-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInfluence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
Vliv formulačních a procesních parametrů na vlastnosti PLGA mikročástic s řízeným uvolňováním léčiva
PLGA mikročástice pro prodloužené uvolňování modelového léčiva ibuprofenu se připravily metodou odpaření rozpouštědla z jednoduché emulze O/V, s různými rychlostmi otáček míchadla (600, 1000 rpm), různou koncentrací emulgátoru (0,1%, 1% PVA) a za použití odlišných organických rozpouštědel (dichlormethan, ethylacetát). Z dosažených výsledků je zřejmé, že zvolené formulační a procesní proměnné ovlivňovaly vlastnosti připravených PLGA mikročástic, a to jejich enkapsulační účinnost, výtěžek, morfologii, velikost částic i uvolňování léčiva. Zvýšení rychlosti otáčení míchadla v procesu odpařování rozpouštědla vedlo ke snížení enkapsulační účinnosti, výtěžku procesu i velikosti mikročástic. Uvolňování léčiva však probíhalo rychleji. Vyšší koncentrace PVA ve vnější fázi emulze měla za následek stejný vliv na sledované vlastnosti s výjimkou rychlosti uvolňování ibuprofenu. Mikročástice připravené při použití dichlormethanu se vyznačovaly vyšší sfericitou, pravidelným tvarem s hladkým povrchem, a vykazovaly tedy lepší kvalitativní vlastnosti ve srovnání s mikročásticemi připravenými při použití ethylacetátu.
Klíčová slova:
mikročástice • odpaření rozpouštědla • PLGA • řízené uvolňování • burst effect
Authors: Jakub Vysloužil • Martina Kejdušová • Kateřina Dvořáčková • David Vetchý
Authors place of work: University of Veterinary and Pharmaceutical Sciences, Department of Pharmaceutics, Faculty of Pharmacy, Brno
Published in the journal: Čes. slov. Farm., 2013; 62, 120-126
Category: Původní práce
Summary
PLGA microparticles for sustained release of ibuprofen as the model drug were prepared by the O/W solvent evaporation method under altering stirring speed (600, 1000 rpm), emulsifier concentration (PVA concentration 0.1%, 1%) and organic solvent selection (dichloromethane, ethyl acetate). The obtained results confirmed the effect of selected formulation and process parameters on the properties of prepared PLGA-based microparticles. An influence on encapsulation efficiency, yield, morphological properties, mean size and drug release was observed. Increased stirring speed within the solvent evaporation process resulted in a decrease of encapsulation efficiency, yield and mean size but the incorporated drug was released faster. Increased PVA concentration in the external emulsion phase brought the same results except the ibuprofen release rate. Microparticles prepared with dichloromethane as the organic solvent exhibited higher sphericity, a more regular shape with a smooth surface, and thus dichloromethane was considered to be a more suitable organic solvent in comparison with ethyl acetate for this purpose.
Keywords:
microparticles • solvent evaporation • PLGA • controlled release • burst effectIntroduction
Microparticles represent a modern and perspective dosage form for controlled delivery of pharmaceuticals. They are defined as solid particles with an approximately round shape and a size between several and hundreds of micrometres. The first subtype, microcapsules have a solid, liquid or gaseous core containing a drug surrounded by a solid shell. The second subtype, microspheres are formed by a matrix without a differentiated core and shell. A drug is spread through the matrix more or less evenly1).
Various methods have been developed to prepare microparticles with sustained drug release. One of the most widely employed techniques is the solvent evaporation method covering several modifications. Selection of a suitable modification is primarily determined by the drug solubility in various solvents2, 3).
O/W single emulsion modification, frequently used for microspheres preparation, consists of several steps. Firstly in this process, the polymer is dissolved in a water immiscible solvent, and the drug intended for encapsulation is dispersed or dissolved in the polymeric solution. Consequently, the resultant solution is emulsified in an aqueous continuous phase to form droplets. Within agitation, the microspheres harden as the solvent evaporation and polymer precipitation occur. Emulsification process mainly affects microparticle size distribution and the conditions of solvent evaporation determine the microparticle morphology, and they are also considered as the critical point for encapsulation efficiency and release behavior of microparticles4). O/W modification is preferably suitable for incorporation of drugs with poor solubility in water5).
Generally, the solvent evaporation method is the most popular technique for preparing poly(lactid-co-glycolic acid) (PLGA) microspheres. This method yields in uniform particle size and has good reproducibility6). PLGA microparticles offer various benefits: 1. possibility to accurately control the resulting drug release kinetics over periods of days to months; 2. complete biodegradability (avoiding the removal of empty remnants upon drug exhaust); 3. good biocompatibility, even if directly administered into the brain tissue7).
The main objective of this study was to prepare biodegradable PLGA microparticles with content of a model drug practically insoluble in water and to observe potential influences of formulation and process variables on the characteristics of the prepared microparticles. For this reason solvent evaporation from O/W single emulsion was chosen.
Experimental part
Materials
Ibuprofen - IBU (Zentiva, Prague, Czech Republic) was used as the poor soluble model drug, PLGA Resomer® RG 504 H (Boehringer Ingelheim, Pharma GmbH & Co, Ingelheim am Rhein, Germany) was used as a polymer carrier. Dichloromethane – DM (Penta, Praha, Czech Republic) and ethyl acetate – EA (Dr. Kulich Pharma, Hradec Králové, Czech Republic) were used as solvents, and polyvinyl alcohol – PVA (Sigma Aldrich, St. Louis, USA) as an emulsifier. Phosphate buffer of pH 6.8 (dodecahydrate sodium hydrogenphosphate, potassium dihydrogenphosphate – both Merck KGaA, Darmstadt, Germany) was applied as the dissolution medium. All materials were of Ph. Eur. Quality.
Microparticle preparation
PLGA microparticles were prepared by the O/W modification of the solvent evaporation method. The process was carried out at laboratory temperature and pressure. To form the internal phase of emulsion, 150 mg of the drug and 700 mg of the polymer were dissolved in 5 ml of either dichloromethane or ethyl acetate. The solution was homogenized using an Ultra-Turrax (T25 basic, IKA-Werke, Staufen, Germany) at 20 000 rpm for one minute to ensure homogenous solution. All at once it was emulsified into the 800 ml of aqueous continuous phase containing 0.1% (w/w) or 1% (w/w) PVA. The emulsion was stirred by a mechanical stirrer (Heidolph RZR 2021, Sigma Aldrich, St. Louis, USA) at 600 or 1000 rpm for 2 hours to ensure complete evaporation of the organic solvent. Prepared microparticles were collected on a fine mesh sieve (opening size 80 μm), washed three times with purified water and dried at 25 °C in a cabinet drier (HORO – 048B, Dr. Hofmann GmbH, Ostfildern, Germany). Every sample was prepared three times. Prepared samples were named in accordance with the type and values of altered variables (organic solvent selection, PVA concentration, stirring speed during evaporation). Samples characteristics are shown in Table 1.
Tab. 1. Variables during preparation of microparticles samples 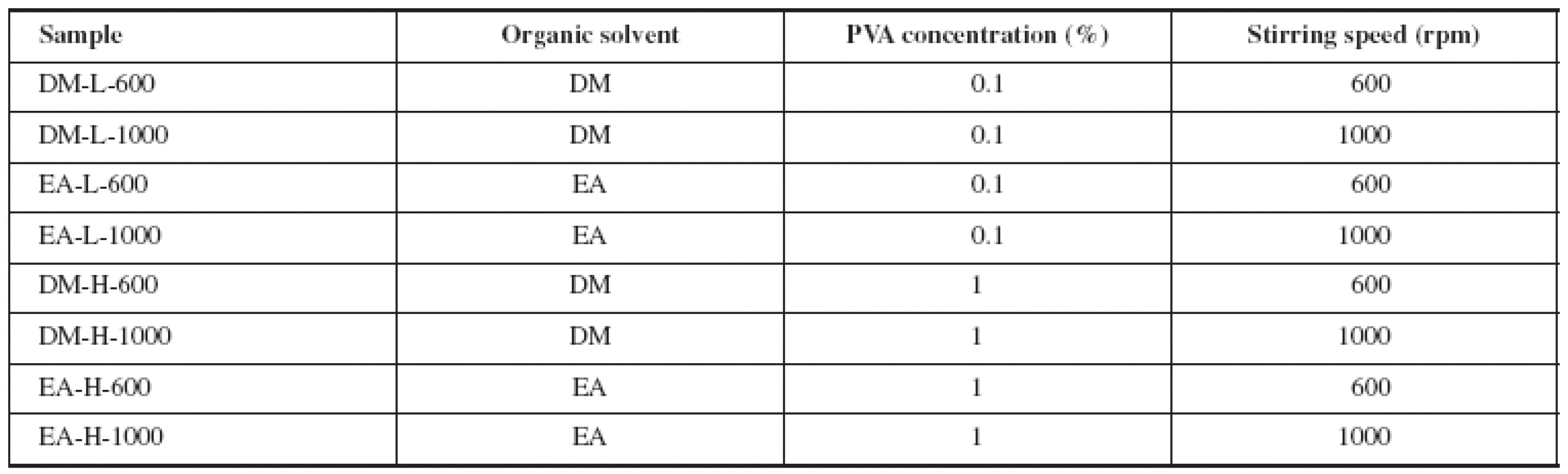
Microparticle characteristics
Drug content
The drug content was determined spectrophotometrically. The appropriate amount of dried PLGA microparticles (~ 10 mg of ibuprofen) was dissolved in 25 ml of the appropriate organic solvent (ethyl acetate or dichloromethane). Absorbance of the samples was measured at 264 nm using an UV/Vis spectrometer (Lambda 25, Perkin Elmer, Waltham, USA). Encapsulation efficiency (EE) and drug load (DL) were calculated from the obtained values by using the following equations8, 9):
where cs corresponds to the actual ibuprofen content and ct represents the theoretical drug load. The assay was carried out in triplicate. The results are expressed as mean values and standard deviation
where w1 is the weight of the drug in the microparticles, w2 is the gross weight of the microparticles. The assay was carried out in triplicate and results are stated as mean values and standard deviations.
Effectiveness of the process was also evaluated by yield, calculated by the following equation:
where w2 is the gross weight of the microparticles, wt is the total weight of the drug and the polymer used for microparticle preparation.
Scanning electron microscopy
Scanning electron microscopy was employed to examine microparticle morphology and surface topography (SEM). The samples were mounted directly onto a SEM sample holder using a double-side sticking tape and then coated with a 10 nm thick layer of Au. Images were taken using a scanning electron microscope MIRA3 (Tescan, Brno, Czech Republic) at an accelerating voltage of 5.0 kV.
Particle distribution
Mean diameter and the particle size distribution of microparticles were measured by a laser diffraction particle size distribution analyser (Horiba Partica LA-300, Horiba Ltd., Tokyo, Japan). Prediluted microparticle sample in a volume of 100 μμl (0.5 g/ml of phosphate buffered saline) was injected into a cuvette-type fraction cell (filled with 10 ml of degassed and filtered phosphate buffered saline, pH of 7.2) equipped with a magnetic stirrer to prevent nonhomogeneous distribution owing to sedimentation of particles. All samples were measured immediately after the application into the cuvette and they were analysed for number-weighted size distribution.
In vitro release studies
The in vitro drug release studies of drug-loaded beads were carried out using a special adjusted basket method in an automatic dissolution apparatus (SOTAX AT 7 On-Line System, Donau Lab, Zürich, Switzerland) at 100 rpm. The dissolution medium volume used was 500 ml of phosphate buffer (pH 6.8) kept at 37.0 ± 0.5 °C. Samples were analyzed using an UV spectrophotometer (Lambda 25, Perkin Elmer, St. Louis, USA) at 264 nm, in connection with the Sotax on-line software. The samples for dissolution test were weighted with respect to their encapsulation efficiency, so that 50 mg of ibuprofen were in each sample. In vitro drug release was observed for 72 hours and it was carried out twice for each sample. Results were expressed as average values and standard deviations.
Results and discussion
Particle size and morphological properties
Effect of the stirring speed
Size of the prepared microparticles ranged between 29.9 and 174.6 μμm. Results (Table 2) clearly show that the prepared microparticles mean size decreased when the stirring speed increased. This effect was also observed in the research work by Sansdrap and Moes. Higher power input leads to distribution of the internal phase into smaller droplets and provides smaller microspheres10).
Effect of PVA concentration
PVA concentration had a significant influence on microparticle size (Table 2) as mean size was decreasing with increasing PVA concentration. Mean size of the samples prepared with 0.1% PVA took values from 77.3 to 174.6 μμm. Mean size of microparticles prepared with 1% PVA ranged between 29.9 and 116.2 μμm. Explanation could be seen in improved stability of the created emulsion, where a higher concentration of PVA was used. This could result in creation of smaller microparticles11). Observable was also enhanced emulsion stabilization, resulting in narrower particle size distribution as showed in Figure 1 and Figure 2. This phenomenon is in agreement with the literature12). PVA concentration also had impact on morphological properties. SEM analysis (Fig. 3) showed that sample DM-L-600 provided approximately spherical microparticles with occasional deformities and holes. In contrast, DM-H-600 sample yielded compact, spherical, regular and more uniform microparticles.
Tab. 2. Microparticle characteristics – encapsulation efficiency, drug loading, yield, mean size and initial drug release 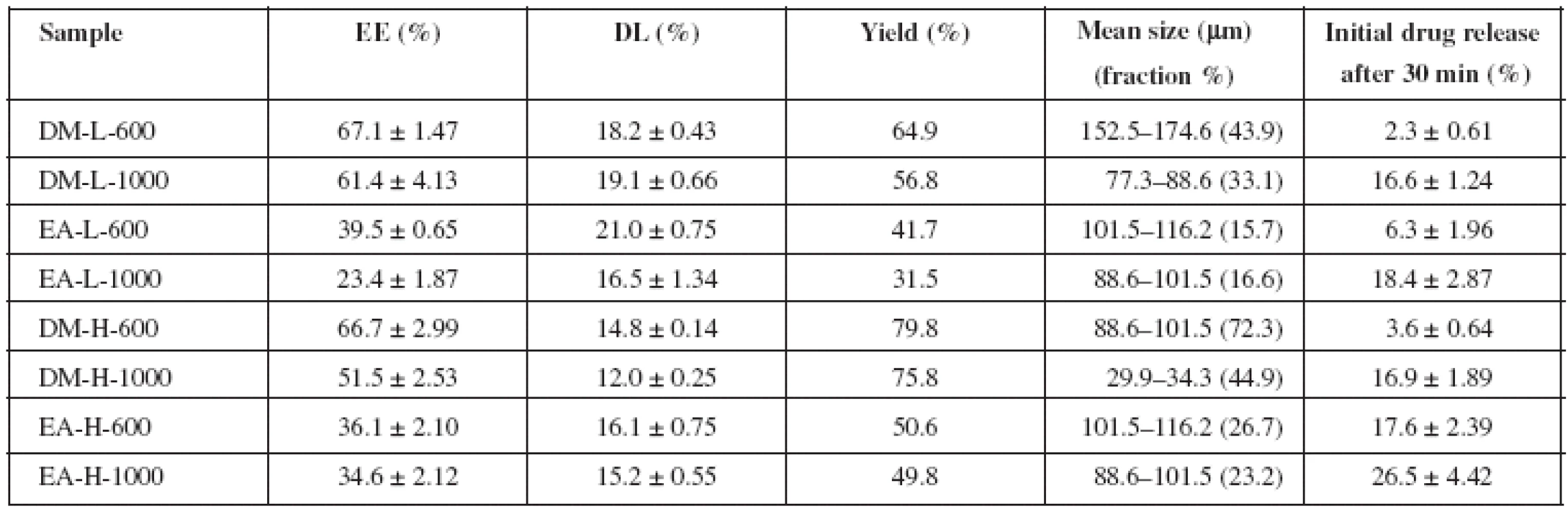
Fig. 1. Particle size distribution of PLGA microparticles; DM samples: a – DM-L-600, b – DM-H-600, c – DM-L-1000, d – DM-H-1000 
Fig. 2. Particle size distribution of PLGA microparticles; EA samples: a – EA-L-600, b – EA-H-600, c – EA-L-1000, d – EA-H-1000 
Fig. 3. SEM photographs of the surface topography of PLGA microparticles: a – DM-L-600, b – DM-H-600, c – EA-L-600, d – EA-H-600 
Effect of the organic solvent choice
Selection of the organic solvent for the microparticle preparation had a significant effect on morphological properties. It is apparent that DM samples provided more regular and spherical microparticles with a relatively smooth surface (Fig. 3a,b). On the other hand, ET samples were of an irregular, non-spherical shape and a rough surface (Fig. 3c,d). An explanation can be found in higher polarity of EA in comparison with DM. This could worsen the formation process resulting in less quality microparticles.
Encapsulation process
Effect of stirring speed
Drug loading of the samples took values from 12.0 to 21.0% (Table 2). Generally, higher rotation speed had a negative effect on DL value, except the DM samples prepared with PVA solution of lower concentration. Similarly, the results of encapsulation efficiency brought strong evidence that this parameter was decreased with an increase in the stirring speed. DM samples prepared with 0.1% PVA may serve as an example. EE decreased from 67.1% to 61.4%, (5.7% decline), when the stirring speed was increased from 600 rpm to 1000 rpm. This effect was also observed in the research work by O’Donnell et McGinity13). Higher power input of the increased stirring speed generates greater energy to the emulsion system, causing more frequent breakdowns of the forming microparticles. EE is thus lower, just as the yield (Table 2).
Effect of PVA concentration
DL was lower in samples prepared with 1% PVA, as shown in Table 2. This observation is in agreement with the literature. High PVA concentration could facilitate distribution of IBU in the external phase14, 15). PVA concentration also influenced EE when DM was used during preparation. From Table 2 it is obvious that EE was only slightly higher in samples prepared at a lower stirring speed (67.1% for DM-L-600 and 66.7% for DM-H-600). A more significant difference was seen in samples obtained at 1000 rpm (61.4% for DM-L-1000 and 51.5% for DM-H-1000). Increasing PVA concentration can lead to higher encapsulation efficiency16), but only to a certain concentration value. Beyond this value, a decrease in EE follows, which could be the explanation of our results, too14, 17, 18). This dependence was not observed in the EA samples. However, EA results might be slightly influenced by the tendency of the internal phase to adhere unpredictably to the shaft of the mechanical stirrer, contributing to a higher loss rate.
Effect of the organic solvent choice
No clear dependence of organic solvent selection on DL was found, when this process was carried out at the stirring speed of 1000 rpm. At the slower rotation speed of 600 rpm, the higher DL was monitored for both PVA concentrations if EA was used as the organic solvent (Table 2). The results provided an unwavering proof that the organic solvent selection is the crucial parameter for encapsulation efficiency. DM samples showed higher EE and yield than their equivalent counterparts prepared with EA. EA has higher polarity than DM and is thus more miscible with water. Higher miscibility of EA with external phase was responsible for creation of less stable emulsion. This fact could lead to more frequent drug leakage from the emulsion internal phase to the external phase, causing lower EE and yield2, 13) (Table 2) .
Drug release behavior of microparticles
The drug dissolution profiles obtained at pH 6.8 are shown in Figure 4 and Figure 5. In general, dissolution curves could be characterized as bi-phasic profiles with more or less significant burst release followed by sustained release of the drug for at least 72 hours. PLGA-based microparticles usually exhibit a tri-phasic profile. However, the influence of several factors such as pore formation or closure, drug solubility, drug amount, polymer molecular weight or its density can transform the tri-phasic profile to the bi-phasic one19-21).
Fig. 4. In vitro release profiles of IBU; DM samples 
Fig. 5. In vitro release profiles of IBU; EA samples (EA-L-600 – SD max. = 2.54) 
Effect of stirring speed
Figure 4 and Figure 5 provide evidence that all samples prepared at 1000 rpm exhibited a higher burst effect than their counterparts prepared at 600 rpm. This was followed by slightly faster drug release with a single exception of ET-H-1000, whose dissolution curve was eventually exceeded by ET-H-600. At 1000 rpm, smaller microparticles were prepared, and thus the samples had a larger surface area per weight unit than the samples prepared at 600 rpm. This fact could explain a more significant burst effect and faster IBU release18). However, more factors could contribute to the higher burst effect, such as more extensive surface porosity and uneven drug spreading in the matrix increasing towards particle surface19).
Effect of PVA concentration
Dissolution profiles clearly showed the influence of PVA concentration on drug release characteristics. Samples prepared with 1% PVA liberated the model drug faster in comparison with the samples prepared with lower PVA concentration. Once more, explanation can be found in the microparticle mean size. Higher PVA concentration ensured production of smaller microparticles with a larger surface per weight unit. This could result in faster IBU release. The effect is more evident in EA samples.
Effect of organic solvent choice
Organic solvent choice was found to have significant influence on dissolution profiles of microparticles. Prepared samples released from 18.0 to 83.0% of IBU within 72 hours. All EA samples possessed a higher burst effect (6.3% drug released in 30 minutes for EA-L-600, 18.4% for EA-L-1000, 17.6% for EA-H-600, 26.5% for EA-H-1000) when compared to their equivalent DM counterparts (2.3% for DM-L-600, 16.6% for DM-L-1000, 3.6% for DM-H-600 and 16.9% for DM-H-1000). Subsequent release was slower in EA samples (18.0% drug released in 72 hours for EA-L-600, 44.5% for EA-L-1000, 75.0% for EA-H-1000) when confronted with DM samples (49.2% for DM-L-600, 59.0% for DM-L-1000, 83.0% for DM-H-1000). The only exception was seen in sample EA-H-600 (79.6%) which exhibited faster drug release than DM-H-600 (57.9%). In general, ethyl acetate samples prepared with 1% PVA experienced considerable improvement in drug release rate. This finding suggests that the result could be a combination of both, PVA concentration and organic solvent selection factors. Distinctions between EA and DM samples could be attributed to different volatility, boiling point and polarity of the solvents used22), providing diverse matrix structures and shape.
Conclusion
PLGA microparticles with sustained drug release of a practically insoluble drug were successfully prepared by the O/W solvent evaporation method. During in vitro dissolution test, samples released different drug amounts ranging between 18.0–83.0% within 72 hours. All parameters under study influenced the microparticle properties, such as encapsulation efficiency, yield, microparticle mean size, morphological properties and drug release. After evaluation of all results, the microparticles preparation at 600 rpm with 1% PVA concentration and dichloromethane as the organic solvent for the internal phase were considered to be optimal. The foundation obtained from this study allowed the incorporation of the drug with its own pharmacological effect.
Acknowledgement
Authors would like to thank TESCAN, a.s., Brno, Czech Republic for SEM analysis performance.
Conflicts of interest: none.
Received 22 February 2013 / Accepted 4 April 2013
PharmDr. Jakub Vysloužil (∗) • M. Kejdušová • K. Dvořáčková • D. Vetchý
University of Veterinary and Pharmaceutical Sciences, Department of Pharmaceutics, Faculty of Pharmacy
Palackého 1/3, 612 42 Brno
e-mail: jakub.vyslouzil@gmail.com
Zdroje
1. Krejčová K., Gryczová E., Rabišková M. Polymeric Microparticles for Oral Administration of Diclofenac Sodium. Chem. Listy 2009; 103, 81–87.
2. Li M., Rouaud O., Poncelet D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008; 363, 26–39.
3. Pérez M. H., Zinutti C., Lamprecht A., Ubrich N., Astier A., Hoffman M., Bodmeier R., Maincent P. The preparations and evaluation of poly(εε-caprolactone) microparticles containing both a lipophilic and a hydrophilic drug. J. Control. Release 2000; 65, 429–438.
4. Rosca I. D., Watari F., Uo M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J. Control. Release 2004; 99, 271–280.
5. Giri T. K., Choudhary Ch., Ajazuddin A., Badwaik H., Tripathi D. K. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm. 2013; 21, 125–141.
6. Tunçay M., Çaliş S., Kaşş H. S., Ercan M. T., Peksoy İ., Hincal A. A. Diclofenac sodium incorporated PLGA (50 : 50) microspheres: formulation considerations and in vitro/in vivo evaluation. Int. J. Pharm. 2000; 195, 179–188.
7. Klose D., Siepmann F., Elkharraz K., Krenzlin S., Siepmann J. How porosity and size affect the drug release mechanism from PLGA-based microparticles. Int. J. Pharm. 2006; 314, 198–206.
8. Paskaris G., Bouropoulos N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads. Int. J. Pharm. 2006; 323, 34–42.
9. Wang S. B., Chen A. Z., Weng L. J., Chen M. Y., Xie X. L. Effect of drug-loading methods on drug load, encapsulation efficiency and release properties of alginate/poly-L-arginine/chitosan ternary complex microcapsules. Macromolecular Bioscience 2004; 4, 27–30.
10. Sansdrap P., Moes A. J. Influence of manufacturing parameters on the size characteristics and the release profiles of nifedipine from poly (DL-lactide-co-glycolide) microspheres. Int. J. Pharm. 1993; 98, 157–164.
11. Yang Y. Y., Chung T., Ng N. P. Morphology, drug distribution and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 2001; 22, 231–241.
12. Mainardes R. M., Evangelista R. C. PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int. J. Pharm. 2005; 290, 137–144.
13. O’Donnell P. B., McGinity J. W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Delivery Rev. 1997; 28, 25–42.
14. Wang C., Ye W., Zheng Y., Liu X., Tong Z. Fabrication of drug-loaded biodegradable microcapsules for controlled release by combination of solvent evaporation and layer-by-layer self-assembly. Int. J. Pharm. 2007; 338, 165–173.
15. El Bahri Z., Taverdet J. L. Elaboration and characterisation of microparticles loaded by pesticide model. Powder technol. 2007; 172, 30–40.
16. Mao S., Xu J., Cai C., Germershaus O.,Schaper A., Kissel T. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int. J. Pharm. 2007; 334, 137–148.
17. Capan Y., Woo B. H., Gebrekidan S., Ahmed S., DeLuca P. P. Influence of formulation parameters on the characteristics of poly(D, L-lactide-co-glycolide) microspheres containing poly(L-lysine) complexed plasmid DNA. J. Control. Release 1999; 60, 279–286.
18. Mao S., Shi Y., Li L., Xu J., Schaper A., Kissel T. Effects of process and formulation parameters on characteristics and internal morphology of poly(D, L-lactide-co-glycolide) microspheres formed by the solvent evaporation method. Eur. J. Pharm. Biopharmaceutics 2008; 68, 214–223.
19. Fredenberg S., Wahlgren M., Reslow M., Axelsson A.: The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems – a review. Int. J. Pharm. 2011; 415, 34–52.
20. Berchane N. S., Carson K. H., Rice-Ficht A. C., Andrews M. J. Effect of meandiameter and polydispersity of PLGmicrospheres on drug release: Experiment and theory. Int. J. Pharm. 2007; 337, 118–126.
21. Berkland C., Kim K. and Pack D. W. PLG Microsphere Size Controls Drug Release Rate Through Several Competing Factors. Pharmaceut. Res 2003; 20, 1055–1062.
22. Sah. H. Microencapsulation techniques using ethyl acetate as a dispersed solvent: effects of its extraction rate on the characteristics of PLGA microspheres. J. Control. Release 1997; 47, 233–245.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2013 Číslo 3- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Warfarin – its synthesis and properties in a twenty-year retrospective*
- Influence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
- A study of a new co-processed dry binder based on spray-dried lactose and microcrystalline cellulose
-
Study of local anaesthetics: Part 201*
Determination of the critical micellar concentration of pentacaine hydrochloride from the measurements of UV absorption of pyrene in methanol solutions - HPLC determination of saccharides after pre-column derivatization in honey samples
- Use of human medicinal preparations in veterinary medicine
- Analysis of the consumption of sedative and hypnotic drugs in Yemen and the Czech Republic
- Prof. RNDr. Milan Melník, DrSc. – 75-ročný
- Štátne vyznamenanie – Dr.h.c., prof. RNDr. Jozefovi Čižmárikovi, PhD.
- Nové knihy
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Warfarin – its synthesis and properties in a twenty-year retrospective*
- Use of human medicinal preparations in veterinary medicine
- Influence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
- HPLC determination of saccharides after pre-column derivatization in honey samples
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání









