-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEnergy evaluation of the compaction process of directly compressible isomalt
Energetické hodnocení lisovacího procesu přímo lisovatelného isomaltu
V práci je porovnávána lisovatelnost dvou přímo lisovatelných isomaltů galenIQ™ 720 a galenIQ™ 721 prostřednictvím energetického hodnocení lisovacího procesu pomocí záznamu „síla-dráha“. Jsou zde hodnoceny energie na tření, energie akumulovaná tabletou, energie dekomprese, energie lisování a plasticita, a to u čistých suchých pojiv, u suchých pojiv s mazadly (0,5 a 1% stearanu hořečnatého a stearylfumarátu sodného) a dále u tabletovin s modelovými léčivy kyselinou acetylsalicylovou a askorbovou. Z výsledků práce vyplynulo, že nižší hodnoty energie na tření i na lisování při stejné lisovací síle vykazuje látka galenIQ 720, proto je lépe lisovatelná než látka galenIQ 721.
Klíčová slova:
přímo lisovatelný isomalt – záznam „síla-dráha“ – mazadla
Authors: Jitka Mužíková; Veronika Pavlasová
Authors place of work: Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmaceutical Technology, Czech Republic
Published in the journal: Čes. slov. Farm., 2011; 60, 11-16
Category: Původní práce
Summary
The paper compares the compressibility of two directly compressible isomalts, galenIQ™ 720 and galenIQ™ 721, using the energy evaluation of the compaction process by means of the force--displacement profiles. It evaluates the energies for friction, energies accumulated by the tablet, energy of decompression, energy of compaction and plasticity in pure dry binders, in dry binders with lubricants (0.5 and 1% of magnesium stearate and sodium stearyl fumarate) and further in the tableting materials containing the model ingredients acetylsalicylic acid and ascorbic acid. The results of the study have revealed that lower values of the energy for friction and compaction with the identical compression force are found by the substance galenIQ 720, which is therefore better compressible than the substance galenIQ 721.
Key words:
directly compressible isomalt – force-displacement profile – lubricantsIntroduction
The measurement of the force-displacement dependence is one of the most widely used methods for the study of the compaction process in the course of tableting. It is the dependence between the trajectory of the upper punch and the compression force. When the upper punch is entering the matrix, the compression force is fluently increasing; after the set value at which the compact is produced is achieved, it rapidly decreases to the initial value. This gives rise to a curve by means of which it is possible to describe the energetic course of compaction (Fig. 1). The supplied energy is necessary for the compression of materials and the formation of solid compacts. The area under the presented curve corresponds to the work of compression, i.e. the energy accumulated in the tablet (E2); the record makes it also possible to determine the energy consumed by friction during compression (E1) and the elastic component of energy (E3). The energetic profiles can be useful as material compression characteristics in pre-formulation studies of compression of various substances. Their contribution primarily lies in the possibility to subsequently correlate the energetic inputs or the work of compression with the deforming and tablet-forming properties of various materials 1, 2).
Fig. 1. Plot of upper punch force versus upper punch displacement during compression and decompression E<sub>1</sub> – energy of friction, E<sub>2</sub> – energy accumulated by the tablet, E<sub>3</sub>- energy of decompression 
The present paper compares, by means of energetic evaluation of the compaction process, two types of directly compressible isomalt, galenIQ 720 and galenIQ 721. The paper directly links up with the previous publication, which evaluated the compressibility of both substances by means of the strength of compacts and in this connection no more significant difference in the compressibility of isomalts was found 3). Isomalt is a mixture of hydrogenated monosaccharides and disaccharides and its principal components are disaccharidic alcohols 1-O-α-D-glucopyranosyl-D-mannitol dihydrate (GPM) and 6-O-α-D-glucopyranosyl-D-sorbitol (GPS) 4). The agglomerated product of isomalt is produced by the German firm Palatinit GmbH under the name of galenIQ™, types 720 and 721 being intended for direct compression. The individual types differ by the ratio of the principal disaccharide alcohols; in type 720 the ratio GPM and GPS is 1 : 1 and in type 721 this ratio is 1 : 3, and as the result it is more soluble in water 5).
EXPERIMENTAL PART
Materials
galenIQ™ 720 – agglomerated isomalt ( 1-O-α-D-glucopyranosyl-D-mannitol dihydrate and 6-O-α-D-glucopyranosyl-D-sorbitol in the ratio of 1 : 1) (Palatinit GmbH, Germany);
galenIQ™ 721 – agglomerated isomalt ( 1-O-α-D-glucopyranosyl-D-mannitol dihydrate and 6-O-α-D-glucopyranosyl-D-sorbitol in the ratio of 1 : 3) (Palatinit GmbH, Germany);
Pruv® – sodium stearyl fumarate (J. Rettenmaier & Söhne GmbH + Co, Rosenberg, Germany);
magnesium stearate (Acros Organics, New Jersey, USA);
ascorbic acid (Northeast General Pharmaceutical Factory, China);
acetylsalicylic acid (Merck KgaA, Darmstadt, Germany).
Preparation of tableting materials and tablets 3)
Tests included tableting materials from dry binders without lubricants, mixtures with lubricants, and mixtures with active ingredients. Altogether 18 tableting materials of the following composition were used (Table 1 and 2).
Tab. 1. Tableting materials without active ingredients 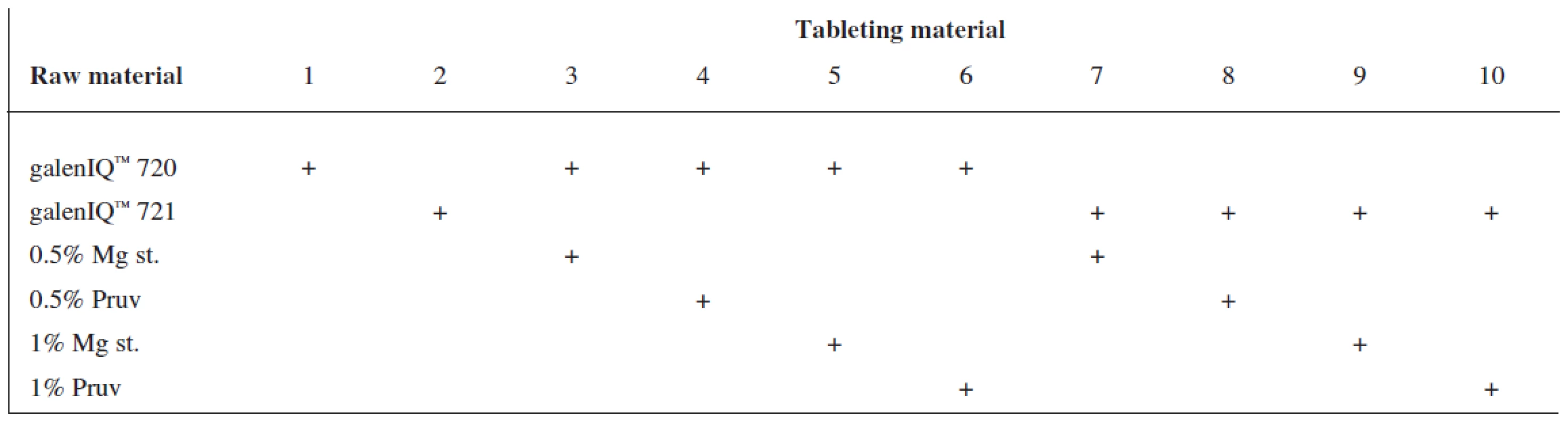
Mg st. – magnesium stearate Tab. 2. Tableting materials with active ingredients 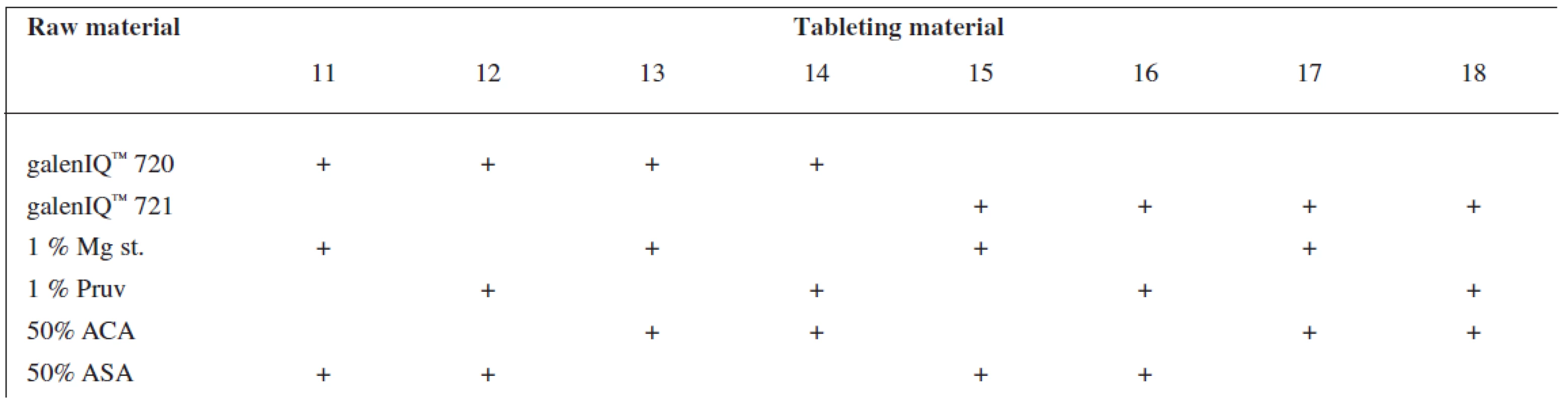
Mg st. – magnesium stearate, ACA – acetylsalicylic acid, ASA – ascorbic acid Dry binders with lubricants were mixed for 5 minutes in a stainless steel cube KB 15S (Erweka GmbH, Hausenstamm, Germany). Mixtures with active ingredients were prepared by mixing the dry binder and the active ingredient for 5 minutes, then the lubricant was added and the material was mixed for 5 minutes again. The rotation rate of the mixing cube was always 17 revolutions/minute. The amount of prepared tableting materials without active ingredients was always 30 g, with active ingredients, 20 g.
Preparation of tablets and energy evaluation of compaction process
All tableting materials were used to produce 10 tablets compressed with the use of a special die with an upper and a lower punch on a material testing equipment T1–FRO 50 TH.A1K Zwick/Roell (Zwick GmbH & Co, Ulm, Germany). Proper compaction took place by applying the pressure on the upper punch. The tablets were of a cylindrical shape without facets with a diameter of 13 mm and weight of 0.5 ± 0.0010 g. Compression velocity was 1 mm/s and compression force 8 kN. The value of preloading was 2 N, the velocity of preloading, 2 mm/s. Mixtures including active ingredients were compressed using a compression force of 10 kN. In all tablets, the “force-displacement” plot was drawn by means of a computer programme testXpert V 9.01 and the compaction process was evaluated as far as energy was concerned, i.e. the energies E1, E2, E3, Ecomp and plasticity were expressed numerically. Energy E1 is the energy consumed by friction, energy E2 is the energy accumulated by the tablet in the course of compression, energy E3 is the energy released during decompression, and Ecomp is E2 + E31).
RESULTS AND DISCUSSION
The study aimed to evaluate the difference in the compressibility of two types of directly compressible isomalts by means of the energetic evaluation of the compaction process. The results of the study are presented in five figures and one table. The figures and the table show the values of the energy consumed for friction (E1), energy accumulated by the tablet after compaction (E2), energy of decompression (E3), plasticity, and the energy of compaction (Ecomp), which is the sum of the energy accumulated by the tablet and the energy of decompression. The figures show these values for the dry binders galenIQ 720 and 721 without and with lubricants at the compression force of 8 kN. The table summarizes the values of the energies of these substances in a mixture with the model active ingredients acetylsalicylic acid and ascorbic acid in the ratio of 1 : 1 always with 1% of lubricants at the compression force of 10 kN.
Figure 2 represents the values of the energy E1 at the compression force of 8 kN for the isomalts under study. This energy is the energy consumed by friction and its value is lower in the case of the substance galen IQ 720. The value of this energy is slightly decreased by the lubricants. Within the type and concentration of lubricants there is no larger difference between the values.
Fig. 2. Values of energy of friction at the compression force of 8 kN E<sub>1</sub> – energy of friction, Mg st. – magnesium stearate 
Figure 3 shows the values of the energy E2. It is the energy accumulated by the tablet after compaction. This energy is again lower in the case of the substance galenIQ 720. Lubricants decrease its value in the substance galenIQ 721. In the substance galenIQ 720 this energy is decreased only by a 1% addition of Pruv.
Fig. 3. Values of energy accumulated by the tablet at the compression force of 8 kN E<sub>2</sub> – energy accumulated by the tablet, Mg st. – magnesium stearate 
Figure 4 represents the values of the energy E3. It is the energy released during decompression, i.e. the energy expressing the elasticity of the material. In this case there were no statistically significant differences in the values of pure dry binders and the mixtures with 0.5% and 1% of magnesium stearate. In the case of the mixture with 0.5% of Pruv and 1% of Pruv, the values were higher in the case of the substance galenIQ 721. The values of this energy are the lowest, the highest values being those of the energy consumed by friction.
Fig. 4. Values of energy of decompression at the compression force of 8 kN E<sub>3</sub> – energy of decompression, Mg st. – magnesium stearate 
Figure 5 shows the values of the energy of compaction (E2 + E3). This energy is higher in the case of the substance galenIQ 721, above all due to a higher value of E2.
Fig. 5. Values of energy of compaction at the compression force of 8 kN E<sub>comp</sub> – energy of compaction, Mg st. – magnesium stearate 
Figure 6 represents the plasticity of both isomalts; higher values are shown by galenIQ 721, plasticity in this case decreases with an addition of lubricants.
Fig. 6. Values of plasticity at the compression force of 8 kN, Mg st. – magnesium stearate 
Table 3 shows the examined values of energies and plasticity for tableting materials containing active ingredients, viz. ascorbic acid and acetylsalicylic acid, in the proportional representation to dry binders of 1 : 1. The active ingredients employed differ in their mechanisms of compaction; acetylsalicylic acid is compacted mainly plastically, ascorbic acid by fragmentation of particles 6). In both active ingredients there occurred a higher value of energy for friction for galen IQ 721, where, in addition, there is no difference in the lubricant employed. In the substance galen IQ 720, there is a lower value of energy in the mixture with 1% of Pruv. Within the type of the active ingredient, higher values occur in the tableting material containing acetylsalicylic acid. As concerns the value of energy E2, i.e. that accumulated in the tablet, there are significantly lower values of this energy in tableting materials containing the isomalt galen IQ 720 and in the case of the mixtures containing acetylsalicylic acid, which is better compressible. In addition, it reveals the difference within the type of the lubricant, which is more marked in the case of ascorbic acid. The values of E2 are always higher with 1% of magnesium stearate. The values of the energy of decompression are higher in the case of the tableting materials containing acetylsalicylic acid, due to the prevailing mechanism of compression by plastic deformation 6). The difference in the effect of lubricants is not statistically significant in the case of both active ingredients. The value of the energy of compaction (E2 + E3) is higher in the case of both active ingredients for galenIQ 721. The values of plasticity are again higher in the case of the substance galenIQ 721 in both active ingredients, in the case of mutual comparison of the active ingredients surprisingly in the mixtures with ascorbic acid, which should be less plastically deformable.
Tab. 3. Values of the energies and plasticity at the compression force of 10 kN: mixtures with active ingredients 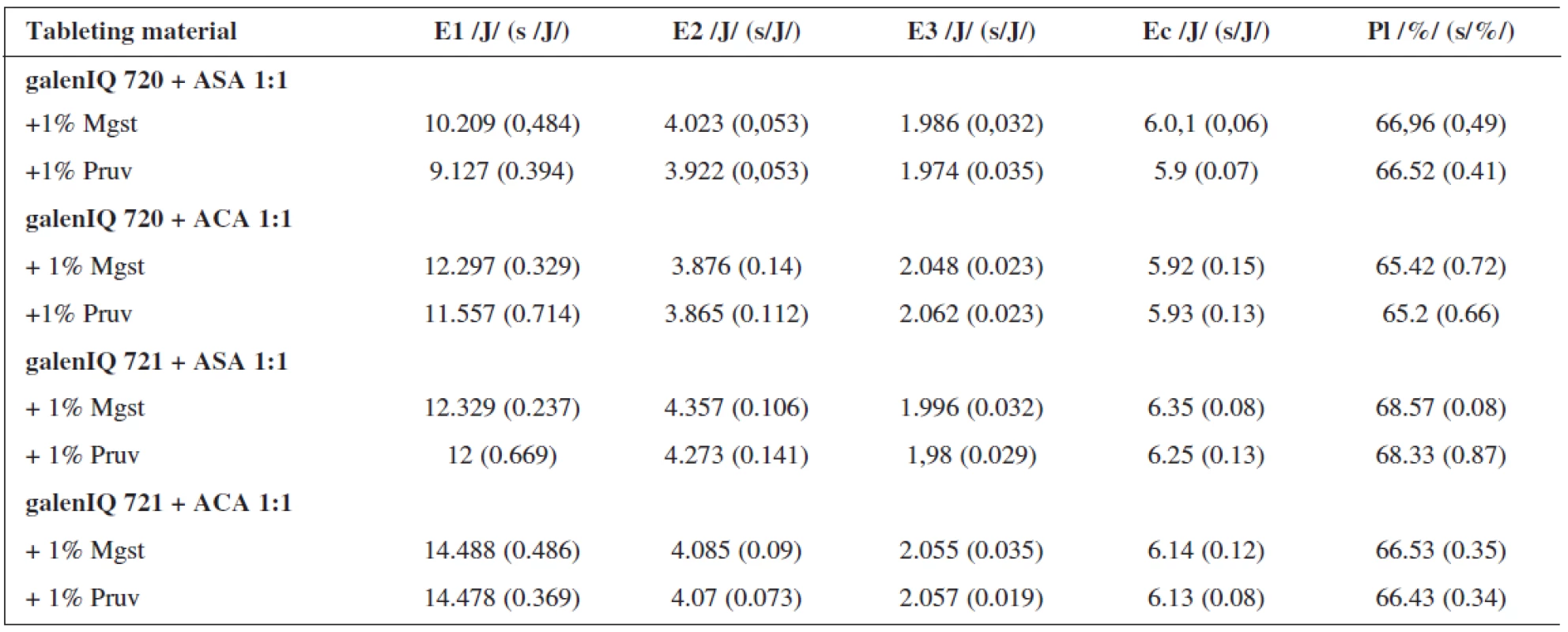
E1 – energy of friction, E2 – energy accumulated by the tablet, E3- energy of decompression, Ec – energie of compaction, Mg st. – magnesium stearate, ASA – ascorbic acid, ACA – acetylsalicylic acid, s – standard deviation It can be concluded that lower values of energy for both friction and compaetion are found in the dry binder galenIQ 720, which is therefore from the energy standpoint better compressible than galenIQ 721.
The study was supported by the grant MSM 0021620822 and by the firm PALATINIT GmbH Germany, which supplied the samples of the dry binders tested.
Received 5 January 2011
Accepted 15 January 2011
Address for correspondence:
PharmDr. Jitka Mužíková, Ph.D.
Department of Pharmaceutical Technology, Charles University in Prague
Faculty of Pharmacy in Hradec Králové
Heyrovského 1203, 500 05 Hradec Králové
e-mail: muzikova@faf.cuni.cz
Zdroje
1. Ragnarsson, G.: Force-diplacement and network measurements. In Alderborn, G., Nyström, Ch., eds. Pharmaceutical powder compaction technology, Inc. New-York: Marcel Dekker 1996; 177–197.
2. Komárek, P., Rabišková, M.: Technologie léků. 3. vyd. Galén: Praha 2006; 228–240.
3. Mužíková, J., Pavlasová, V.: A study of the properties of tablets from directly compressible isomalt. Čes. slov. Farm. 2009; 58, 172–178.
4. Bolhuis, G. K., Armstrong, N. A.: Excipients for direct compaction – an update. Pharm. Dev. Tech. 2006; 11, 111–124.
5. PALATINIT GmbH: galenIQ™ – The smart excipient. Fir. Lit., 2007, http//www.beneo-paltinit.com (1. 11. 2007).
6. Bolhuis, G. K., Chowhan, Z. T.: Materials for direct compaction. In Alderborn, G., Nystrőm, Ch. (eds.). Pharmaceutical Powder Compaction Technology, Inc. New York: Marcel Dekker 1996; 419–500.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2011 Číslo 1- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Energy evaluation of the compaction process of directly compressible isomalt
- Determination of nabumetone and 6-methoxy-2-naphthylacetic acid in plasma using HPLC with UV and MS detection
- Determination of airborne and surface contamination with cyclophosphamide at the Masaryk Memorial Cancer Institute, Brno, Czech Republic
- 60 let časopisu Česká a slovenská farmacie
- Pracovní den sekce technologie lékůČeské farmaceutické společnosti ČLS JEPPokroky v lékových formách
- 51. sympozium z dějin farmacie a veterinární medicíny
- Ocenenie RNDr. Márii Švejnochovej
- Životní jubileum doc. PharmDr. Miloslavy Rabiškové, CSc.
- On teaching the chemistry of pharmaceutical auxiliary substances within the framework of pharmaceutical education in the Czech and Slovak Republics
- K životnímu jubileu prof. RNDr. Karla Waissera, DrSc.
- Prof. Ing. Ján Mocák, DrSc. – nositeľ Weberovej ceny SFS
- Ocenenie doc. RNDr. Josefovi Kučerovi, PhD.
- Study on 99mTc-MAG3 and 99mTc-DMSA renal accumulation using in vitro cellular model
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Životní jubileum doc. PharmDr. Miloslavy Rabiškové, CSc.
- On teaching the chemistry of pharmaceutical auxiliary substances within the framework of pharmaceutical education in the Czech and Slovak Republics
- Study on 99mTc-MAG3 and 99mTc-DMSA renal accumulation using in vitro cellular model
- Pracovní den sekce technologie lékůČeské farmaceutické společnosti ČLS JEPPokroky v lékových formách
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



