechanism of the action will require further studies to be conducted.
The complexity of this problem is further enhanced by the structural variability of the individual saponins; different results have been observed in the same model, for instance, for ginsenosides Rb1, Rg1 and notoginsenoside R1 where all three ingredients reported an anti-inflammatory effect (reduced amount of adhered leukocytes, inhibition of mast cells degranulation, and others), however, at various levels and at various intensity92), or the above-mentioned possibility of active metabolite formation93). Another problem is obtaining different results in in vivo and in vitro studies94).
Clinical studies, adverse effects
For the purposes of evaluating the action of ginseng on the immune system, a number of studies have been conducted with both trained and untrained persons, observing a number of parameters monitoring the general condition of the tested person, as well as values associated with immune system functions. As already mentioned above, the action on the immune system is associated with saponins and with polysaccharides. Table 2 summarizes some studies associated with immune system evaluation and based on physical stimulation of the tested person95).
Thanks to the popularity of ginseng, a number of clinical studies have been conducted for evaluation of the clinical effect of ginseng in upper respiratory tract therapy using both extracts rich in polysaccharides and saponin-containing extracts.
Ginseng extracts (100 mg of aqueous extract and 100 mg of standardized extract of P. ginseng) were tested on healthy volunteers with the aim of evaluating the immune response parameters, such as polymorphonuclear chemotaxis, phagocytosis index, activity of NK cells, and others. 8 weeks of administration 2 ⋅ 100 mg of both extracts had a significant immunomodulation effect as compared to the placebo104). Pilot studies proved the ginseng effect in patients suffering from acute attacks of chronic bronchitis where it was proven that administration of G115 extract significantly accelerates bacterial clearance (non-blinded study)105). Interesting results were also provided by the study evaluating efficiency of Ginsana G115 extract on the occurrence of influenza disease in vaccinated patients, indicating reduced influenza occurrence and immune system stimulation91).
Tab. 2. Summary of selected clinical studies assessing the effect of ginseng on performance and the immune system 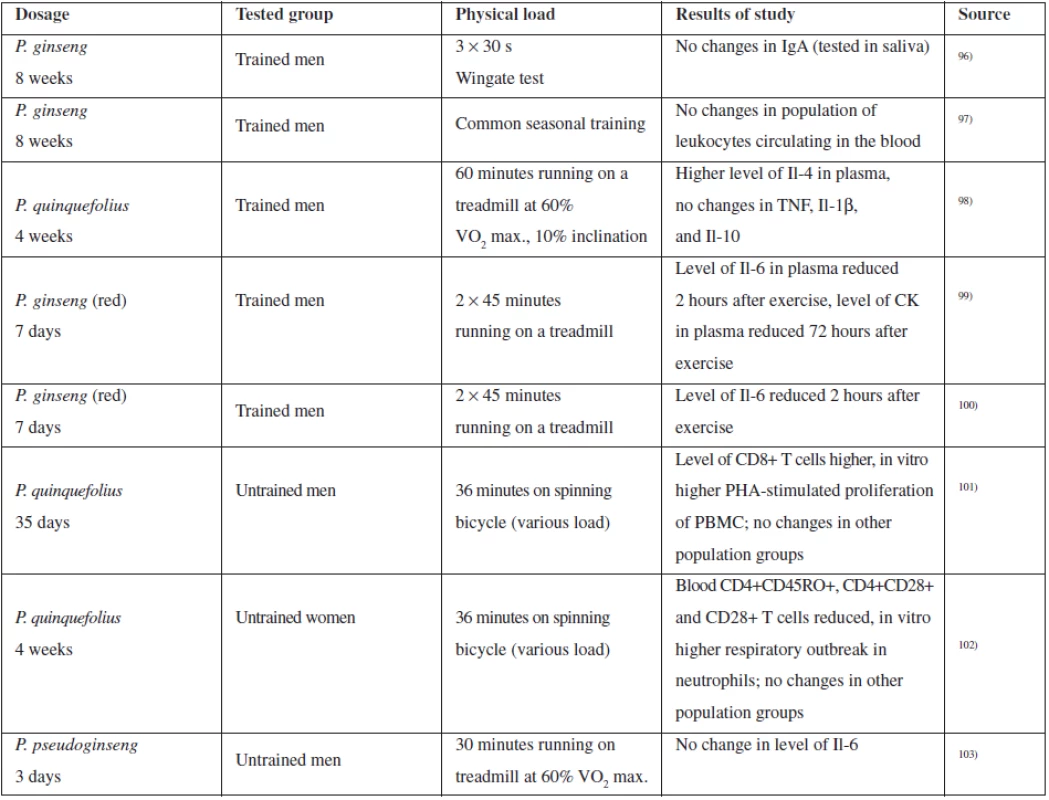
A relevant series of clinical tests was conducted mainly with COLD-fX extract rich in polysaccharides, although there may be a problem with the test validity due to the study compensation by the producer106–108). Other tests were conducted with Ginsana G115 extract (standardized extract from P. ginseng root) without any connection with the producer. 3 tests were conducted with the aim of identifying the effectiveness of the therapy, in particular in connection with the prevention of the occurrence of respiratory disease in older patients, two studies included middle-aged patients. Table 3 provides an overview of the studies, extract types, dosage and results.
The results of the study indicate that the ability of ginseng to reduce the occurrence and severity of acute respiratory diseases requires further verification and that the effect is observed with reduced duration of acute respiratory diseases in preventive application for 8–16 weeks. As continuation of the studies with COLD-fX preparation, another double-blinded study evaluating long-term administration of the extract (4 months) and effect on viral respiratory diseases108) was conducted. Disease distribution was similar between the placebo group and the group administered extract; however, the course of diseases was slighter with the group administered ginseng. Marker monitoring resulted in observation that administration of COLD-fX increased the proportional quantity of Th cells and NK cells and increased the IgA level in plasma108). Another clinical study evaluating P. quinquefolius standardized extract (80% of polysaccharides) on a group of paediatric patients did not prove an effect (double-blinded randomized study, 75 persons); however, the study did not report the occurrence of any adverse effect109).
Adverse effects: Gastrointestinal intolerance in all studies, no severe adverse effect, DM2 development probably without causal connection. More tests, mainly of long-term administration, required with respect to the relatively low numbers of patients in the studies. Insomnia occurred rarely91).
Pelargonium sidoides
Geranium Pelargonium sidoides DC. (Geraniaceae, syn. Pelargonium sidaefolium Thunb.), also known by the traditional names of Umckaloabo, Uvendle, Kalwerbossie is a perennial plant originating from South Africa, where it mainly grows in the Eastern Cape Province and Lesotho Highlands111). The roots of this geranium are succulent and red, the leaves have a round cordial shape, featuring a long footstalk and glandular trichomes. The flowers are quinate, dark red to black coloured (Fig. 5)112, 113). Several preparations have been created on the basis of P. sidoides, containing EPs® 7630 extract. The preparations available on the domestic market include, but are not limited to, Kaloba®, a preparation known abroad (in Germany) is, for instance, Umckaloabo®.
In the area from where it originates, P. sidoides is used by the locals for the treatment of various difficulties: aqueous extract from the roots and leaves is used to cure diarrhoea, gastritis and respiratory tract infections. It is also used to treat dysentery, colic, coughs, tuberculosis and gonorrhoea111, 114, 115). Following watering and processing into a brown paste, the powdered plant material is used as acne therapy cream111, 116).
Traditional use for veterinary purposes has also been noted: root brew as antihelminthics for calves111, 117), boiled leaves for protection of wounds against larvae or for prevention of horse diarrhoea111, 115). Extract made by watering of roots is used for dysentery treatment of cattle111, 116).
The current application of P. sidoides extracts mainly focuses on the therapy of respiratory tract infections. The EMA evaluated the use of pelargonium root (Pelargonii radix) as traditional herbal medicinal product for the symptomatic treatment of common cold118). An interesting fact is that the scope of P. sidoides use has successfully expanded beyond the limits of common use in folk medicine. In upper respiratory tract infection therapy the currently mentioned areas include, for instance, acute bronchitis, asthma, sinusitis and tonsillopharyngitis. The clinical research which has mainly resulted in the formulation of EPs® 7630 (Umckaloabo®) product, whose effectiveness continues to be verified, has been basically organized and conducted according to this use.
Fig. 5. P. sidoides (Derek Ramsey (c) 2006 
Ingredients
Thanks to the described pharmacologic activity, research in the area of the phytochemistry of P. sidoides has been extensive; however, it is still not sure whether it has been possible to identify the ingredients actually responsible for the biological effects. The ingredients can be classified to a certain extent based on their presence in the above-ground and underground part, but also according to the method of extract preparation. Ethanol extract of P. sidoides root mostly contains oligomers and polymers of proanthocyanidins based on gallocatechin and epigallocatechin molecules. The biological activity of extracts is to a large extent also associated with the presence of highly oxidized coumarins (such as 7-hydroxy-5,6-dimethoxycoumarin, 6,8-dihydroxy-5,7 - -dimethoxycoumarin). Other remarkable molecules may include sulfoxycoumarins. Other present ingredients may also include phenolic acids, phenylpropanoids and benzopyrans111, 119). Phenolic acids, phenylpropanoids, coumarins (such as scopoletin, umckalin, frantin, magnolioside) and rather commonly appearing flavonoids (such as quercetin, vitexin, orientin, isovitexin, isoorientin)111, 119) have been found in the aerial part of P. sidoides. The main representatives of P. sidoides compounds are displayed in Figure 6.
Fig. 6. Selected P. sidoides content compounds 
Tab. 3. Summary of selected clinical studies of the effect of ginseng on upper respiratory tract catarrh<sup>110)</sup> 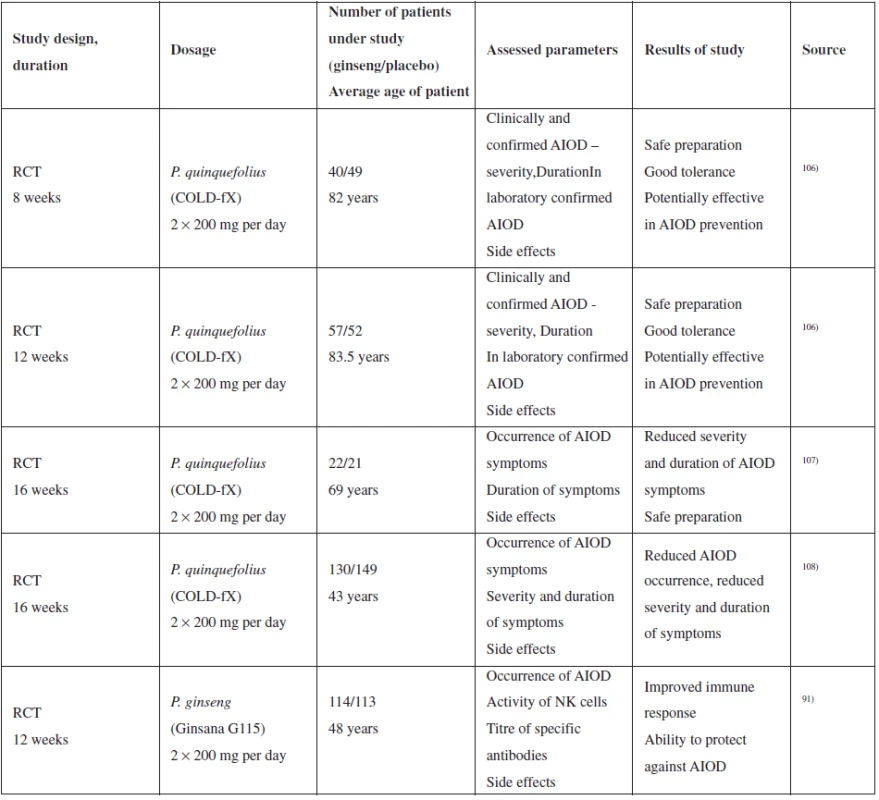
Action mechanism
As regards verification of the action mechanism and in vitro activity of P. sidoides ingredients and extracts, a number of anti-microbial activity tests by micro-dilution methods have been conducted, as well as tests of microorganism adhesive capacity, viral neuraminidases inhibition, and others. In vitro studies have proven antiviral, antibacterial and immunomodulation activity of extracts and individual selected ingredients111). As examples, we can mention the following experiments and activities: the above-mentioned P. sidoides extract EPs®7630 was tested for activity towards various virus types, identifying antiviral effect of various intensity against two influenza A viruses, RSV, human coronavirus HCo-229E, parainfluenza virus 3, coxsackie A9 and herpes simplex viruses111). It identified the most intensive activity towards H1N1 A/Luxembourg/46/2009 virus120, 121) and H3N2 virus121), which indicates potential in the treatment of upper respiratory tract infections. The antiviral effect is mainly accredited to proanthocyanidins and epigallocatechins present in the extract. Theisen et al. found that the most effective are oligo - and polymers, less effective are dimer and the least intensive antiviral effect is attributed to monomers of gallocatechin and epigallocatechin. The antiviral action mechanism consists in the inhibition of neuraminidase and virus bond to the membrane of the host cell111, 120). In the antibacterial activity tests, butanol extract of P. sidoides reported higher activity than its individual components, a possible and most likely explanation of such phenomena includes synergic action of the present complex of active substances111, 122). Based on not very promising MIC values, some authors conclude that the ingredients of P. sidoides have no direct influence on bacteria and they probably have a different mechanism of action. This may concern the influence of host-bacteria interaction and influence of phagocytosis. Studies conducted by Beil and Kilian and Conrad et al show that the ingredients of EPs®7630 extracts avoid adhesion of bacteria on the membrane of the host cell123, 124).
Immunomodulation activity described in P. sidoides extracts mainly consists in enhancement of the non-specific immune response. Gallic acid, umckalin and 6,8-dihydroxy-5,7-dimethoxycoumarin from P. sidoides induce NO production in macrophages. The induction rate depends on the EPs®7630 dose used to treat macrophages before125, 126). Phenolic substances, mainly gallic acid, are associated with the induction of TNF and interferon formation127–129).
In vivo studies conducted with the aim of clarifying the action mechanisms of P. sidoides focused on antiviral activity testing. Theisen and Muller proved the antiviral action of EPs®7630 on an animal model, however, only with inhalation thereof115). Upon inhalation, the EPs®7630 extract significantly increased the survival of mice infected by H1N1 A/Puerto Rico/8/34 virus. Application by stomach probe did not prove any antiviral effect. As the cause, the authors declare the low biological availability caused by degradation of oligomers of proanthocyanidins in the digestive tract to less effective monomers and dimer before they become absorbed. On the other hand, after inhalation, the complete spectrum of phenolic substances from EPs®7630 directly reaches the site of the respiratory infection120). However, as indicated by the above-mentioned summary, both in vitro and in vivo studies have not fully clarified the effect of P. sidoides; the mechanism of action remains unclear and the research, mainly in area of anti-infection effects, needs to be expanded.
Clinical studies, adverse effects
The fact that P. sidoides containing preparations is an attractive phytopreparation with therapeutic potential is demonstrated by a number of clinical studies dealing with evaluation of the effect on acute respiratory diseases of the upper respiratory tract. EPs®7630 effectiveness was evaluated with the following diseases: acute bronchitis (9 clinical tests in total), acute rhinosinusitis (1 clinical test), common cold (1 clinical test), chronic obstructive pulmonary disease (1 clinical test) and asthmatic attacks during viral infection (1 clinical test)111). Most studies were designed as randomized, double-blinded and placebo-controlled. Results were measured as a change in score of symptoms of the subject diseases. A summary of the selected clinical studies is provided in Table 4.
Tab. 4. Summary of selected clinical studies of the effect of P. sidoides on upper respiratory tract catarrh 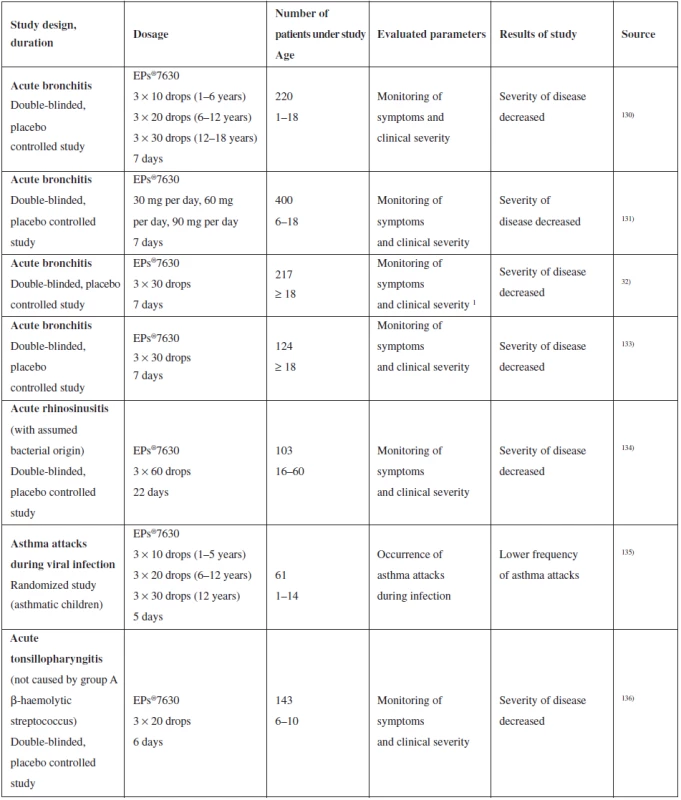
The results obtained indicate the possible effectiveness of phytomedicines on the basis of P. sidoides in the treatment of respiratory infections caused by bacteria or viruses111), on the other hand, meta-analysis published by Timmer et al. refers to the low quality of data obtained in clinical studies, as there were few studies conducted for each individual disease and more importantly, they were initiated and managed by the producer137). Promising results of in vitro tests indicate certain therapeutic potential of P. sidoides. Precise documentation and evaluation thereof requires more experiments to be conducted, both in vivo and those of a clinical character. They will lead not only to better clarification of the action mechanism, but also to specification of indication of phytomedicines made from P. sidoides.
As far as adverse effects are concerned, no toxic effect was observed in in vivo tests120). There were some adverse effects reported during clinical tests, such as gastrointestinal intolerance, nervous, respiratory and hearing system difficulties, exanthema, tracheitis, epistaxis and allergic skin reactions111). However, none of the adverse effects were reported as severe and were of a temporary character. Due to lack of data, P. sidoides must not be used during pregnancy and during lactation, and there are no interactions known from the current data. However, full evaluation requires further studies to be conducted.
Thymus vulgaris
Thymus vulgaris L. from the Lamiaceae family is a strongly aromatic subshrub, whose common name is thyme (Fig. 7). It is naturally spread mainly in the western part of South Europe (Spain, France, Italy)138). Traditional therapeutic use of T. vulgaris includes coughs and upper respiratory tract disease associated with reduced patency thereof (familiarly, blocked nose)139). The EMA evaluated T. vulgaris (T. zygis, resp.) herba for its traditional use as herbal medicinal product for treatment of productive cough associated with cold140). Other similar indications include upper respiratory tract catarrh and bronchitis symptoms141). Other indications not related to the respiratory tract include digestive problems, and therefore it is used as a stomachic142).
Fig. 7. T. vulgaris (By Henry Brisse) 
Ingredients
Thyme is a typical representative of plants of the Lamiaceae family which contain a relatively high amount of lipophilic oil stored in glands on the surface of the leaves and stem. 6 chemotypes of T. vulgaris are distinguished by oil composition, whereas only oil rich in thymol meets the definition required by the European Codex. The dried drug usually contains up to max 2.5% of oil (minimum required amount of 1.2% with min. 40% of thymol and carvacrol), the main components are thymol, carvacrol, p-cymene, γ-terpinene, borneol, linalool, β-myrcen and terpinen-4-ol. Some monoterpenes can appear as glycosides (such as p-cymene-9-ol)139, 142, 143).
Other thyme ingredients are flavonoids. They describe simple flavones (apigenin, luteolin) and their glycosides, as well as methylated flavones, such as thymonin. The content of flavonoids ranges up to 1%. Phenolic ingredients present in thyme also include caffeic acid and rosmarinic acid,. Some other ingredients present include triterpenes (ursolic and oleanolic acid derivates)139, 144). The selected representatives of thyme compounds are displayed in Figure 8.
Fig. 8. Selected representatives of thyme compounds 
Action mechanism
Antimicrobial activity of thyme extracts, oil and individual ingredients have been well and repeatedly confirmed in in vitro tests139, 145). They have observed not only action against common pathogens causing upper respiratory tract catarrh, but also against cariogenic and periodontopathogenic bacteria (mainly Porphyromonas gingivalis, Selenomonas artemidis, Streptococcus sobrinus). The probable mechanism of this action is the perforation of the bacterial membrane146, 147).
Oils are almost always complex mixture of a number of substances, and therefore their biological effects are often described as a result of the synergism of all present molecules or they reflect activities of the main ingredients (present in the highest concentrations)148). Synergic action is advantageous, because bacteria can adapt and preserve function of their membrane in presence of sub-inhibition concentration of anti-bacterial ingredients. This may raise resistance which the complex effect of oil may help suppress149). The general rule is that properties of the main ingredients reflect the biophysical and biological characteristics of oil relatively well (as we can see, for instance, with Origani etheroleum and carvacrol/ thymol150)), and the level of their effects depends on their concentration151, 152). The complexity of ingredients present in the oil thus strongly affects the odour, texture, colour, and penetration into the cell153), lipophilicity or hydrophilicity, interaction with the cell wall and membrane and cell distribution148). This is the reason why it is better in many cases to analyse the activity of the entire oil and compare it with the activity of the pure main ingredients.
The presence of thymol and carvacrol in T. vulgaris is associated with antibacterial activity of the plant. The antibacterial and antiseptic effect of thymol and carvacrol is well-known154). Their synergic action has been described formerly and has been well explored155), the synergy mechanism has also been clarified156, 157). By interaction with the lipid double layer of cytoplasmic membranes, thymol and carvacrol cause loss of integrity and leakage of cellular material. This effect (in general, by membrane decomposition or formation of a high number of pores) can, in general, increase membrane permeability also for other antimicrobial compounds. Carvacrol acts on B. cereus by exhausting the intracellular stock of ATP, changes membrane potential and increases membrane permeability for protons and potassium. Carvacrol further integrates in the lipid single-layer of cell membrane, changes its fluidity and damages its function158). Evidence of other mechanisms of antibacterial action exists, such as interaction with DNA. Besides, it has been found that carvacrol inhibits formation of bacterial biofilm that is one of the bacterial resistance mechanisms155). Thymol, aromatic monoterpenic phenol of p-menthane type, isomeric to carvacrol, is causing a significant antimicrobial interaction with both an internal and external part of the cytoplasmic cell membrane by incorporation into the lipid double-layer. Such interactions change the properties of the cell membrane and result in higher permeability/ disintegration159–161). Besides, thymol can also up - or down-regulate expression of genes coding the outer membrane protein synthesis. It is not only able to inhibit enzymes involved in protection against thermal load, but it can also influence ATP synthesis or the metabolic pathway of citrate (Krebs cycle)162, 163).
The general rule is that lipophilic flavonoids (flavonoids aglycones or methoxylated and prenylated flavonoids) synthesize plants as a part of protection against microbial infection, and therefore, they could be used for antimicrobial therapy in humans. The lipophilic flavonoids of thymonin have an antibacterial effect and are a part of, for instance, propolis, a well-known antimicrobial active material164, 165). Antibacterial flavonoids most likely have more final destinations in cells, rather than one specific place of action. One of their activities at molecular level is the formation of complexes with proteins non-specifically not only via hydrogen bonds and hydrophobic interactions, but also via covalent bond formation. Their antimicrobial activities thus can be in relation to their ability to inactivate various proteins, such as microbial adhesines, enzymes, transport proteins of the cell cover and others165). Lipophilic flavonoids can fill microbes even by disintegrating microbial membranes165). Some flavonoids also have an antiviral effect166).
Thyme is associated also with spasmolytic activity. In in vitro tests, the relaxation effect of thyme extracts, flavonoids and oil on smooth muscles of the trachea and ilea have been observed167, 168). Of the various fractions tested, the strongest relaxation effect observed was the n-hexane fraction (lipophilic, containing essential oil components) and the weakest one with aqueous fraction. The n-hexane fraction mainly contains thymol, carvacrol and other monoterpenes, or, as the case may be, lipophilic flavonoids to which can thus be attributed the relaxation effect169). Van den Broucke and Lemli explain the spasmolytic effect of flavones in T. vulgaris by their ability to inhibit the effect of specific agonists (acetylcholine, histamine and L-noradrenaline), as well as of non-specifically acting substances (BaCl2)170).
Therapy of upper respiratory tract catarrh can also be supported by the above-described anti-inflammatory activity. Thymol is in vitro able to inhibit COX-1 in a concentration similar to indomethacin171, 172). In vitro testing of carvacrol found out that it inhibited not only COX-1, but also COX-2173). Flavonoids, such as apigenin, luteolin and thymonin are also mentioned in literature in connection with anti-inflammatory action174–177).
Clinical studies, adverse effects
Thyme, its extracts, oils and individual ingredients are well-known in the treatment of upper respiratory tract catarrh. Thyme activity, however, has been documented only by a limited number of clinical studies (Table 5).
Tab. 5. Summary of selected clinical studies of the effect of T. vulgaris on upper respiratory tract catarrh 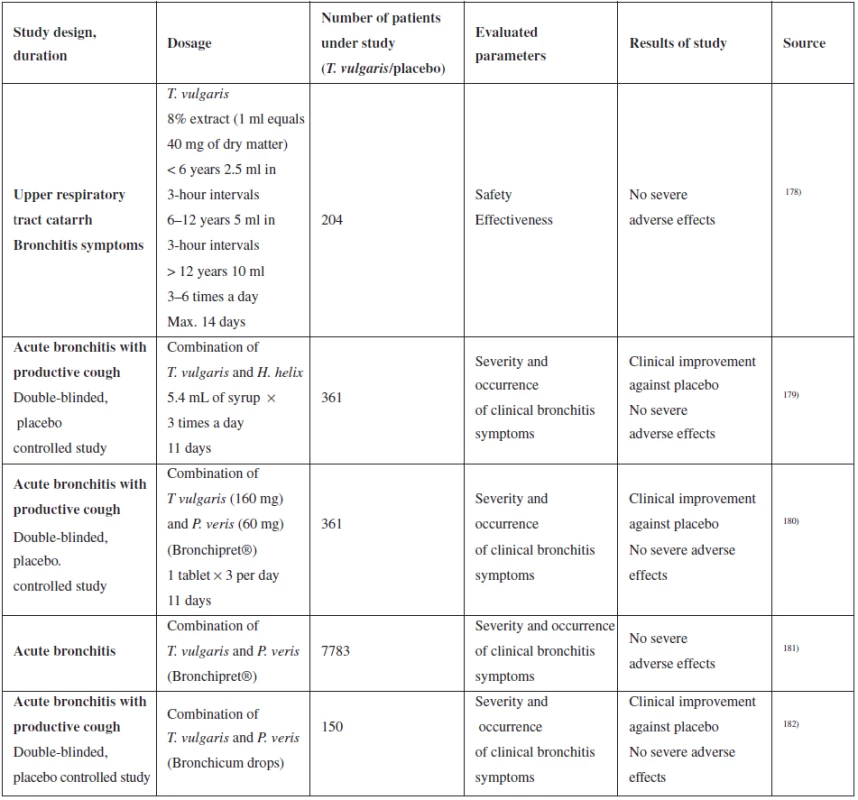
Ernst et al. generated an extensive multi-centre supervisory study, in which they were comparing the effectiveness of Bronchipret (extracts of thyme and primrose root) with other phytomedicines, ambroxol and N-acetylcysteine in acute bronchitis therapy181). The study was designed as “matched-pair comparison” of 7,783 patients. Patients with acute bronchitis were diagnosed upon the first visit to the doctor and divided into two groups – one group received treatment with Bronchipret, the other with any other secretolytic. The second group was then retrospectively divided into 3 sub-groups by specific medicinal product (phytomedicine, N-acetylcysteine, ambroxol). The patients were re-examined after ten days. They documented clinical symptoms of acute bronchitis and their changes during treatment, as well as adverse effects. The data was then used for comparing the success of the treatment with the tested product in 3 control groups. The authors conclude that the clinical effectiveness of Bronchipret is not less than one of synthetic products (N-acetylcysteine, ambroxol), however, they describe a trend to better results in the group of adult patients181). The above-described study has several discrepancies. It is not blinded, so it is impossible to eliminate the patient’s trust or distrust of the prescribed medication (placebo and nocebo effect). Also, the numbers of patients in each of the three control groups are several times lower than the number of patients using the tested drug, which reduces the statistical significance of comparison of the results.
Several studies of patients have evaluated the safety and occurrence of adverse effects. No severe adverse effects appeared during testing, study by Ismail et al even lower occurrence of adverse effects compared to ambroxol and N-acetylcysteine183). Although clinical studies prove that administration of medicinal products with thyme is safe, treatment of children under 4 years of age is recommended only under a physician’s supervision. With respect to lack of data, T. vulgaris must not be used during pregnancy and lactation.
Adverse effects of thyme and oil extracts are associated, for instance, with neurological symptoms, such as headaches and dizziness. Swallowing a large amount of thyme oil may induce an attack or coma (Basch et al 2004)139). However, we need to realize the fact that, for instance, LD50 for rats is fixed at 2.84 g/kg, which would correspond to 196 g for an adult, with the content of approximately 2% of oil in a consumed dose of less than 10 kg of thyme. Other adverse effects of thyme include described conjunctivitis after exposure to thyme dust, contact dermatitis after exposure to thyme dust or thymol139, 144, 184) and professional asthma and allergisation (cultivation and production personnel)139). In an ex vivo test of thyme extract, a bond to receptors for progesterone and estradiol has been observed; endocrinal effects of T. vulgaris in people are still unclear139, 185). Due to the probable content of phytoestrogens, there is a theoretical risk of interaction with hormonal contraception or with preparations containing phytoestrogens139, 185).
Conclusion
Extracts of ginseng (P. quinquefolium) and purple coneflower (Echinacea spp.) stimulate the immune system and improve resistance of the organism to infection agents causing upper respiratory tract catarrh. Their action can be supported by extracts of thyme (T. vulgaris) or umckaloabo geranium (P. sidoides), which have a major antibacterial influence and can contribute to already running disease. In terms of phytochemistry, these medicinal plants have been explored relatively well and the mechanism of their action has been described in a number of both in vitro and in vivo studies.
The literature listed below proves that extracts obtained from these medicinal plants represent an alternative to conventional treatment of upper respiratory tract catarrh. According to clinical studies, their application brings benefits in the form of reduced frequency and severity of symptoms of the disease and can also reduce the occurrence of complications which need to be managed by antibiotic therapy.
Conflict of interests: none.
Received 1 August 2016
Accepted 12 August 2016
doc. PharmDr. Karel Šmejkal, PhD. • V. Rjašková
University of Veterinary and Pharmaceutical Sciences Brno
Faculty of Pharmacy, Department of Natural Drugs
Palackého 1946/1, 612 42 Brno, Czech Republic
e-mail: karel.mejkal@post.cz
Zdroje
1. Kopřiva F. Diagnostika a léčba bronchitídy u dětí. Pediatr. pro Praxi 2007; 2, 106–108.
2. Pauk N. Terapie akutní bronchitídy. Interní Med. 2011; 13, 327–328.
3. Tan B. K., Vanitha J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: a review. Curr. Med. Chem. 2004; 11, 1423–30.
4. Das S., Bordoloi R., Newar N. A review on immune modulatory effect of some traditional medicinal herbs. J. Pharm. Chem. Biol. Sci. 2014; 2, 33–42.
5. Cundell D. R. Herbal Phytochemicals as Immunomodulators. Curr. Immunol. Rev. 2014; 10, 64–81.
6. Shukla S., Bajpai V. K., Kim M. Plants as potential sources of natural immunomodulators. Rev. Environ. Sci. Bio. 2014; 13, 17–33.
7. Manayi A., Vazirian M., Saeidnia S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015; 9, 63–72.
8. Gupta M., Sharma D., Sharma A., Kumari V., Goshain O. P. A review on purple cone flower (Echinacea purpurea L. Moench. J. Pharm. Res. 2012; 8, 4076–4081.
9. Community herbal monograph on Echinacea purpurea (L. Moench, radix) EMA/HMPC/577784/2008. http://www. ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2011/01/WC500101497.pdf
10. Community herbal monograph on Echinacea angustifolia DC., radix. EMA/HMPC/688216/2008. http://www.ema.europa.eu/ docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2012/05/WC500127890.pdf
11. Community herbal monograph on Echinacea pallida (Nutt. Nutt., radix) EMEA/HMPC/332350/2008. http://www. ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2009/12/WC500018248.pdf
12. European Union herbal monograph on Echinacea purpurea (L. Moench, herba recens) EMA/HMPC/48704/2014. http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2015/04/WC500185437.pdf
13. Gajalakshmi S., Vijayalakshmi S., Devirajeswari V. Echinaceae puprpurea – a potent immunostimulant. Int. J. Pharm. Sci. Rev. Res. 2012; 14, 47–52.
14. Barnes J., Anderson L. A., Gibbons S., Phillipson J. D. Echinacea species (Echinacea angustifolia (DC. Hell.) Echinacea pallida (Nutt.) Echinacea purpurea (L.) Moench. a review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005; 57, 929–954.
15. Woelkart K., Dittrich P., Beubler E., Pinl F., Schoop R., Suter A., Bauer R. Pharmacokinetics of the main alkamides after administration of three different Echinacea purpurea preparations in humans. Planta Med. 2008; 74, 651–656.
16. Shekarchi M., Hajimehdipoor H., Khanavi M., Roostaie A. The effects of plant age and harvesting time on chicoric and caftaric acids content of E. purpurea (L. Moench). Iranian J. Pharm. Sci. 2012; 8, 203–208.
17. Thomsen M. O., Frette X. C., Christensen K, B., Christensen L. P., Grevsen K. Seasonal Variations in the Concentrations of Lipophilic Compounds and Phenolic Acids in the Roots of Echinacea purpurea and Echinacea pallida. J. Agric. Food Chem. 2012; 60, 12131–12141.
18. Barrett B. Medicinal properties of Echinacea: a critical review. Phytomedicine 2003; 10, 66–86.
19. Rininger J. A., Kickner S., Chigurupati P., McLean A., Franck Z. Immunopharmacological activity of Echinacea preparations following simulated digestion on murine macrophages and human peripheral blood mononuclear cells. J. Leukocyte Biol. 2000; 68, 503–510.
20. Eichler F., Krüger G. R. F. Effects of non-specific immunostimulants (Echinacin, isoprinosine, and thymus factors. on the infection and antigen expression in herpesvirus–6 exposed human lymphoid cells. In Vivo 1994; 8, 565–576.
21. Bauer R., Remiger P., Jurcic K., Wagner H. Beeinflussung der Phagozytose-Aktivität durch Echinacea-Extrakte. Influence of Echinacea extract on phagocytotic activity. Z. Phytother. 1989; 10, 43–48.
22. Bauer R., Wagner H. Echinacea species as potential immunostimulatory drugs. In: Wagner H., Farnsworth N. R. (eds.) Economic and Medicinal Plant Research. New York: Academic Press Limited 1991; 253–318.
23. Bauer R. Chemistry, analysis and immunological investigations of Echinacea phytopharmaceuticals. In: Wagner H. (ed.) Immunomodulatory Agents from Plants. Basel, Boston, Berlin: Birkhauser Verlag 1999; 41–88.
24. Woelkart, K., Bauer, R. The role of alkamides as an active principle of echinacea. Planta Med. 2007; 73, 615–623.
25. Woelkart K., Xu W., Pei Y., Makriyannis A., Picone R. P., Bauer R. The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Med. 2005; 71, 701–705.
26. Karsch-Völk M., Barrett B., Kiefer D., Bauer R., Ardjomand-Woelkart K., Linde K. Echinacea for preventing and treating the common cold. Cochrane Database Syst. Rev. 2014; 2, CD000530.
27. Schapowal A., Klein P., Johnston S. L. Echinacea reduces the risk of recurrent respiratory tract infections and complications: a meta-analysis of randomized controlled trials. Adv. Ther. 2015; 32, 187–200.
28. Baetgen D. Erfolge in der Keuchhusten-Behandlung mit Echinacin®. Successful treatment of whooping cough with Echinacea. Therapiewoche 1984; 34, 5115–5119.
29. Baetgen D. Behandlung der akuten Bronchitis im Kindesalter. Praxisstudie mit einem Immunstimulans aus Echinacea purpurea. Treatment of acute bronchitis in children. A study of an immunostimulatory agent from Echinacea purpurea in a primary care setting. Translator unknown. Therapiewoche Pädiatrie 1988; 1, 65–70.
30. Melchart D., Linde K., Worku F., Bauer R., Wagner H. Immunomodulation with echinacea – a systematic review of controlled clinical trials. Phytomedicine 1994; 1, 245–254.
31. Turner R. B., Riker D. K., Gangemi J. D. Ineffectiveness of Echinacea for prevention of experimental rhinovirus colds. Antimicrob. Agents Chemother. 2000; 44, 1708–1709.
32. Melchart D., Walther E., Linde K., Brandmaier R., Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: A double-blind, placebo-controlled randomized trial. Arch. Family Med. 1998; 7, 541–545.
33. Schöneberger D. Einfluß der immunstimulierenden Wirkung von Preßsaft aus Echinacea purpurea auf Verlauf und Schweregrad von Erkältungskrankheiten. The influence of the immunostimulating effects of pressed juice from Echinacea purpurea on the course and severity of cold infections. Forum Immunologie 1992; 8, 18–22.
34. Grimm W., Muller H. H. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on incidence and severity of colds and respiratory infections. Am. J. Med. 1999; 106, 138–143.
35. Bräunig B., Dorn M., Knick E. Echinaceae purpureae radix: Zur Stärkung der körpereigenen Abwehr bei grippalen Infekten. Strengthening of the endogenous resistence to influenzal infections. Z. Phytother. 1992; 13, 7–13.
36. Melchart D., Linde K. Clinical investigations of Echinacea phytopharmaceuticals. In: Wagner H. (ed.) Immunomodulatory Agents from Plants. Basel, Boston, Berlin: Birkhauser Verlag 1999; 105–118.
37. Brinkeborn R. M., Shah D. V., Degenring F. H. Echinaforce® and other Echinacea fresh plant preparations in the treatment of the common cold. Phytomedicine 1999; 6, 1–6.
38. Schmidt U., Albrecht M., Schenk N. Pflanzliches Immunstimulans senkt Haufigkeit grippaler Infekte. Natur - und Ganzheitsmedizin 1990; 3, 277–281.
39. Cohen H. A., Varsano I., Kahan E., Sarrell E. M., Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled, multicenter study. Arch. Pediatr. Adolesc. Med. 2004; 158, 217–221.
40. Taylor J. A., Weber W., Standish L., et al. Efficacy and safety of echinacea in treating upper respiratory tract infections in children: a randomized controlled trial. JAMA 2003; 290, 2824–2830.
41. Jawad M., Schoop R., Suter A., Klein P., Eccles R. Safety and efficacy profile of Echinacea purpurea to prevent common cold episodes: a randomized, doubleblind, placebo-controlled trial. Evid Based Complement. Alternat. Med. 2012; 2012, 841315.
42. Gallo M., Sarkar M., Au W., Pietrzak K., Comas B., Smith M, Jaeger T. V., Einarson A., Koren G. Pregnancy outcome following gestational exposure to Echinacea. Arch. Intern. Med. 2000; 160, 3141–3143.
43. Qi L.-W., Wang C.-Z., Yuan C.-S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry 2011; 72, 689–699.
44. Yuan C.-S., Wang C.-Z., Wicks S. M., Qi L.-W. Chemical and pharmacological studies of saponins with a focus on American ginseng. J. Ginseng Res. 2010; 34, 160–167.
45. Do J. H., Lee H. O., Lee S. K., Noh K. B., Lee S. D., Lee K. S. Comparisons of acidic polysaccharide content in various ginseng species and parts. J. Ginseng Res. 1993; 17, 145–147.
46. Liao J.-F., Shen Y.-C., Huang Y.-T. Pharmacology of Polysaccharides from Ginseng Species. Int. J. Biomed. Pharm. Sci. 2012; 6, 63–69.
47. Han B. H., Park M. H., Han Y. N., Woo L. K., Sankawa U., Yahara S., Tanaka O. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982; 44 : 146–149.
48. Karikura M., Miyase T., Tanizawa H., Taniyama T., Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VI. The decomposition products of ginsenoside Rb2 in the stomach of rats. Chem. Pharm. Bull. 1991; 39, 400–404.
49. Karikura M., Miyase T., Tanizawa H., Taniyama T., Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and -Rb2 in the digestive tract of rats. Chem. Pharm. Bull. 1991; 39, 2357–2361.
50. Hasegawa H., Sung J. H., Matsumiya S., Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996; 62, 453–457.
51. Wakabayashi C., Murakami K., Hasegawa H., Murata J., Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem. Biophys. Res. Commun. 1998; 246, 725–730.
52. Suda K., Murakami K., Murata J., Hasegawa H., Saiki I. Induction of apoptosis in Lewis lung carcinoma cells by an intestinal bacterial metabolite resulted from orally administered ginseng protopanaxadiol saponins. J. Trad. Med. 2000; 17, 236–244.
53. Hasegawa H., Suzuki R., Nagaoka T., Tezuka Y., Kadota S., Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural killer cytotoxicity by fatty acid conjugate of protopanxatriol. Biol. Pharm. Bull. 2002; 25, 861–866.
54. Hasegawa H., Lee K. S., Nagaoka T., Tezuka Y., Uchiyama M., Kadota S., Saiki I. Pharmacokinetics of ginsenoside deglycosylated by intestinal bacteria and its transformation to biologically active fatty acid ester. Biol. Pharm. Bull. 2000; 23, 298–304.
55. Sievenpiper J. L., Arnason J. T., Leiter L. A., Vuksan V. Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: the role of ginsenosides. J. Am. Coll. Nutr. 2004; 23, 248–258.
56. Dascalu A., Sievenpiper J. L., Jenkins A. L., Stavro M. P., Leiter L. A., Arnason T., Vuksan V. Five batches representative of Ontario–grown American ginseng root produce comparable reductions of postprandial glycemia in healthy individuals. Can. J. Physiol. Pharmacol. 2007; 85, 856–864.
57. Kang S., Min H. Ginseng, the ‘Immunity Boost’: The Effects of Panax ginseng on Immune System. J. Ginseng Res. 2012; 36, 354–368.
58. Rivera E., Ekholm Pettersson F., Inganäs M., Paulie S., Grönvik K. O. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccine 2005; 23, 5411–5419.
59. Yang Z., Chen A., Sun H., Ye Y., Fang W. Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine 2007; 25, 161–169.
60. Sun J., Song X., Hu S. Ginsenoside Rg1 and aluminum hydroxide synergistically promote immune responses to ovalbumin in BALB/c mice. Clin. Vaccine Immunol. 2008; 15, 303–307.
61. Song X., Chen J., Sakwiwatkul K., Li R., Hu S. Enhancement of immune responses to influenza vaccine (H3N2). by ginsenoside Re. Int. Immunopharmacol. 2010; 10, 351–356.
62. Song X., Hu S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine 2009; 27, 4883–4890.
63. Lee E. J., Ko E., Lee J., Rho S., Ko S., Shin M. K., Min B. I., Hong M. C., Kim S., Y., Bae H. Ginsenoside Rg1 enhances CD4+ T-cell activities and modulates Th1/Th2 differentiation. Int. Immunopharmacol. 2004; 4, 235–244.
64. Lee J. H., Han Y. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4+ T cell. Int. Immunopharmacol. 2006; 6, 1424–1430.
65. Kenarova B., Neychev H., Hadjiivanova C., Petkov V. D. Immunomodulating activity of ginsenoside Rg1 from Panax ginseng. Jpn. J. Pharmacol. 1990; 54, 447–454.
66. Song X., Zang L., Hu S. Amplified immune response by ginsenoside–based nanoparticles (ginsomes.. Vaccine 2009; 27, 2506–2511.
67. Tung N. H., Quang T. H., Son J. H., Koo J. E., Hong H. J., Koh Y. S., Song G. Y., Kim Y. H. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch. Pharm. Res. 2011; 34, 681–685.
68. Luo Y. M., Cheng X. J., Yuan W. X. Effects of ginseng root saponins and ginsenoside Rb1 on immunity in cold water swim stress mice and rats. Zhongguo Yao Li Xue Bao 1993; 14, 401–404.
69. Su W., Sun A. J., Xu D. L., Zhang H. Q., Yang L., Yuan L. Y., Jia J. G., Zou Y. Z., Wu Y. L., Wang K. Q., Ge J. B. Inhibiting effects of total saponins of panax ginseng on immune maturation of dendritic cells induced by oxidized-low density lipoprotein. Cell Immunol. 2010; 263, 99–104.
70. Joh E. H., Lee I. A., Jung I. H., Kim D. H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation-the key step of inflammation. Biochem. Pharmacol. 2011; 82, 278–286.
71. Byeon S. E., Lee J., Kim J. H., Yang W. S., Kwak Y. S., Kim S. Y., Choung E. S., Rhee M. H., Cho J. Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediat. Inflamm. 2012; 2012, ID 732860.
72. Tomoda M., Hirabayashi K., Shimizu N., Gonda, R., Ohara, N. The core structure of ginsenan PA, a phagocytosis-activating polysaccharide from the root of Panax ginseng. Biol. Pharm. Bull. 1994; 17, 1287–1291.
73. Kim K. H., Lee Y. S., Jung I. S., Park S. Y., Chung H. Y., Lee I. R., Yun Y. S. Acidic polysaccharide from Panax ginseng, Ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with IL-2. Planta Med. 1998; 64, 110–115.
74. Sonoda Y., Kasahara T., Mukaida N., Shimizu N., Tomoda M., Takeda T. Stimulation of interleukin-8 production by acidic polysaccharides from the root of Panax ginseng. Immunopharmacology 1998; 38, 287–294.
75. Assinewe V., Arnason J., Aubry A., Mullin J., Lemaire I. Extractable polysaccharides of Panax quinquefolius L. North American ginseng. root stimulate TNFα production by alveolar macrophages. Phytomedicine 2002; 9, 398–404.
76. Jang H. I., Shin H. M. Wild Panax ginseng. Panax ginseng C. A. Meyer. protects against methotrexate-induced cell regression by enhancing the immune response in RAW 264.7 macrophages. Am. J. Chin. Med. 2010; 38, 949–960.
77. Azike C. G., Charpentier P. A., Hou J., Pei H., King Lui E. M. The Yin and Yang actions of North American ginseng root in modulating the immune function of macrophages. Chin. Med. 2011; 6, 21.
78. Ahn J. Y., Choi I. S., Shim J. Y., Yun E. K., Yun Y. S., Jeong G., Song J. Y. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll–like receptor–mediated inflammatory signals. Eur. J. Immunol. 2006; 36, 37–45.
79. Kim D. Y., Yang W. M. Panax ginseng ameliorates airway inflammation in an ovalbumin-sensitized mouse allergic asthma model. J. Ethnopharmacol. 2011; 136, 230–235.
80. Ro J. Y., Ahn Y. S., Kim K. H. Inhibitory effect of ginsenoside on the mediator release in the guinea pig lung mast cells activated by specific antigen-antibody reactions. Int. J. Immunopharmacol. 1998; 20, 625–41.
81. Biondo P. D., Goruk S., Ruth M. R., O’Connell E., Field C. J. Effect of CVT–E002 (COLD-fX. versus a ginsenoside extract on systemic and gut–associated immune function. Int. Immunopharmacol. 2008; 8, 1134–1142.
82. Lee J. H., Shim J. S., Chung M. S., Lim S. T., Kim K. H. Inhibition of pathogen adhesion to host cells by polysaccharides from Panax ginseng. Biosci. Biotechnol. Biochem. 2009; 73, 209–212.
83. Lee J. H., Shim J. S., Lee J. S, Kim M. K., Chung M. S., Kim K. H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr. Res. 2006; 341, 1154–1163.
84. Fukuyama N., Shibuya M., Orihara Y. Antimicrobial polyacetylenes from Panax ginseng hairy root culture. Chem. Pharm. Bull. 2012; 60, 377–380.
85. Chan L. Y., Kwok H. H., Chan R. W., Peiris M. J., Mak N. K., Wong R. N., Chan M. C., Yue P. Y. Dual functions of ginsenosides in protecting human endothelial cells against influenza H9N2-induced inflammation and apoptosis. J. Ethnopharmacol. 2011; 137, 1542–1546.
86. Kim J. Y., Kim H. J., Kim H. J. Effect of oral administration of Korean red ginseng on infl uenza A (H1N1. virus infection. J. Ginseng. Res. 2011; 35, 104–110.
87. Quan F. S., Compans R. W., Cho Y. K., Kang S. M. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine 2007; 25, 272–282.
88. Kim J., Han B. J., Kim H., Lee J. Y., Joo I., Omer S., Kim Y. S., Han Y. Th1 immunity induction by ginsenoside Re involves in protection of mice against disseminated candidiasis due to Candida albicans. Int. Immunopharmacol. 2012; 14, 481–486.
89. Song X., Zang L., Hu S. Amplified immune response by ginsenoside-based nanoparticles ginsomes. Vaccine 2009; 27, 2306–2311.
90. Song X., Chen J., Sakwiwatkul K., Li R., Hu S. Enhancement of immune responses to influenza vaccine (H3N2. by ginsenoside Re. Int. Immunopharmacol. 2010; 10, 351–356.
91. Scaglione F., Cattaneo G., Alessandria M., Cogo R. Efficacy and safety of the standardised Ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold. Drugs Exp. Clin. Res. 1996; 22, 65–72.
92. Sun K., Wang C. S., Guo J., Horie Y., Fang S. P., Wang F., et al. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 2007; 81, 509–518.
93. Park E. K., Shin Y. W., Lee H. U., Kim S. S., Lee Y. C., Lee B. Y., et al. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosynthesis of RAW 264.7 cells induced by lipopolysaccharide. Biol. Pharm. Bull. 2005; 28, 652–656.
94. Smolinsk A. T., Pestka J. J. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin, chamomile, ginsenoside Rb 1. ginseng, and parthenolide feverfew. Food Chem. Toxicol. 2003; 41, 1381–1390.
95. Senchina D. S., Hallam J. E., Cheney D. J. Multidisciplinary perspectives on mechanisms of activity of popular immune-enhancing herbal supplements used by athletes. Front. Biol. 2013; 8, 78–100.
96. Engels H. J., Fahlman M. M., Wirth J. C. Effects of ginseng on secretory IgA, performance, and recovery from interval exercise. Med. Sci. Sports Exerc. 2003; 35, 690–696.
97. Gaffney B. T., Hügel H. M., Rich P. A. The effects of Eleutherococcus senticosus and Panax ginseng on steroidal hormone indices of stress and lymphocyte subset numbers in endurance athletes. Life Sci. 2001; 70, 431–442.
98. Hsu M. Effect of American ginseng supplementation on cytokines and oxidative stress following an acute downhill running. Med. Sci. Sports Exerc. 2010; 42, S788.
99. Jung H., Kang H., Chang Y., Kim T. Effect of Panax ginseng supplementation on muscle damage and inflammation after treadmill running in humans. Med. Sci. Sports Exerc. 2011; 43(Suppl 1), S432.
100. Park S., Jung H., Hong S., Kang Y., Lee G., Kim S., Kang H., Lee C. Effects of red ginseng intake on interleukin-6 (IL-6) and cortisol responses after high-intensity exercise. Med. Sci. Sports Exerc. 2008; 40(Suppl), S432.
101. Biondo P., McCargar L., Harber V., Field C. A randomized controlled trial of ginseng supplementation on the immune response to a moderate exercise stress protocol in non-athletic women. Open Nutr. J. 2010; 4, 1–10.
102. Biondo P. D., Robbins S. J., Walsh J. D., McCargar L. J., Harber V. J., Field C. J. A randomized controlled crossover trial of the effect of ginseng consumption on the immune response to moderate exercise in healthy sedentary men. Appl. Physiol. Nutr. Metab. 2008; 33, 966–975.
103. Lau W., Liang M., Sokmen B., Spalding T., Quezada L., Chuang W. Effect of Panax notoginseng (Chinese ginseng) and cycling exercise on IL-6 and cortisol in untrained non-diabetic men. Med. Sci. Sports Exerc. 2011; 43(Suppl 1), S432.
104. Scaglione F., Ferrara F., Dugnani S., Falchi M., Santoro G., Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp. Clin. Res. 1990; 16, 537–542.
105. Scaglione, F., Weiser, K., Alessandria, M. Effects of the Standardised Ginseng Extract G115® in Patients with Chronic Bronchitis A Nonblinded, Randomised, Comparative Pilot Study. Clin. Drug Invest. 2001; 21, 41–45.
106. McElhaney J. E., Gravenstein S., Cole S. K., Davidson E., O’Neill D., Petitjean S., et al. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J. Am. Geriatr. Soc. 2004; 52, 13–19.
107. McElhaney J. E., Goel V., Toane B., Hooten J., Shan J. J. Efficacy of COLD-fX in the prevention of respiratory symptoms in community-dwelling adults: a randomized, double-blinded, placebo controlled trial. J. Altern. Complement. Med 2006; 12, 153–157.
108. Predy G. N., Goel V., Lovlin R., Donner A., Stitt L., Basu T. K. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. Canadian Med. Assoc. J. 2005; 173, 1043–1048.
109. Vohra S., Johnston B. C., Laycock K. L., Midodzi W. K., Dhunnoo I., Harris E., Baydala L. Safety and tolerability of North American ginseng extract in the treatment of pediatric upper respiratory tract infection: a phase II randomized, controlled trial of 2 dosing schedules. Pediatrics 2008; 122, e402–410.
110. Seida J. K., Durec T., Kuhle S. North American (Panax quinquefolius) and Asian ginseng (Panax ginseng) preparations for prevention of the common cold inhealthy adults: a systematic review. Evid. Based Complement. Alternat. Med. 2011; 2011, 282151.
111. Moyo M., van Staden J. Medicinal properties and conservation of Pelargonium sidoides DC. J. Ethnopharmacol. 2014; 152, 243–255.
112. Navrátilová Z. Léčivý muškát Pelargonium sidoides. Prakt. Lék. 2012; 8, 290–292.
113. Kolodziej H. Pelargonium reniforme and Pelargonium sidoides: their botany, chemistry and medicinal use. In: Lis Balchin M (ed.) Geranium and Pelargonium. In Medicinal and Aromatic Plants – Industrial Profiles. New York, London: Taylor & Francis 2002; 262–290
114. Colling J., Groenewald J.-H., Makunga N. P. Genetic alteration for increased coumarin production lead to metabolic changes in the medicinally important Pelargonium sidoides DC (Geraniaceae). Metab. Eng. 2010; 12, 561–572.
115. Brendler T., van Wyk B.-E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J. Ethnopharmacol. 2008; 119, 420–433.
116. Lewu F. B., Adebola P. O., Afolayan A. J. Commercial harvesting of Pelargonium sidoides in the Eastern Cape, South Africa: striking a balance between resources conservation and livelihoods. J. Arid. Environ. 2007; 70, 380–388.
117. Hutchings A., Scott A. H., Lewis G., Cunningham A. Zulu Medicinal Plants. Pietermaritzburg: Natal University Press 1996.
118. Community herbal monograph on Pelargonium sidoides DC and/or Pelargonium reniforme Curt., radix. EMA/HMPC/ 560961/2010. http://www.ema.europa.eu/docs/en_GB/document _library/Herbal_-_Community_herbal_monograph/2013/02/WC 500138439.pdf
119. Kolodziej H. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo®. Phytomedicine 2007; 14, 9–17.
120. Theisen L. L., Muller C. P. EPs®7630 (Umckaloabo®), an extract from Pelargonium sidoides roots, exerts anti-influenza virus activity in vitro and in vivo. Antiviral Res. 2012; 94, 147–156.
121. Michaelis M., Doerr H. W., Cinatl J. Jr. Investigation of the influence of EPs® 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine 2011; 18, 384–386.
122. Mativandlela S. P. N., Meyer J. J. M., Hussein A. A., Lall N. Antitubercular activity of compounds isolated from Pelargonium sidoides. Pharm. Biol. 2007; 45, 645–650.
123. Beil W., Kilian P. EPs®7630, an extract from Pelargonium sidoides roots inhibits adherence of Helicobacter pylori to gastric epithelial cells. Phytomedicine 2007; 14, 5–8.
124. Conrad A., Jung I., Tioua D., Lalleman C., Carrapatoso F., Engels I., Daschner F. D., Frank U. Extract of Pelargonium sidoides (EPs®7630) inhibits the interactions of group A-streptococci and host epithelia in vitro. Phytomedicine 2007; 14, 52–59.
125. Kayser O., Kolodziej H., Kinderlen A. F. Immunomodulatory principles of Pelargonium sidoides. Phytother. Res. 2001; 15, 122–126.
126. Thäle C., Kiderlen A. F., Kolodziej H. Anti-infective mode of action of EPs®7630 at the molecular level. Planta Med. 2008; 74, 675–681.
127. Kolodziej H., Kinderlen A. F. In vitro evaluation of antibacterial and immunomodulatory acitvities of Pelargonium reinforme, Pelargonium sidoides and the related herbal drug preparation EPs®7630. Phytomedicine 2007; 14, 18–26.
128. Kolodziej H., Burmeister A., Trun W., Radtke O. A., Kinderlen A. F., Ito H., Hatano T., Yoshida T., Foo L. L. Tannins and related compounds induce nitric oxide synthase and cytokines genes expression in Leishmania major – infected macrophage-like RAW 264.7 cells. Bioorg. Med. Chem. 2005; 13, 6470–6476.
129. Radtke O. A., Kinderlen A. F., Kayser O., Kolodziej H. Gene expression profiles of inducible nitric oxide synthase and cytokines in Leishmania major – infected macrophage-like RAW 264.7 cells treated with gallic acid. Planta Med. 2004; 70, 924–928.
130. Kamin W., Ilyenko L. I., Malek F. A., Kieser M. Treatment of acute bronchitis with EPss 7630: randomized, controlled trial in children and adolescents. Pediatr. Int. 2012; 54, 219–226.
131. Kamin W., Maydannik V. G., Malek F. A., Kieser M. Efficacy and tolerability of EPss 7630 in patients (aged 6–18 years old) with acute bronchitis: a randomized, double-blind, placebo-controlled clinical dose-finding study. Acta Paediatr. 2010; 99, 537–543.
132. Matthys H., Funk P. EPSs 7630 improves acute bronchitic symptoms and shortens time to remission: Results of a randomised, double-blind, placebo-controlled, multicentre trial. Planta Med. 2008; 74, 686–692.
133. Chuchalin A. G., Berman B., Lehmacher W. Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPss 7630) a randomised, double-blind controlled trial. Explore 2005; 1, 437–445.
134. Bachert C., Schapowal A., Funk P., Kieser M. Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPss 7630: a randomized, double-blind, placebo-controlled trial. Rhinology 2009; 47, 51–58.
135. Tahan F., Yaman M. Can the Pelargonium sidoides root extract EPss 7630 prevent asthma attacks during viral infections of the upper respiratory trac tin children? Phytomedicine 2013; 20, 148–150.
136. Bereznoy V. V., Riley D. S., Wasmer G., Heger M. Efficacy of extract of Pelargonium sidoides in children with acute non-group A betaemolytic streptococcus tonsillopharyngitis: a randomized, double-blind, placebo-controlled trial. Altern. Ther. Health Med. 2003, 9, 68–79.
137. Timmer A., Günther J., Rücker G., Motschall E., Antes G., Kern W. V. Pelargonium sidoides extract for acute respiratory tract infections. Cochrane Database Syst. Rev. 2008; 3, CD006323.
138. Leugnerová G. 2008. Thymus vulgaris L. – mateřídouška obecná, tymián/dúška tymiánová. Dostupné na: http://botany.cz/ cs/thymus-vulgaris/, datum citace 25.1.2016
139. Basch E., Ulbricht C., Hammerness P., Bevins A., Sollars D. Thyme (Thymus vulgaris L.). Thymol. J. Herb. Pharmacother. 2004; 4, 49–67.
140. Community herbal monograph on Thymus vulgaris L. and Thymus zygis L., herba. EMA/HMPC/342332/2013. http:// www. ema.europa.eu/docs/en_GB/document_library/Herbal_Community_herbal_monograph/2014/06/WC500167812.pdf
141. Blumenthal M. (ed.) The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Boston: Integrative Medicine Communications 1998.
142. Czygan F. C., Hiller K. Thymi herba. In: Wichtl M (ed.) Herbal drugs and Phytopharmaceuticals. Medpharm Scientific Publishers 2004; 607–610.
143. Bruneton J. Pharmacognosy, Phytochemistry, Medicinal Plants. 2nd ed. Secaucus, NJ: Lavoisier Publishing 1999.
144. Spiewak R., Skorska C., Dutkiewics J. Occupational airborne contact dermatitis caused by thyme dust. Contact Dermatitis 2001; 44, 235–239.
145. Manou I., Bouillard L., Devleeschouwer M. J., et al. Evaluation of the preservative properties of Thymus vulgaris essentials oil in topically applied formulations under a challenge test. J. Appl. Microbiol. 1998; 84, 368–376.
146. Twetman S., Petersson L. G. Interdental caries incidence and progression in relation to mutant streptococci suppression after chlorhexidine-thymol varnic treatments in schoolchildren. Acta Odontol. Scand. 1999; 57, 144–148.
147. Shapiro S., Guggenheim B. The action of thymol on oral bacteria. Oral Microbiol. Immunol. 1995; 10, 241–246.
148. Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils – A review. Food Chem. Toxicol. 2008; 46, 446–475.
149. Di Pasqua R., Betts G., Hoskins N., Edwards M., Ercolini D., Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007; 55, 4863–4870.
150. Ipek E., Zeytinoglu H., Okay S., Tuylu B. A., Kurcuoglu M., Baser K. H. C. Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem. 2005; 93, 551–556.
151. Franzios G., Mirotsou M., Hatziapostolou E., Kral J., Scouras Z. G., Mavragani-Tsipidou P. Insecticidal and genotoxic activities of mint essential oils. J. Agric. Food Chem. 1997; 45, 2690–2694.
152. Santana-Rios G., Orner G. A., Amantana A., Provost C., Wu S. Y., Dashwood R. H. Potent antimutagenic activity of white tea in comparison with green tea in the Salmonella assay. Mutat. Res. 2001; 495, 61–74.
153. Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Med. 2006; 72, 311–316.
154. Radulovic N. S., Blagojevic P. D., Stojanovic-Radic Z. Z., Stojanovic N. M. Antimicrobial Plant Metabolites: Structural Diversity and Mechanism of Action. Curr. Med. Chem. 2013; 20, 932–952.
155. Friedman M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol., a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014; 62, 7652–7670.
156. Langeveld W. T., Veldhuizen E. J. A., Burt S. A. Synergy between essential oil components and antibiotics: A review. Cr. Rev. Microbiol. 2014; 40, 76–94.
157. Bassolé I. H. N., Juliani H. R. Essential oils in combination and their antimicrobial properties. Molecules 2012; 17, 3989–4006.
158. Nowotarska S., Nowotarski K., Friedman M. Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers. Molecules 2014; 19, 7497–7515.
159. Helander I. M., Alakomi H., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E. J., Gorris L. G. M., von Wright A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998; 46, 3590–3595.
160. Lambert R. J. W., Skandamis P. N., Coote P. J., Nychas G. J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001; 91, 453–462.
161. Walsh S. E., Maillard J. Y., Russell A. D., Catrenich C. E., Charbonneau D. L., Bartolo R. G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and negative bacteria. J. Appl. Microbiol. 2003; 94, 240–247.
162. Horváth G., Kovács K., Kocsis B., Kustos I. Effect of thyme (Thymus vulgaris L.) essential oil and its main constituents on the outer membrane protein composition of Erwinia strains studied with microfluid chip technology. Chromatographia 2009; 70, 1645–1650.
163. Di Pasqua R., Mamone G., Ferranti P., Ercolini D., Mauriello G. Changes in the proteome of Salmonella enterica serovar Thompson as stress adaptation to sublethal concentrations of thymol. Proteomics 2010; 10, 1040–1049.
164. Liu Y., Song X., He J., Zheng X., Wu H. Synthetic derivatives of chrysin and their biological activities. Med. Chem. Res. 2014; 23, 555–563.
165. Cushnie T. P., Lamb A. J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005; 26, 343–356.
166. Sithisarn P., Michaelis M., Schubert-Zsilavecz M., Cinatl J. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 2013; 97, 41–48.
167. Reiter M., Brandt W. Relaxant effects on tracheal and ileal smooth muscles of the guinea pig. Arzneimittelforschung 1985; 35, 408–414.
168. Meister A., Bernhardt G., Christoffel V., et al. Antispasmodic activity of Thymus vulgaris extract on the isolated guinea-pig trachea: discrimination between drug and ethanol effects. Planta Med. 1999; 65, 512–516.
169. Keyhanmanesh R., Boskabady M. H. Relaxant effects of differrent fractions from Thymus vulgaris on guinea-pig tracheal chains. Biol. Res. 2012; 45, 67–73.
170. van den Broucke C. O., Lemli J. A. Pharmacological and chemical investigation of thyme liquid extracts. Planta Med. 1981; 41, 129–135.
171. Marsik P., Kokoska L., Landa P., Nepovim A., Soudek P., Vanek T. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1 - and -2-catalyzed prostaglandin E2 biosyntheses. Planta Med. 2005; 71, 739–742.
172. de Cássia da Silveira e Sá R., Andrade L. N., de Sousa D. P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013; 18, 1227–1254.
173. Landa P., Kokoska L., Pribylova M., Vanek T., Marsik P. In vitro Anti-inflammatory Activity of Carvacrol: Inhibitory Effect on COX–2 Catalyzed Prostaglandin E2 biosynthesis. Arch. Pharm. Res. 2009; 32, 75–78.
174. Patil R. H., Babu R. L., Naveen Kumar M., Kiran Kumar K. M., Hegde S. M., Nagesh R., Ramesh G. T., Sharma S. C. Anti-Inflammatory Effect of Apigenin on LPS-Induced Pro-Inflammatory Mediators and AP-1 Factors in Human Lung Epithelial Cells. Inflammation 2016; 39, 138–147.
175. Arango D., Diosa-Toro, M., Rojas-Hernandez L. S., Cooperstone J. L., Schwartz S. J., Mo X., Jiang J., Schmittgen T. D., Doseff A. I. Dietary apigenin reduces LPS-induced expression of miR-155 restoring immune balance during inflammation. Mol. Nutr. Food Res. 2015; 59, 763–772.
176. Seo H.-S., Sikder M. A., Lee H. J., Ryu J., Lee C. J. Apigenin inhibits tumor necrosis factor-α-induced production and gene expression of mucin through regulating nuclear factor-kappa B signaling pathway in airway epithelial cells. Biomol. Therapeutics 2014; 22, 525–531.
177. Kritas S. K., Saggini A., Varvara G., Murmura G., Caraffa A., Antinolfi P., Toniato E., Pantalone A., Neri G., Frydas S., et al. Luteolin inhibits mast cell-mediated allergic inflammation. J. Biol. Reg. Homeostat. Agents 2013; 27, 955–959.
178. Kaas P. J. Atemwegsinfekte im Kindesalter: Anwendungsbeobachtung bestätigt Effizienz einer Thymian zubereitung. Natura Med. 2003; 18, 2–4.
179. Kemmerich B., Eberhardt R., Stammer H. Efficacy and tolerability of a fluid extract combination of thyme herb and ivy leaves and matched placebo in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled clinical trial. Arzneimittelforschung 2006; 56, 652–660.
180. Kemmerich B. Evaluation of efficacy and tolerability of a fixed combination of dry extracts of thyme herb and primrose root in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled multicentre clinical trial. Arzneimittelforschung 2007; 57, 607–615.
181. Ernst E., März R., Sieder C. A controlled multi-centre study of herbal versus synthetic secretolytic drugs for acute bronchitis. Phytomedicine 1997; 4, 287–293.
182. Gruenwald J., Graubaum H. J., Busch R. Efficacy and tolerability of a fixed combination of thyme and primrose root in patients with acute bronchitis. A double-blind, randomized, placebo-controlled clinical trial. Arzneimittelforschung 2005; 55, 669–676.
183. Ismail C., Willer G., Steindl H. Bronchipret bei akuter Bronchitis. Schweiz. Zschr. Ganzheitsmedizin. 2003; 15, 171–175.
184. Lorenzi S., Placucci F., Vincezi C., et al. Allergic contact dermatitis due to thymol. Contact Dermatitis 1995; 33, 439–440.
185. Zava D. T., Dollbaum C. M., Blen M. Estrogen and progestin aktivity of foods, herbs, and spices. Proc. Soc. Exp. Biol. Med. 1998; 217, 369–378.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2016 Číslo 4- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Životné jubileum Dr.h.c. prof. RNDr. Václava Suchého, DrSc.
- Obsahové látky listov Cornus officinalis Sieb. et Zucc.*
- Bunkové a molekulové mechanizmy účinku horčín: aktuálne poznatky a perspektíva*
- Použití rostlinných extraktů jako efektivní alternativní terapie zánětů cest dýchacích
- Fenolové látky v listových inzerciách Mentha × villosa Huds. cv. Snežná*
- In memoriam – doc. RNDr. PhMr. Jarmila Vlčková, CSc.
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Použití rostlinných extraktů jako efektivní alternativní terapie zánětů cest dýchacích
- Bunkové a molekulové mechanizmy účinku horčín: aktuálne poznatky a perspektíva*
- Životné jubileum Dr.h.c. prof. RNDr. Václava Suchého, DrSc.
- Fenolové látky v listových inzerciách Mentha × villosa Huds. cv. Snežná*
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




