-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMucoadhesive films as perspective oral dosage form
Mukoadhezivní filmy jako perspektivní lékové formy pro orální podání
Mukoadheze představuje specifický proces vytváření vazeb během těsného kontaktu mezi biologickými povrchy pokrytými slizovou vrstvou a mukoadhezivním materiálem. V posledních letech vstupují do popředí zájmu farmaceutického průmyslu moderní lékové formy založené na tomto procesu. Filmy složené z mukoadhezivních polymerů (deriváty celulosy, polyakryláty, polyoxyethylen atd.) připravené některou ze zavedených metod (odpařování rozpouštědla, extruze taveniny atd.) mohou být perspektivními kandidáty pro orální aplikaci mnoha léků, především pro jejich flexibilitu a pohodlné použití. K dalším výhodám této lékové formy patří zlepšení relativně krátké doby setrvání konvenčních orálních lékových forem na sliznici a dodání přesné dávky léčiva na místo aplikace. Kromě toho mohou také překrytím chránit povrch slizniční léze, což napomáhá ke snížení bolestivosti a zlepšení účinnosti léčby. Cílem tohoto článku je poskytnout přehled o principech vzniku mukoadhezivních vazeb a o inovativní lékové formě – mukoadhezivních filmech z hlediska jejich složení, přípravy a praktického využití.
Klíčová slova:
sliznice dutiny ústní • principy mukoadheze • mukoadhezivní lékové formy • filmy • disky
Authors: Hana Landová; Zdeněk Daněk; Jan Gajdziok; David Vetchý; Jan Štembírek
Published in the journal: Čes. slov. Farm., 2013; 62, 4-11
Category: Přehledy a odborná sdělení
Summary
Mucoadhesion is a specific phenomenon of creating bonds during intimate contact between biological surfaces covered by a mucus layer and a mucoadhesive material. In recent years come to the forefront of interest in the pharmaceutical industry modern dosage forms based on this specific process. Films (discs, patches) composed of mucoadhesive polymers (cellulose derivatives, polyacrylates, polyoxyethylene, etc.) prepared by established methods (solvent casting, hot melt extrusion, etc.) could be perspective candidates for oral administration of many drugs due to their flexibility and comfortable use. In addition, they can circumvent the relatively short residence time of conventional oral dosage forms on the mucosa and provide a precisely measured drug dose to the application site. Moreover, they can also help to protect the wound surface, thus help to reduce pain and improve effectiveness of the therapy. The aim of this article is to give an overview about the principles of creation of mucoadhesive bonds and about novel dosage form – mucoadhesive films in terms of their composition, preparation and practical usage.
Keywords:
oral mucosa • mucoadhesion principles • mucoadhesive dosage forms • films • patches • discs
Mucoadhesion/bioadhesion
Mucus is composed of mucin glycoproteins (0.5–5%), lipids, inorganic salts, nucleic acids, enzymes and water (more than 95%). The mucin glycoproteins are the most important structure-forming components of the mucous, resulting in its gel-like characteristic, cohesive and adhesive properties1, 2).
In 1986, Longer and Robinson defined bioadhesion as the phenomenon between two materials (a synthetic or natural macromolecule and mucus and/or epithelial surface), which are held together for extended period of time by interfacial forces3). In general, “bioadhesion” is a superior term used to describe adhesive interactions with any biological or biologically derived material, and “mucoadhesion” is used only when describing a bond involving mucus or mucosal surface4).
The process involved in the mucoadhesion phenomenon has been described in three steps: an intimate contact with the tissue resulting from a good wetting of the mucosal surface and swelling of the mucoadhesive polymer; interpenetration of the polymer chains and entanglement with those of mucus; and finally the formation of weak chemical bonds between entangled chains5).
To date, no single-valued theory has been accepted to explain mucoadhesion as a phenomenon occurring via one plain mechanism. However, several theories have been developed and used to describe the complex phenomenon of mucoadhesion. Some of these theories are founded on physical interactions (diffusion theory) while others are based on chemical interactions, such as electrostatic, hydrophobic, hydrogen bonding and van der Waals interactions (adsorption and electronic theories)6).
- Electronic theory – the mucoadhesive polymer and mucin glycoproteins have typically different electronic characteristics, resulting in the formation of the electrical double layer at the interface. Attraction across the electrical double layer leads to adhesion of the two surfaces7).
- Adsorption theory – the formation of mucoadhesive bonds could be a result of secondary surface forces such as van der Waals forces, hydrogen bonds and hydrophobic bonds. For bioadhesive polymers with carboxyl groups, hydrogen bonding is considered to be the dominant force at the interface8, 9).
- Diffusion theory – is based on the diffusion and interpenetration of the adhesive polymeric chains and the substrate to a sufficient depth while creating a semipermanent adhesive bond. The penetration rate depends on concentration gradients and diffusion coefficients of interacting polymers (mucoadhesive polymer and glycoprotein chains of the mucus), which are affected by their molecular weight and cross-linking density10).
- Fracture theory – relates the force required for the detachment of polymers from the mucus to the strength of their adhesive bonds. It has been found that the strength of adhesion decreases with increasing cross-linking density of the polymer11).
- Wetting theory – is primarily applicable to liquid or semi-solid mucoadhesive systems and relates the ability of a mucoadhesive polymer to spread over a tissue. This theory uses surface tensions at the interfaces to calculate the spreading coefficient12).
The mucoadhesion process probably involves all of the above-mentioned mechanisms and the decisive factor establishing the dominant one is the type of the particular mucoadhesive polymer.
Mucoadhesive films
Films or patches are the most recently developed dosage form for buccal administration. In the scientific literature it is possible to find equivalent terms “patches”, “films” and also “discs”. Some reviews include films (especially these forming in situ) into the semi-solid form13). Films are laminates usually consisting of two or three layers and, thanks to their flexibility and comfortable use, are preferred over adhesive tablets. Small thickness of the film with non-irritating properties and strong mucoadhesiveness of the polymer demand only minimal changes in the patients’ normal activities such as eating, drinking or speaking. In addition, they can circumvent the relatively short residence time of oral gels on the mucosa and provide a measured dose of drug to the application site. Moreover, they can also help protect the wound surface or cover mucosal defects of the oral cavity, which leads to pain reduction14). Flexible patches of various sizes allow their adaptation to the morphology of the oral cavity and size of the defect. Structure of films, of used bioadhesive polymers and of other excipients and methods of preparation are described further.
Structure of mucoadhesive films
Till now, a relatively wide range of mucoadhesive films for oral use have been studied. Nafee et al. developed a single-layer buccal patch. The mucoadhesive layer contained polyvinyl alcohol, hydroxyethycelullose or chitosan, respectively. This type of patches with no supporting layer enables multidirectional controlled release of antiseptic and may be used to reach drug concentrations above the minimum inhibitory concentration in the oral cavity for a prolonged period of time15).
Thin non-erodible mucoadhesive discs consisting of two layers were reported by McQuinn et al. A homogenous mixture of drug and mucoadhesive polymers (carbopol, polyisobutylene and polyisopropylene) was compressed to an appropriately thin mucoadhesive layer. A hydrophobic polymer, ethylcellulose, was then applied to one side of this film. This backing layer slows down the diffusion of saliva into the drug layer, thus enhancing the adhesion time and reducing drug loss caused by its administration into the oral cavity (unidirectional drug release). In addition to this, the backing layer prohibits adhesion to tissues from the opposite side16).
Robinson et al. reported the use of buccal patches consisting of three layers: an impermeable backing layer; a release rate limiting middle membrane containing the drug; and a mucoadhesive basement layer containing the bioadhesive polymer polycarbophil for mucosal adhesion. This patch has been tested in human buccal mucosa and was shown to remain in place for up to 15 hours without any obvious discomfort, irrespective of food or drink consumption17).
Mucoadhesive polymers used in formulation of mucoadhesive films
To date, a wide variety of mucoadhesive materials have been used for the development of new pharmaceutical preparations, including synthetic and natural polymers. In general, “polymer” is the term used to describe a long molecule – a chain consisting of structural units (monomers), which are repeated and connected by covalent bonds. The differences between monomers can affect properties such as solubility, flexibility and strength. Bioadhesive polymers should have certain physicochemical characteristics including hydrophilicity, visco-elastic properties, flexibility for interpenetration with mucus and epithelial tissue, and numerous hydrogen bond-forming groups such as hydroxylic -OH, carboxylic -COOH, or amide -CONH2. Some authors reviewed that mucoadhesive polymers should have the following characteristics13, 18, 19):
- Be non-toxic, non-irritant and free from leachable impurities (including the degradation products).
- Show bioadhesive properties in both dry and liquid state.
- Be able to incorporate both oil - and water-soluble drugs for the purpose of controlled drug delivery.
- Have a good spreadability, solubility, biodegradability, wetting and swelling properties.
- Quickly adhere to the buccal mucosa and possess sufficient mechanical strength.
- Exhibit strong interaction with the mucosal epithelial tissue.
- Be sufficiently cross-linked but not to the degree of suppression of bond forming groups.
- Have biocompatible pH and good visco-elastic properties.
- Possess peel, tensile and shear strengths at the bioadhesive range.
- Be unaffected by the hydrodynamic conditions, food and pH changes.
- Demonstrate local enzyme inhibition and penetration enhancement properties.
- Have required impact on drug release.
- Have optimum molecular weight.
- Possess adhesively active groups.
- Possess required spatial conformation.
- Be easily incorporated in various dosage forms.
- Demonstrate acceptable shelf life.
- Be easily available and economically acceptable.
- Not aid in development of secondary infections such as dental caries.
In general, several criteria such as the origin, aqueous solubility, or charge can be used for classification of adhesive polymers. The most commonly used synthetic polymers are poly(acrylic acid)-based derivatives (carbomer, polycarbofil, etc.). Cellulose derivatives (carboxymethylcellulose, hydroxypropylmethylcellulose, methylcellulose, etc.) or chitosan are typical representatives of semi-synthetic mucoadhesive polymers. Natural mucoadhesive polymers are agarose, gelatin, hyaluronic acid, pectin, and various gums such as guar, xanthan, gellan carrageenan, or sodium alginate14, 18).
Polymer charge also affects its bioadhesive properties. Cationic and anionic polymers adhere to the mucous membrane more effectively than neutral polymers13). Examples of cationic mucoadhesive polymers are chitosan or dextran; anionic polymers are for example polyacrylates, carboxymethylcellulose, polyacrylic acid, or sodium alginate. Poly(vinylalcohol), poly(vinylpyrrolidone), poly(ethylene oxide), hydroxypropylmethylcellulose, or methylcellulose belong to neutral polymers with mucoadhesive properties14).
In recent literature, newer “second generation” of mucoadhesives (for example thiolated polymers), specific for its capability of forming stronger chemical interactions – even covalent bonds – with the mucus or/and the cell surface, eventually targeting specific receptors, is widely discussed. Thiolated polymers are enhanced derivatives of a polymer such as chitosan, poly (acrylic acid), etc. containing characteristic free thiol groups on the polymeric backbone. These groups form covalent disulphide bonds with cysteine-rich subdomains of mucus glycoproteins20). Another class of compounds with the ability of strong and quick direct binding onto the mucosal cell surface rather than the mucus itself is called lectins. These proteins or glycoproteins have been isolated from animals, plants or are of microbial origin. They bind to sugar-moieties of the cell membrane with significant specificity. An example of non-toxic lectin is tomato lectin isolated from Lycopersicum esculetum. Lectin-mediated bioadhesive polymers can improve drug delivery via specific binding and can increase the residence time of the dosage form21).
Other excipients used in formulation of mucoadhesive films
Plasticizers are other crucial excipients in film formulation. They significantly improve properties such as flexibility and reduce fragility of the film. Glycerol, propylene glycol, low molecular weight polyethylene glycols, phthalates and citrate derivatives, or castor oil are some of the commonly used plasticizers22).
Problems with lower drug absorption through the epithelial barrier (if systemic absorption of the drug is required) can be overcome using enhancers23). Although absorption enhancers belong to various chemical classes, they should be in general safe and non-toxic, pharmacologically and chemically inert, non-irritant, and non-allergenic. They can be divided into several groups: surfactants (sodium lauryl sulphate, polyoxyethylene, lecithine), bile salts (sodium glycocholate, sodium taurocholate, sodium deoxycholate), chelators (EDTA, citric acid, sodium salicylate), fatty acids (oleic acid, capric acid, lauric acid), alcohols (ethanol, propylene glycol), and others (azone, dextran sulfate, sulfoxides)24).
Protein and polypeptide drugs are prone to enzymatic degradation. Enzyme inhibitors can reduce this problem. In particular, competitive inhibitors of proteolytic enzymes are used. Examples of protease inhibitors investigated in buccal mucosal delivery are aprotinin, betastin, or puromycin24). Some mucoadhesive polymers such as poly(acrylic acid) derivatives or chitosans show these properties, too25).
Sweetening agents, natural as well as artificial sweeteners, are used to improve the palatability of the formulations used in the oral cavity. Common natural sweeteners are sucrose, dextrose, fructose, glucose, maltose, and polyhydric alcohols (polyols) such as sorbitol, mannitol, or maltitol. The artificial sweeteners such as saccharin, cyclamate, aspartame, acesulfame-K, or sucralose are several hundred to several thousand times sweeter than sucrose, but they usually have an unpleasant aftertaste effect22).
Technology of mucoadhesive film manufacturing
The most widely used technology for formulation of mucoadhesive films is the solvent casting method. This method is quite simple and no special equipment is needed. A prepared casting solution or suspension is transferred to a casting mould and the solvent evaporated. The final steps are cutting the dosage form and packaging. Problems that may occur when employing this technology include bad rheological properties of the solution or suspension, entrapped air bubbles, insufficient content uniformity, or residual solvents in the final dosage form26).
Another technology, hot melt extrusion, has been widely used in the pharmaceutical industry to manufacture tablets, granules, and pellets over the last 20 years. Recently, Repka et al. investigated the use of hot-melt extrusion for manufacturing mucoadhesive buccal films27). Extrusion is the process of converting a blend of pharmaceutical ingredients into a product of uniform shape and density. Molten raw material is forced through an orifice (the die) under controlled conditions to yield a more homogeneous material in different shapes. Extrusion can be operated as a continuous process with a consistent product flow28). This procedure has many advantages in comparison with the solvent casting method, such as shorter processing time and greater time-effectiveness, no need of solvents (and therefore no solvent residues in the final dosage form), high stability, and improved solubility and bioavailability of poorly soluble drugs. The relevant disadvantages are a requirement of thermal stability of all components at the processing temperature, the fact that components must be almost moisture free, and investment into specialized equipment29).
Use of the buccal mucoadhesive films
Permanent exposure of the oral mucosa to external factors leads to various disorders including RAS, which affect, in the course of life, the majority of population and are manifesting by painful lesions on the mucous membrane. They can be treated locally by a wide range of topical oral drug systems. Drugs investigated for use in buccal mucoadhesive films for local treatment are listed in Table 1. For example, mentioned can be: anaesthetics (lidocaine, tetracaine), antibiotics (ciprofloxacin, ofloxacin, tetracycline), antifungal drugs (miconazole, nystatin, cotrimazole), antiseptics (chlorhexidine, cetylpyridinium chloride), or non-steroidal anti-inflammatory drugs (ibuprofen, flufenamic acid, benzydamine).
Tab. 1. List of investigated buccal mucoadhesive films/patches for local action 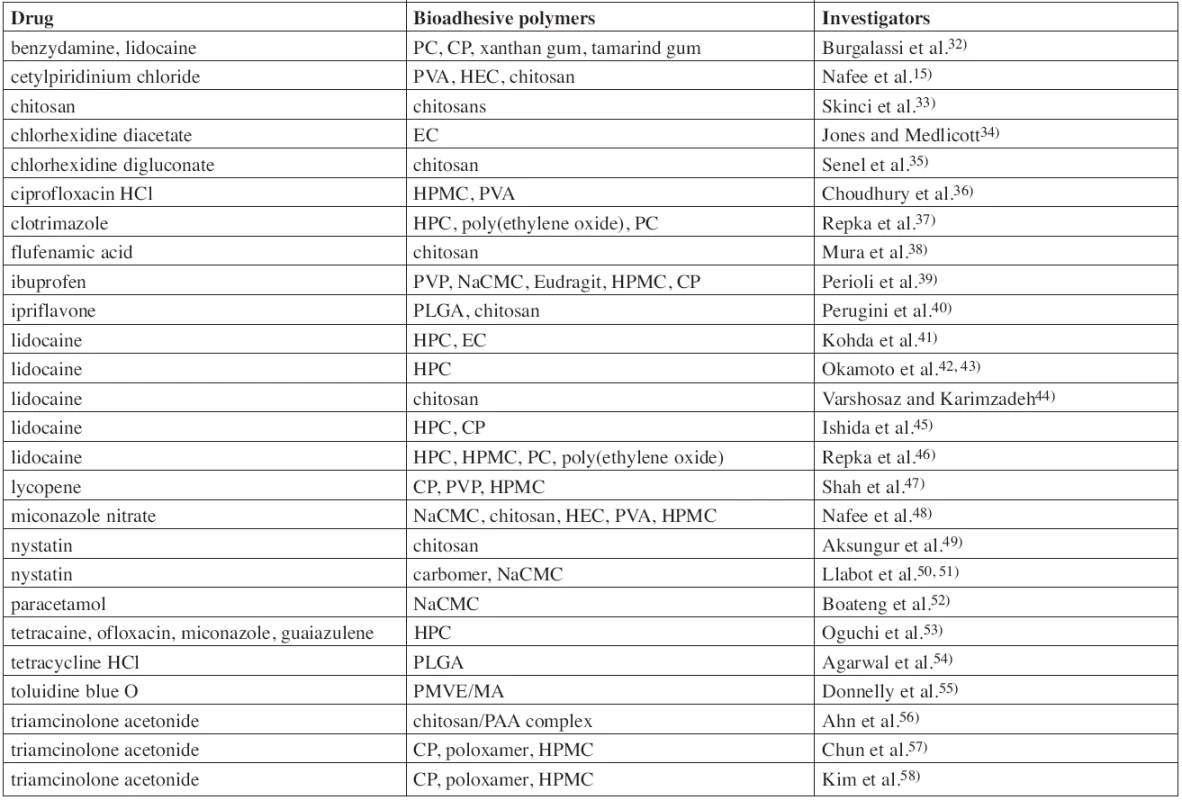
The region of the oral cavity and its mucosa is attractive not only for application of locally acting drugs, but also as a route for systemic administration of drugs. Direct access from the buccal mucosa to the systemic circulation through the internal jugular vein bypasses the hepatic first pass metabolism, which leads to increased bioavailability of the drug. Other advantages such as low enzymatic activity, good accessibility, painless administration and easy drug withdrawal in the case of adverse side effects predetermine buccal mucoadhesive films as a promising object for further research30). Development of new mucoadhesive films should deal with a low permeability of the buccal mucous membrane and other disadvantages such as the continuous saliva secretion (500–2000 mL/day), fast turnover of the mucus, or need of food and liquid intake during administration31). The benefits are however potentially significant and for this reason numerous drugs were investigated as possible active ingredients of buccal mucoadhesive films for systemic action (Table 2.). These included peptidic hormones (insulin, oxytocin, protirelin, calcitonine), analgesics (especially opioid drugs like fentanyl or buprenorphine), antihypertensive drugs (metoprolol, propranolol, carvedilol, nifedipine, losartan), bronchodilators (salbutamol, terbutaline), anti-diabetic drugs (glibenclamide, glipizide), histamine antagonists (H1 – fexofenadine, chlorpheniramine; H2 – famotidine), and others.
Tab. 2. List of investigated buccal mucoadhesive films/patches for systemic action 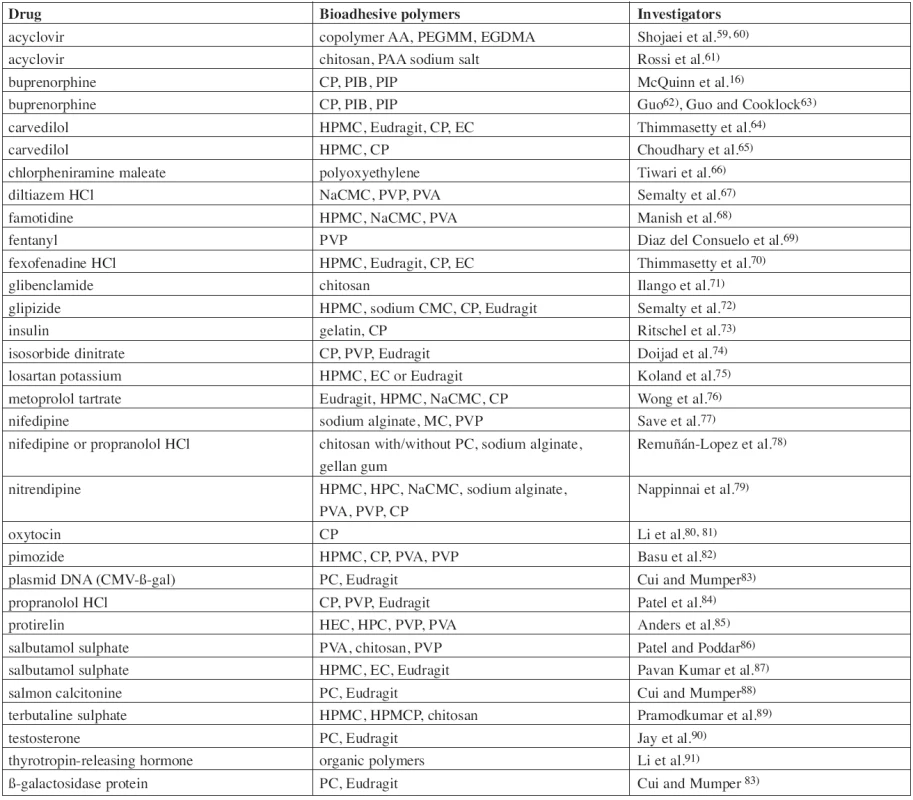
Abbreviations
- CMC – carboxymethylcellulose
- CP – carbopol
- EC – ethylcellulose
- EGDMA – ethylenglycol dimethacrylate
- HEC – hydroxyethylcellulose
- HCl – hydrochloride
- HPC – hydroxypropylcellulose
- HPMC – hydroxypropylmethylcellulose
- HPMCP – hydroxypropylmethylcellulose phthalate
- MC – methylcellulose
- NaCMC – sodium carboxymethylcellulose
- PAA – poly(acrylic acid)
- PC – polycarbophil
- PEGMM – polyethyleneglycol monomethylether monomethacrylate
- PIB – polyisobutylene
- PIP – polyisoprene
- PLGA – poly(D, L-lactide-co-glycolide)
- PMVE/MA – poly(methylvinylether-co-maleic anhydrid)
- PVA – polyvinyl alcohol
- PVP – poly(vinylpyrrolidone)
Conflicts of interest: none.
This article was completed with support of a Ministry of Health of Czech Republic research project NT11396 and IGA VFU Brno, Czech Republic, research project No. 78/2012/FaF.
Received 27 November 2012 / Accepted 3 December 2012
H. Landová • PharmDr. Jan Gajdziok, Ph.D. (✉) • D. Vetchý
Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences
Palackého 1/3, 612 42 Brno, Czech Republic
e-mail: gajdziokj@vfu.cz
Z. Daněk
Clinic of Oral and Maxillofacial Surgery, University Hospital Brno, Czech Republic
J. Štembírek
Department of Maxillo-Facial surgery, University hospital Ostrava, Czech Republic
Zdroje
1. Smart J. D. The basics and underlying mechanism of mucoadhesion. Adv. Drug Deliv. Rev. 2005; 57, 1556–1568.
2. Peppas N. A., Sahlin J. J. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials 1996; 17, 1553–1561.
3. Longer M. A., Robinson J. R. Fundamental apects of bioadhesion. Pharm. Int. 1986; 7, 114–117.
4. Chickering D. E., Mathiowitz E. Definitions, mechanisms, and theories of bioadhesion. In: Mathiowitz, E., Chickering, D. E., Lehr, C. M. eds. Bioadhesive drug delivery systems: Fundamentals, novel approaches, and developement. 1st ed. New York: Marcel Dekker Inc. 1999.
5. Duchźne D., Touchard F., Peppas N. A. Pharmaceutical and medical aspects of bioadhesive systems for drug administration. Drug Dev. Ind. Pharm. 1988; 14, 283–318.
6. Serra L., Doménech J., Peppas N. A. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009; 71, 519–528.
7. Derjaguin B. V., Aleinikova I. N., Toporov Y. P. On the role of electrostatic forces in the adhesion of polymer particles to solid surfaces. Progr. Surf. Sci. 1994; 45, 119–123.
8. Kinloch A. J. The science of adhesion: Part 2 Mechanics and mechanisms of failure. J. Mater. Sci. 1982; 17, 617–651.
9. Kealble D. H., Moacanin J. A surface energy analysis of bioadhesion. Polymer. 1977; 18, 475–482.
10. Wake W. C. Theories of adhesion and uses of ahesives: a review. Polymer. 1978; 19, 291–308.
11. Ahagon A., Gent A. N. Effect of interfacial bonding on the strength of adhesion. J. Polymer. Sci. Polymer. Phys. Ed. 1975; 13, 1285–1300.
12. Lehr C. M., Bouwstra J. A., Boddé H. E., Junginger H. E. A surface energy analysis of mucoadhesion: Contact angle measurements on polycarbophil and pig intestinal mucosa in physiologically relevant fluids. Pharm. Res. 1992; 9, 70–75.
13. Sudhakar Y., Kuotsu K., Bandyopadhyay A. K. Buccal bioadhesive drug delivery – A promising option for orally less efficient drugs. J. Contr. Release. 2006; 114, 15–40.
14. Salamat-Miller N., Chittchang M., Johnston T. P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005; 57, 1666–1691.
15. Nafee N. A., Boraie M. A., Ismail F. A., Mortada L. M. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta Pharm. 2003; 53, 199–212.
16. McQuinn R. L., Kvam D. C., Maser M. J., Miller A. L., Oliver S. Sustained oral mucosal delivery in human volunteers of buprenorphine from a thin non-eroding mucoadhesive polymeric disk. J. Contr. Release. 1995; 34, 243–250.
17. Robinson J. R., Lomger M. A., Veillard M. Bioadhesive polymers for controlled drug delivery. Ann. NY. Acad. Sci. 1987; 507, 307–314.
18. Lee J. W., Park J. H., Robinson J. R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000; 89, 850–866.
19. Roy S. K., Prabhakar B. Bioadhesive polymeric platforms for transmucosal drug delivery systems – a review. Trop. J. Pharm. Res. 2010; 9, 91–104.
20. Bernkop-Schnürch A., Schwarz V., Steininger S. Polymer with thiol groups: A new generation of mucoadhesive polymers? Pharm. Res. 1999; 16, 876–881.
21. Lehr C-M. Lectin-mediated drug delivery: The second generation of bioadhesives. J. Control. Release. 2000; 65, 19–29.
22. Dixit R. P., Puthli S. P. Oral strip technology: Overview and future potential. J. Control. Release. 2009; 139, 94–107.
23. Ganem-Quintanar A., Kalia Y. N., Falson-Rieg F., Buri P. Mechanisms of oral permeation enhancement. Int. J. Pharm. 1997; 156, 127–142.
24. Veuillez F., Kalia Y. N., Jacques Y., Deshusses J., Buri P. Factors and strategies for improving buccal absorption of peptides. Eur. J. Pharm. Biopharm. 2001; 51, 93–109.
25. Lueßen H. L., Lehr C.-M., Rentel C.-O., Noach A. B. J., de Boer A. G., Verhoef J. C., Junginger H. E. Bioadhesive polymers for the peroral delivery of peptide drugs. J. Control. Release. 1994; 29, 329–338.
26. Morales J. O., McConville J. T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011; 77, 187–199.
27. Repka M. A., Repka S. L., McGinity J. W. Bioadhesive hot-melt extruded film for topical and mucosal adhesion applications and drug delivery and process for preparation thereof. US Patent Office, Patent No 6375963 B1. 2002.
28. Chokshi R., Zia H. Hot melt extrusion technique: A review. Iran J. Pharm. Res. 2004; 3, 3–16.
29. Repka M. A., Koleng J. J., Zhang F. Hot-melt extrusion technology. In: Swarbrick, J., Boylan, J. C. eds. Encyclopedia of Pharmaceutical Technology, 2nd ed. New York: Marcel Dekker Inc. 2002.
30. Alur H. H., Johnston T. P., Mitra A. K. Peptides and proteins: Buccal absorption. In: Swarbrick, J., Boylan, J. C. eds. Encyclopedia of Pharmaceutical Technology, 2nd ed. New York: Marcel Dekker Inc. 2002.
31. Gandhi R. B., Robinson J. R. Oral cavity as a site for bioadhesive drug delivery. Adv. Drug Deliv. Rev. 1994; 13, 43–74.
32. Burgalassi S., Panichi L., Saettone M. F., Jacobsen J., Rassing M. R. Development and in vitro/in vivo testing of mucoadhesive buccal patches releasing benzydamine and lidocaine. Int. J. Pharm. 1996; 133, 1–7.
33. Ikinci G., Senel S., Akincibay H., Kas S., Ercis S., Wilson C. G., Hincal A. A. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int. J. Pharm. 2002; 235, 121–127.
34. Jones D. S., Medlicott N. J. Casting solvent controlled release of chlorhexidine from ethylcellulose films prepared by solvent evaporation. Int. J. Pharm. 1995; 114, 257–261.
35. Senel S., Ikinci G., Kas S., Yousefi-Rad A., Sargon M. F., Hincal A. A. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int. J. Pharm. 2000; 193, 197–203.
36. Choudhury A., Das A., Dhangar S., Kapasiya S., Kanango A. Development and characterization buccoadhesive film of ciprofloxacin hydrochloride. Int. J. PharmTech. Res. 2010; 2, 1050–1057.
37. Repka M. A., Prodduturi S., Stodghill S. P. Production and characterization of hot-melt extruded films containing clotrimazole. Drug Dev. Ind. Pharm. 2003; 29, 757–765.
38. Mura P., Corti G., Cirri M., Maestrelli F., Mennini N., Bragagni M. Development of mucoadhesive films for buccal administration of flufenamic acid: Effect of cyclodextrin complexation. J. Pharm. Sci. 2010; 99, 3019–3029.
39. Perioli L., Ambrogi V., Angelici F., Ricci M., Giovagnoli S., Capuccella M., Rossi C. Development of mucoadhesive patches for buccal administration of ibuprofen. J. Contr. Release. 2004; 99, 73–82.
40. Perugini P., Genta I., Conti B., Modena T., Pavanetto F. Periodontal delivery of ipriflavone: new chitosan/PLGA film delivery system for a lipophilic drug. Int. J. Pharm. 2003; 252, 1–9.
41. Kohda Y., Kobayashi H., Baba Y., Yuasa H., Ozeki H., Kanay Y., Sagara E. Controlled release of lidocaine hydrochloride from buccal mucosa-adhesive films with solid dispersion. Int. J. Pharm. 1997; 158, 147–155.
42. Okamoto H., Taguchi H., Iida K., Danjo K. Development of polymer film dosage forms of lidocaine for buccal administration: I. Penetration rate and release rate. J. Contr. Release. 2001; 77, 253–260.
43. Okamoto H., Taguchi H., Iida K., Danjo K. Development of polymer film dosage forms of lidocaine for buccal administration: II. Comparison of preparation methods. J. Pharm. Sci. 2002; 91, 2424–2432.
44. Varshosaz J., Karimzadeh S. Development of cross-linked chitosan films for oral mucosal delivery of lidocaine. Res. Pharm. Sci. 2007; 2, 43–52.
45. Ishida M., Nambu N., Nagai T. Mucosal dosage form of lidocaine for toothache using hydroxypropyl cellulose and carbopol. Chem. Pharm. Bull. (Tokyo) 1982; 30, 980–984.
46. Repka M. A., Gutta K., Prodduturi S., Munjal M., Stodghill S. P. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur. J. Pharm. Biopharm. 2005; 59, 189–196.
47. Shah D., Gaud R. S., Misra A. N. Formulation of a water soluble mucoadhesive film of lycopene for treatment of leukoplakia. Int. J. Pharmaceut. Sci. Rev. Res. 2010; 2, 6–10.
48. Nafee N. A., Ismail F. A., Boraie N. A., Mortada L. M. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int. J. Pharm. 2003; 264, 1–14.
49. Aksungur P., Sungur A., Ünal S., Iskit A. B., Alper B., Squier C. A., Senel S. Chitosan delivery systems for treatment of oral mucositis: in vitro and in vivo studies. J. Contr. Release. 2004; 98, 269–279.
50. Llabot J. M., Palma S. D., Manzo R. H. Allemandi D. A. Design of novel antifungal mucoadhesive films: Part I. Pre-formulation studies. Int. J. Pharm. 2007; 330, 54–60.
51. Llabot J. M., Palma S. D., Manzo R. H. Allemandi D. A. Design of novel antifungal mucoadhesive films: Part II. Formulation and in vitro biopharmaceutical evaluation. Int J Pharm 2007 336 : 263–268.
52. Boateng J. S., Auffret A. D., Matthews K. H., Humphrey M. J., Stevens H. N. E., Eccleston G. M. Characterization of freeze-dried wafers and solvent evaporated films as potential drug delivery systems to mucosal surfaces. Int. J. Pharm. 2010; 389, 24–31.
53. Oguchi M., Shikama N., Sasaki S., Gomi K., Katsuyama Y., Ohta S., Hori M., Takei K., Arakawa K., Sone S. Mucosa-adhesive water-soluble polymer film for treatment of acute radiation-induced oral mucositis. Int. J. Radiat. Oncol. Biol. Phys. 1998; 40, 1033–1037.
54. Agarwal R. K., Robinson D. H., Maze G. I., Reinhardt R. A. Development and characterization of tetracycline-poly (lactide/glycolide. films for treatment of periodontitis. J. Contr. Realese. 1993; 23, 137–146.
55. Donnelly F. A., McCarron P. A., Tunney M. M., Woolfson A. D. Potential of photodynamic therapy in treatment of fungal infections of the mouth: Design and characterisation of a mucoadhesive patch containing toluidine blue O. J. Photochem. Photobiol. B. Biol. 2007; 86, 59–69.
56. Ahn J. S., Choi H. K., Chun M. K., Ryu J. M., Jung J. H., Kim Y. U., Choa C. S. Release of triamcinolone acetonide from mucoadhesive polymer composed of chitosan and poly(acrylic acid) in vitro. Biomaterials. 2002; 23, 1411–1416.
57. Chun M. K, Kwak B. T., Choi H. K. Preparation of buccal patch composed of carbopol, poloxamer and hydroxypropyl methylcellulose. Arch. Pharm. Res. 2003; 26, 973–978.
58. Kim T. H., Ahn J. S., Choi H. K., Choi Y. J., Cho C. S. A novel mucoadhesive polymer film composed of Carbopol, poloxamer and hydroxypropylmethylcellulose. Arch. Pharm. Res. 2007; 30, 381–386.
59. Shojaei A. R., Berner B., Li X. Transbuccal delivery of acyclovir: I. In vitro determination of routes of buccal transport. Pharm. Res. 1998; 15, 1182–1188.
60. Shojaei A. R., Zhou S., Li X. Transbuccal delivery of acyclovir: II. Feasibility, system design, and in vitro permeation studies. J. Pharm. Pharmaceut. Sci. 1998; 1, 66–73.
61. Rossi,S., Sandri G., Ferrari F., Bonferoni M. C., Caramella C. Buccal delivery of acyclovir from films based on chitosan and polyacrylic acid. Pharmaceut. Dev. Tech. 2003; 8, 199–208.
62. Guo J-H. Bioadhesive polymer buccal patches for buprenorphine controlled delivery: Formulation, in vitro adhesion and release properties. Drug Dev. Ind. Pharm. 1994; 20, 2809–2821.
63. Guo J-H, Cooklock K. M. Bioadhesive polymer buccal patches for buprenorphine controlled delivery: Solubility consideration. Drug Dev. Ind. Pharm. 1995; 21, 2013–2019.
64. Thimmasetty J., Pandey G. S., Sathes Babu P. R. Design and in vivo evaluation of carvedilol buccal mucoadhesive patches. Pak. J. Pharm. Sci. 2008; 21, 1–8.
65. Choudhary A., Tiwari G., Pandey M., Kymonil K. M., Saraf S. A. Formulation and characterization of carvedilol buccal mucoadhesive patches. Int. J. Res. Pharmaceut. Sci. 2010; 1, 396–401.
66. Tiwari D., Sause R., Madan P. L. Evaluation of polyoxyethylene homopolymers for buccal bioadhesive drug delivery device formulations. AAPS PharmSci. 1999; 1, 1–8.
67. Semalty A., Bhojwani M., Bhatt G. K., Gupta G. D., Shrivastav A. K. Design and evaluation of mucoadhesive buccal films of diltiazem hydrochloride. Indian J. Pharmaceut. Sci. 2005; 67, 548–552.
68. Manish K., Garima G., Pushpendra K. Design and in vitro evaluation of mucoadhesive buccal films containing famotidine. Int. J. Pharm. Pharmaceut. Sci. 1990; 2, 86–90.
69. Diaz del Consuelo I., Falson F., Guy R. H., Jacques Y. Ex vivo evaluation of bioadhesive films for buccal delivery of fentanyl. J. Contr. Release. 2007; 112, 135–140.
70. Subrahmanyam C. V. S., Thimmasetty J., Manish Kumar D. S., Manjunath K., Shivanand K., et al. Design and evaluation of fexofenadine HCl buccal mucoadhesive patches. Biomed. 2007; 2, 78–83.
71. Ilango R., Kavimani S., Mullaicharam A. R., Jayakar B. In-vitro studies on buccal strips of glibenclamide using chitosan. Indian J. Pharmaceut. Sci. 1997; 59, 232–235.
72. Semalty M., Semalty A., Kumar G., Juyal V. Development of mucoadhesive buccal films of glipizide. Int. J. Pharmaceut. Sci. Nanotechnology. 2008; 1, 184–190.
73. Ritschel W. A., Ritschel G. B., Forusz H., Kraeling M. Buccal absorption of insulin in the dog. Res. Commun. Chem. Pathol. Pharmacol. 1989; 63, 53–67.
74. Doijad R. C., Manvi F. V., Malleswara Rao V. S. N., Patel P. S. Buccoadhesive drug delivery system of isosorbide dinitrate: Formulation and evaluation. Indian J. Pharmaceut. Sci. 2006; 68, 744–748.
75. Koland M., Charyulu R. N., Prabhu P. Mucoadhesive films of losartan potassium for buccal delivery: Design and characterization. Indian J. Pharm. Educ. Res. 2010; 44, 315–323.
76. Wong C. F., Yuen K. H., Peh K. K. Formulation and evaluation of controlled release Eudragit buccal patches. Int. J. Pharm. 1999; 178, 11–22.
77. Save T., Shah M. U., Ghamande A. R. Comparative study of bucoadhesive formulations and sublingual capsules of nifedipine. J. Pharm. Pharmacol. 1994; 46, 192–195.
78. RemuĖán-López C., Portero A., Vila-Jato J. L., Alonso M. J. Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery. J. Contr. Release. 1998; 55, 143–152.
79. Nappinnai M., Chandanbala R., Balaijirajan R. Formulation and evaluation of nitrendipine buccal films. Indian J. Pharmaceut. Sci. 2008; 70, 631–635.
80. Li C., Bhatt P. P., Johnston T. P. In vitro release and permeation of oxytocin from mucoadhesive buccal patch. Pharmaceut. Dev. Tech. 1996; 1, 357–364.
81. Li C., Bhatt P. P., Johnston T. P. Transmucosal delivery of oxytocin to rabbits using a mucoadhesive buccal patch. Pharmaceut. Dev. Tech. 1997; 2, 265–274.
82. Basu B., Garala K., Thimmasetty J. Formulation and evaluation of pimozide buccal muccoadhesive patches. Int. J. Pharmaceut. Sci. Nanotechnology. 2010; 2, 739–748.
83. Cui Z., Mumper R. J. Bilayer films for mucosal (genetic. immunization via the buccal route in rabbits. Pharmaceut. Res. 2002; 19, 947–953.
84. Patel V. M., Prajapati B. G., Patel M. M. Effect of hydrophilic polymers on buccoadhesive eudragit patches of propranolol hydrochloride using factorial design. AAPS PharmSciTech. 2007; 8, E1–E8.
85. Anders R., Merkle H. P. Evaluation of laminated muco-adhesive patches for buccal drug delivery. Int. J. Pharm. 1989; 3, 231–240.
86. Patel R. S., Poddar S. S. Development and characterization of mucoadhesive buccal patches of salbutamol sulphate. Curr. Drug. Deliv. 2009; 6, 140–144.
87. Pavan Kumar G. V., Ramakrishna V., William G. J., Konde A. Formulation and evaluation of buccal films of salbutamol sulphate. Indian J. Pharmaceut. Sci. 2005; 67, 160–164.
88. Cui Z., Mumper R. J. Buccal transmucosal delivery of calcitonin in rabbits using thin-film composites. Pharmaceut. Res. 2002; 19, 1901–1906.
89. Pramodkumar T. M., Shivakumar H. G. Novel core in cup buccoadhesive systems and films of terbutaline sulphate – development and in vitro evaluation. Asian J. Pharmaceut. Sci. 2006; 1, 175–187.
90. Jay S., Fountain W., Cui Z., Mumper R. J. Transmucosal delivery of testosterone in rabbits using novel bi-layer mucoadhesive wax-film composite disks. J. Pharm. Sci. 2002; 91, 2016–2025.
91. Li C., Koch R. L., Raul V. A., Bhatt P. P., Johnston T. P. Absorption of Thyrotropin-releasing hormone in rats using a mucoadhesive buccal patch. Drug Dev. Ind. Pharm. 1997; 23, 139–246.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2013 Číslo 1- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Oral mucosa and therapy of recurrent aphthous stomatitis
- 55 rokov Katedry farmaceutickej chémie FaF UK v Bratislave
- Design and development of diltiazem hydrochloride transmucosal drug delivery system
- Diclofenac sodium entrapment and release from halloysite nanotubules
- Česká a slovenská farmacie – 62. ročník
- Pediatric oral solutions with propranolol hydrochloride for extemporaneous compounding: the formulation and stability study
- Mucoadhesive films as perspective oral dosage form
- Testing of the potentially probiotic lactobacilli for use in food supplements
- Za RNDr. PhMr. Zdeňkem Hanzlíčkem
- Organization and management of nationalized pharmaceutical industry in Slovakia from 1945 to 1948
- Erratum
- Pracovní den Sekce technologie léků České farmaceutické společnosti ČLS JEP
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Pediatric oral solutions with propranolol hydrochloride for extemporaneous compounding: the formulation and stability study
- Oral mucosa and therapy of recurrent aphthous stomatitis
- Organization and management of nationalized pharmaceutical industry in Slovakia from 1945 to 1948
- Mucoadhesive films as perspective oral dosage form
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



