-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDevelopment of a spectrophotometric method for carmoisine determination in quality control of equipment cleaning
Authors: Anna Materiienko; Volodymyr Grudko; Victoriya Georgiyants
Authors place of work: The National University of Pharmacy, Kharkiv, Ukraine
Published in the journal: Čes. slov. Farm., 2016; 65, 32-33
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Pharmaceutical products can be contaminated by other active pharmaceutical ingredients, by cleaning agents, or by other materials. Cleaning processes in pharmaceutical industry have been regulated by the implementation of the Good Manufacturing Practice rules. Cleaning procedures include sampling and testing for allowable trace amounts of active pharmaceutical ingredients or excipients that were a part of the previous preparation1, 2).
There are two methods of sampling that are considered to be acceptable: direct surface sampling (swab method) and indirect sampling (use of rinse solutions). The acceptance limits of contaminants and residues are determined in the International Conference on Harmonisation (ICH) guidelines on their specific toxicity in tested impurities3, 4).
The suitability of the material to be used for sampling and the suitability of the sampling medium should be determined. The ability to recover samples accurately may be affected by the choice of the sampling material. A major complication when using rinse solutions is probably very dilute solutions since the quantitative content of the active substance is not always possible to determine by the available analytical methods1, 3).
Carmoisine (E 122) (Fig. 1) is a red-coloured synthetic food azo dye, a derivative of diazosulfonaphthalenes, a crystalline substance that is soluble in water. It is used to provide a certain colour for many medicines as for individual matters and mixed with other dyes5).
Methods for determining the dyes in food and medicines by HPLC are known6). But these methods are laborious, they require a lot of time and use expensive equipment that carries great expenses to pharmaceutical manufacturers.
Experimental methods
The goal of our work is to develop an express spectrophotometric procedure for determining the residual quantities of carmoisine dye in rinse solutions to control the completeness of the process equipment cleaning.
During carrying out the experimental researches, the following materials and methods were used – the synthetic food dye carmoisine (series 657334(1-20)-657383(1-20)) and the medicinal substance myramistin (series 010514). Analytical researches were performed on a spectrophotometer Evolution 60S. For operation we used an AXIS ANG200 analytical balances, Class A measuring glassware and excipients which meet the requirements of the State Pharmacopoeia of Ukraine (SPhU)7) which are harmonized with the European ones8).
To develop the control methods of equipment cleaning, we propose to use an extractive photometry method using the reaction of formation of the ion associate of carmoisine with myramistin. Due to the earlier results9), for the process of concentration of the substance contained in a large volume of liquid it is possible to use an extraction of forming ion associate by a minimum volume of an extractant. We found previously that the forming ion associate is extracted well with chloroform. We decided to use a mixture of butanol and chloroform for extraction. The sample solution was used to choose the optimal ration of chloroform and butanol.
Preparation of sample solution: 0.1 ml of 14 ∙ 10–3 M carmoisine solution was placed into a 1000.0 ml volumetric flask, 2.5 ml of 1 ∙ 10–3 M myramistin solution was added and adjusted to the mark with water R. The resulting sample solution was transferred to a separating funnel and extracted with a portion of organic solvent of 50.0 ml. The obtained extract was measured on a spectrophotometer at 523 nm wavelength against a blank solution.
The results are presented in Table 1.
Tab. 1. The optimum ratio of extragents 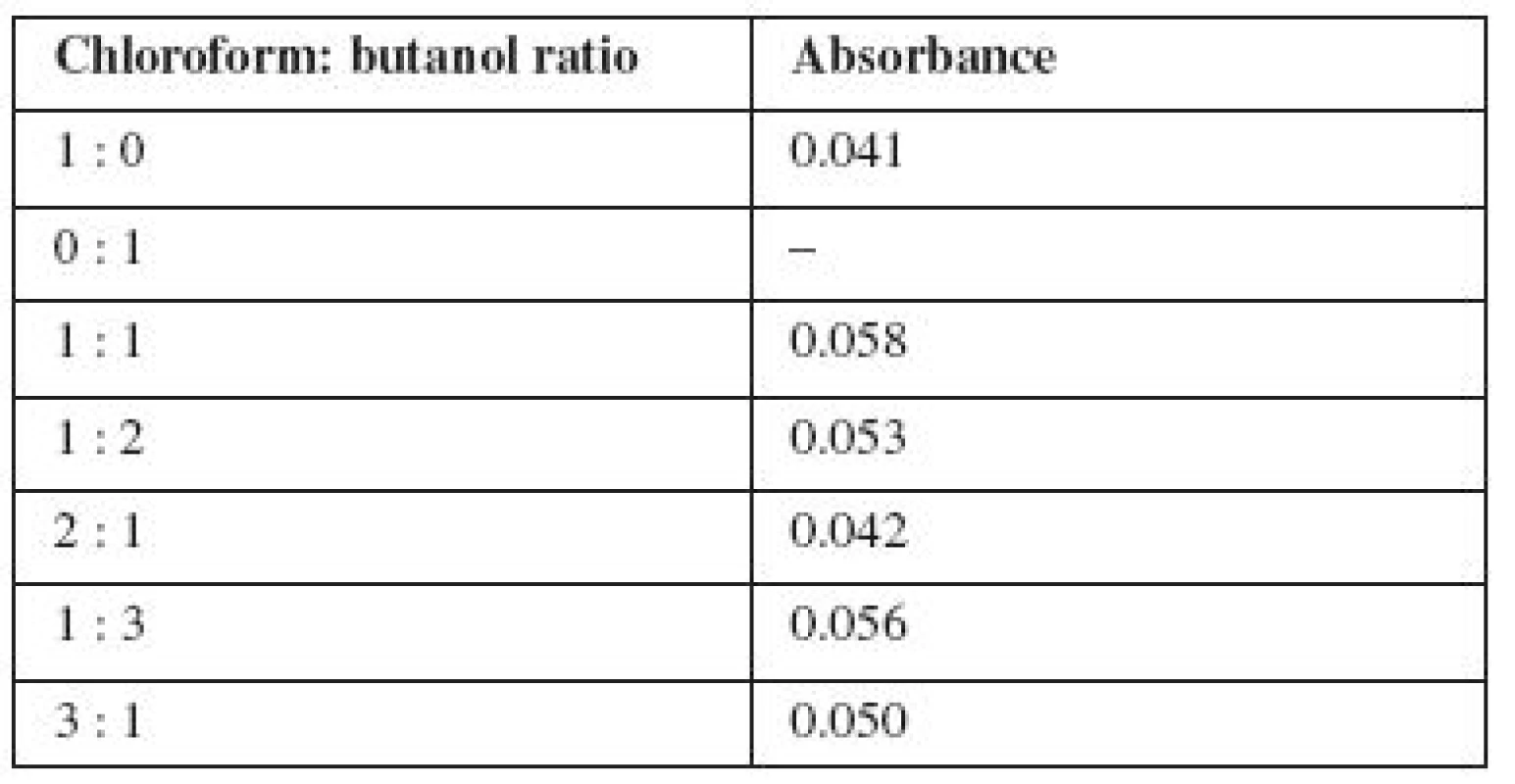
One of the major requirements that determine the possibility of using spectral methods to quantify the matter is subordination of light absorption solution of Beer-Lambert law.
To test this subordination, 0.0500 g of a standard dye sample (accurately weighed) quantitatively with 60 ml of water R was transferred to a volumetric flask of 100.0 ml, adjusted to the mark with the same solvent and thoroughly mixed. The aliquots of 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0 ml, respectively, of the resulting solution were taken and placed into a 100.0 ml volumetric flask and adjusted to the mark with the same solvent. The measurement of absorbance was carried out at 517 nm wavelength in a ditch with a layer thickness of 10 mm against a blank solution.
During the control of cleaning equipment, definition of dye residues was carried in a solution with a very low concentration. We have also tested that the light absorption of carmoisine and myramistin mixture solutions subordinates the Beer-Lambert law in a concentration of 1–10 ∙ 10–6 M.
Results and discussion
Analysis of data in Table 1 shows that the optimal ratio of chloroform and butanol in the organic phase depends on the partial solubility of butanol and chloroform in water. Accordingly, it is proposed to use chloroform and butanol ratio of 1 : 1 for the extraction of the ion associate of carmoisine with miramistin from rinse solutions.
The results suggest that the solution of carmoisine subordinated the Beer-Lambert law in concentrations of 0.5–5.0 mg/100 ml. The specific absorbance is 446 ± 3.
The results suggest that the solution of the associate subordinated the Beer-Lambert law in concentrations 1 ∙ 10–6 – 10 ∙ 10–6 M. The molar absorptivity is 11214 ± 460.
Method of determination the carmoisine in quality control of equipment cleaning
Place a 1000.0 ml of rinse solution into a separating funnel, add 2.5 ml of 1 ∙ 10–3 M myramistin solution and mix. Add 50.0 ml of a mixture solution of chloroform and butanol (in the ratio 1 : 1) to the obtained solution, shake it intensively for 2 minutes and leave for 5 minutes to separate the layers. Measure the absorbance on a spectrophotometer at 523 nm wavelength against blank solution, which is used as a mixture of chloroform with butanol.
Preparing the solution of dye standard sample
0.0500 g of standard dye sample (accurately weighed) is quantitatively transferred to a volumetric flask of 100.0 ml, adjusted to the mark with the water R and thoroughly mixed. The aliquot of 1.0 ml of the resulting solution is taken and placed in a 100.0 ml volumetric flask and adjusted to the mark with the same solvent.
Calculate the residual quantities of carmoisine in rinse solution (in mg) using the formula:
where А absorbance of test solution, Ast absorbance of standard solution, Сst concentration of standard solution, mg/ml.
In order to obtain the metrological characteristics of the method developed for the determination of residual quantities of carmoisine dye in rinse solutions, we used the method of modelling samples.
Preparation of sample solution: 0.1 ml of 14 ∙ 10–3 M carmoisine solution was placed into a 1000.0 ml volumetric flask, 2.5 ml of 1 ∙ 10–3 M myramistin solution was added and adjusted to the mark with water R. The resulting sample solution was transferred to a separating funnel and extraction was performed by the developed method described above.
The results are given in Table 2.
Tab. 2. The metrological characteristics of the developed method (P, % = 95, n = 6) 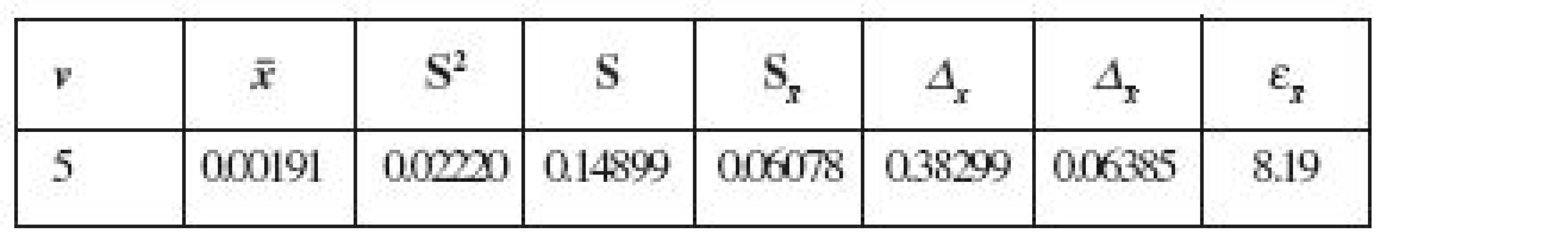
Conclusions
- A method of determination of residual quantities of carmoisine in rinse solutions from the surface of the pharmaceutical equipment was developed.
- A simple and expressive procedure, which is characterized by satisfactory metrological characteristics, was proposed.
- The method can be recommended for the determination of residual quantities in the control of fullness cleaning of process equipment for pharmaceutical companies.
Conflict of interest: none.
Anna Materiienko
The National University of Pharmacy
Pushkinska str. 53,
61002 Kharkiv,
Ukraine
e-mail: anna.materienko@gmail.com
Zdroje
1. PIC/S document PI006-3. Validation master plan installation and operational qualification. Non-sterile process validation. Cleaning validation 2007; 27 p.
2. U. S. Food and Drug Administration. Guide to inspections validation of cleaning processes. http://www.fda.gov/ICECI/Inspections/ InspectionGuides/ucm074922.htm
3. Shklyaev S. A. Analytical procedures provide cleaning of process equipment from residues of the active ingredients in pharmaceutical company. Kyiv: Pharmaceutical Journal 2012; 5, 33–37.
4. Zavyalova I. E., Sharahova E. F. Organizational-methodical approaches to validation of cleaning processes of equipment in the production of medicines. Siberia: Siberian Medical Review 2010; 63(3), 57–62.
5. Smirnov E. V. Food dyes. Directory St. Petersburg: ed. Profession 2009.
6. Minioti K. S., Sakellariou C. F., Thomaidis N. S. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Elsevier: Analytica Chimica Acta 2007; 583(1), 103–110.
7. European Pharmacopoeia. Sixth edition. Volume 2.2. http://online6.edqm.eu/ep802/
8. State Pharmacopoeia of Ukraine. State Enterprise “Scientific and expert pharmacopoeia center”. 1st ed. Kharkiv: RIREH 2001.
9. Materiienko А. S., Grudko V.O., Georgiyants V. A., Khanin V. A. The study of properties an ion associate of food azo dye carmoisine with myramistin. Der Pharma Chemica 2015; 7(6), 237–244.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2016 Číslo 1- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Antimikrobiální, protiparazitické a protinádorové vlastnosti Hibiscus sabdariffa (L.) a jeho sloučeniny: in vitro a in vivo studie
- Orální filmy jako perspektivní léková forma
- Vliv velikosti oka extruzní přepážky na vlastnosti jader určených k přípravě pelet s řízeným uvolňováním glukosy
- Potential anticancer agent hypericin and its model compound emodin: interaction with DNA
- Vzťah dysbalancie lipidov a chronického zápalu sprostredkovaný PPAR
- Development of a spectrophotometric method for carmoisine determination in quality control of equipment cleaning
- Thin-layer chromatography application for the standardization of Sophora flowers
- 35. technologické dni a pohľad do histórie tohto podujatia
- Farmakognózia. Biologicky aktívne rastlinné metabolity a ich zdroje.
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Orální filmy jako perspektivní léková forma
- Farmakognózia. Biologicky aktívne rastlinné metabolity a ich zdroje.
- Antimikrobiální, protiparazitické a protinádorové vlastnosti Hibiscus sabdariffa (L.) a jeho sloučeniny: in vitro a in vivo studie
- Vzťah dysbalancie lipidov a chronického zápalu sprostredkovaný PPAR
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





