-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEntry and competition of retail pharmacies: A case study of OTC drugs sales and ownership deregulation1
Rozhodovanie o vstupe a konkurencia na trhu lekární: Prípadová štúdia voľnopredajných liekov a deregulácie vlastníctva
Táto štúdia poskytuje nové empirické dôkazy o zmenách v intenzite konkurencie a štruktúre trhu lekární po deregulácii vstupných podmienok. Štúdia skúma vývoj maloobchodného trhu lekární v Portugalsku, ktorý prešiel regulačnými zmenami v rokoch 2004 a 2007. V dôsledku týchto zmien boli predaj voľne predajných liekov a vlastníctvo lekární liberalizované, avšak obmedzenia vstupu súvisiace s veľkosťou trhu a umiestnením nových lekární ostali v platnosti v nezmenenej podobe.
Empirická stratégia vychádza z modelov vstupu, ktoré umožňujú porovnanie nevyhnutnej veľkosti trhu pre vstup tej-ktorej lekárne na trh pred a po zmenách v regulácii. Takéto porovnanie umožňuje zistiť, či sa konkurencia zintenzívnila s deregulovanými OTC liekmi.
Zo štúdie vyplynuli tri hlavné zistenia. Po prvé, nevyhnutná veľkosť trhu sa znížila bez ohľadu na počet lekární na tomto trhu, čo naznačuje, že priestor na realizáciu ziskov je širší ako pred dereguláciou. Po druhé, hoci vstupné prahy mali nižšiu hodnotu, ich relatívny nárast s každým ďalším konkurentom vstupujúcim na trh bol v roku 2020 strmší ako v roku 2004, čo naznačuje zintenzívnenie cenovej konkurencie. Po tretie, súčasné pravidlo 3500 pacientov na lekáreň je pravdepodobne príliš reštriktívne a lekárne by sa vedeli presadiť aj na menších trhoch.
Klíčová slova:
regulácia – model vstupu – trhová konkurencia – maloobchodné lekárne
Authors: Matúš Bilka; António Portugal Duarte; Martin Lábaj
Published in the journal: Čes. slov. Farm., 2023; 72, 11-20
Category: Přehledy a odborná sdělení
doi: https://doi.org/https://doi.org/10.5817/CSF2023-1-11Summary
This study provides new empirical evidence on the changes in competition and entry decisions of pharmacies after regulatory changes. It investigates the development of the retail pharmacy market in Portugal, which underwent major regulatory changes in 2004 and 2007. Sale of OTC drugs and ownership of pharmacies were liberalized while entry restrictions related to market size and the location of new pharmacies prevailed.
Our empirical strategy was based on entry models and provided indirect information on the toughness of competition and entry decisions of firms in the market. We estimated and compared the entry thresholds and their ratios before and after liberalization. Such a comparison allows to see if competition got tenser with OTC drugs deregulated.
There were three main findings from the study. First, the entry thresholds decreased regardless of the number of pharmacies in the market, suggesting that room for the realization of profits is broader than it was in the past. Second, although the entry thresholds were lower in value, their increase was steeper with each incumbent in 2020, suggesting harsher price competition with new entrants. Third, the current rule of 3,500 patients per pharmacy is likely overly restrictive, pharmacies could break-even even in smaller markets.
Keywords:
regulation – entry model – market competition – retail pharmacy
Introduction
Deregulation and its impact on competition
Globally, the importance of the healthcare sector, measured as its share in gross domestic product (GDP), has been rising continuously. The OECD suggested that between 1960 and 2013 the share of health spending in GDP for OECD countries more than doubled, from 4% to 8.9%1). Retail pharmacies play an important role within the healthcare sector. Pharmacies are visited approximately twice as frequently as primary care physicians2). They dispense medicine and provide complementary services to doctors3). They also provide product-based services, vaccination, and consultations and can thus substitute for multiple tasks of physicians. The importance of pharmacies in our everyday lives can be expected to rise even more as the population in Europe continues to get older. All of this leads to the increased significance of the questions related to the regulation of retail pharmacies.
The results of studies conducted in the US have reported positive effects from deregulation and increased competition on the price accessibility of medication. Brooks et al.4) analysed the effect of the retail pharmacy market structure on utilization, services, and prices. The authors reported that higher concentrations of pharmacies led to broader provision of medication reviews (critical examinations of a patient’s medicine mix, with the aim of optimizing its impact and minimizing drug-related problems). This was related to the willingness of pharmacies in a competitive environment to differentiate from the competition and provide additional services. Furthermore, in line with economic theory, the prices of prescribed drugs were lower in areas with higher competition. Chen5) suggested that pharmacies in areas with a greater density of competitors had lower prescription drugs prices than pharmacies with greater distances to their competitors did. Caution is needed when interpreting price effects, as they are difficult to measure in many countries because the regulations often introduced the distribution of patients into several different segments based on insurance coverage and thus limited the possibilities for pharmacists to set prices.
On the other hand, studies from Europe have identified several drawbacks to deregulation, which did not necessarily lead to better drug accessibility or service provision. Heinsohn and Flessa6) conducted a survey of the competitive environment among pharmacies in Germany. Their results suggested that even though from 2004 onwards the government abolished the policy of prohibiting branch pharmacies (one owner can have up to three public pharmacies after the change in legislation) and allowed price competition on non-prescription (OTC – over-thecounter) drugs and thus increased competition on the market, the market did not necessarily become more competitive. Pharmacies tried to bind their customers with customer loyalty schemes and there was a limited entry of additional pharmacies into monopoly markets. On the other hand, they were more prone to investing in customer satisfaction. Other evidence supporting limited effects from OTC deregulation in Germany can be found in Stargardt et al.7). Studying the prices of selected OTC drugs, the authors concluded that two years after deregulation only a few pharmacies had enhanced their individual pricing strategies and price competition had a very small scale.
A similar study was conducted for the Spanish pharmacy market by Barbarisi et al.8) following the deregulation around 2000. The study brought forward a specific problem related to the market structure, known as cannibalism. In 1996, national threshold levels were set to 250 meters among pharmacies and 2,800 inhabitants per pharmacy. To take into consideration regional differences, however, the right to modify the rules was transferred to autonomous communities. Navarre, one such community, reduced the criteria to 150 meters and 700 inhabitants. This led to an inflow of new players on the market and almost doubled the total number of pharmacies. Using a geographical information system and several quantitative indicators, the authors concluded that the accessibility of drugs increased only by a limited scope. Regarding the market structure, however, there was an increase in cannibalization between pharmacies where old players were often not able to maintain an adequate market niche. This is because new players preferred to locate closer to older pharmacies rather than to new entrants. Fernandez et al.9) also identified cannibalization in the Spanish pharmacy market arising from stronger competition on the market.
Another extensive paper written by Vogler et al.10) provided a summary of several other countries deregulating their pharmacy markets. The list includes Ireland, the Netherlands, Norway, and England. While the steps taken ranged from the abolition of a monopoly through the legalization of integration to the alleviation of entry tests, the study suggested a common motion among the developed countries towards more liberalized market structures. The results support the evidence from Germany and Spain discussed above. The authors pointed out that even though deregulation has been used as a means to increase the accessibility of drugs and bring down the prices of OTC products, such efforts often fall short. Liberalization does not necessarily bring a more competitive environment. As the main reason, the authors saw the market dominance of the new actors, often wholesale companies and chains. Even the issue of accessibility remained questionable in their study because new pharmacies tended to be established at attractive locations, where competition is already present, and omit rural, sparsely populated areas where a pharmacy would most increase drug accessibility.
Empirical framework to study the toughness of competition
The entry models originating from Bresnahan and Reiss11) aim at the examination of entry decisions and the toughness of competition in the market. They enable the study of the relationship among the number of firms in the market, market size, and competition. The main implication of the models is that if the perfirm population required to support a given number of firms in a market grows with the number of firms, then competition must be getting tougher. Intense competition reduces profit margins and a larger population is necessary to generate the sales required to cover entry costs. The empirical strategy based on entry models has modest data requirements. Data on market size (population), the number of firms in the market, and market characteristics are usually publicly available, which makes this approach a preferable research design to studying entry behaviour and competition in markets where data on marginal costs and prices are not available.
Nilsson12) focused on competition and market entry with the Swedish pharmacy market. His work was conducted following 2009 changes to legislation. Before 2009, the only player on the pharmacy market was Apoteket AB, protected by the Swedish government. Since 2009, thanks to deregulation and market reforms, private companies may enter the market. As a method, Nilsson used statistical regression, estimating market entries based on a set of independent variables. His results suggested that one additional pharmacy decreased the likelihood of a new player entering the market by almost 33%. At the same time, the author found that an additional pharmacy in the area increased the probability of market exit by other pharmacies. Both results are in line with the theory – that higher competition leads to lower profits. In a similar line, Arentz et al.13) divided pharmacy stores within Germany into 4,115 geographic markets, estimated models of entry based on the two-stage game applied in Bresnahan and Reiss11), and identified economic forces driving market entry. The authors found evidence for the notion that the tougher the competition, the lower the profits earned by all competitors, in turn discouraging new players from entering the market. They also concluded that new entrants would face fixed entry costs, decreasing the net profit level and affecting firms’ entry decisions.
More recently, Aue14) used a structural dynamic entry model to document qualitative and quantitative changes in the spatial distribution of pharmacies in Germany. Aue’s estimates implied that one additional active pharmacy within 900 metres reduced profit margins almost by 1%. The impact on profits, resulting from lower margins and decreased local demand, would be higher.
Lábaj et al.15) assessed changes in competitive behaviour in the markets of healthcare professionals during a transition of regulations in Slovakia. The authors reported that for a pharmacy to enter a nonregulated market in 2010 around 3,300 inhabitants was needed to break even. For the second, third, or fourth entrant, the market necessary to break even had a relatively stable size, up to 3,750, suggesting that the margins were not changed significantly by the entry of a new pharmacy. At the beginning of deregulation, in 1995, the number was 3,845 inhabitants, and it rose sharply for the second and third entrants. Regarding deregulation, the authors concluded that “the removal of entry restrictions and the liberalization of ownership rights led to a reduction in the break-even population, more entry and an intensification of competition”15). Another study focusing on market entry to the retail pharmacy market in Slovakia was conducted by Lábaj and Mandžák16), who estimated entry thresholds for selected healthcare providers. Their results provided several important findings. First, the increase in the total number of pharmacies lead to their diffusion into smaller markets. Diffusion was also supported by Mandžák17). Second, to enter the pharmacy market the threshold of patients per pharmacy was 1,700 inhabitants within a municipality. For the second firm to enter, the market population per pharmacy had to increase by 30%. Interestingly, for a higher number of entrants than two, the intensity of competition did not change.
Portuguese pharmacy market
The market of the retail pharmacies in Portugal is a regulated market, with rules set on the establishment and ownership of pharmacies as well as on price creation for specific kinds of drugs. Regarding the ownership of pharmacies prior to 2007, only qualified pharmacists were allowed to own a pharmacy. However, Decree Law No. 307/200718) allowed pharmacy ownership to have no constraint other than a maximum of four pharmacies per owner.
Regarding the opening of a new retail pharmacy, the location is regulated. The Ministry of Health decides if a new pharmacy is justifiable by checking two rules. There has to be proof of at least 3,500 new clients in the location and the new pharmacy has to be opened at a minimum distance of 350 meters from the nearest competitor. It has been noted by de Almeida Simões et al.19) that this provides established pharmacies with considerable monopolistic power over prescription drugs. As will be discussed in the data section, however, in reality there are many markets with fewer than 3,500 clients per pharmacy. The demographic and spatial rules have not undergone any changes over time. In 2018, Padeiro20) analysed the accessibility of retail pharmacies for elderly people in Lisbon and found that there were areas of pharmaceutical deprivation, suggesting that despite proclaimed good coverage, accessibility might be a problem. This brings the strictness of the regulation into question.
On the other hand, price regulation has been relatively lively, changing considerably over time. Regarding OTC drugs, the market underwent major deregulation in 2005, when price fixation was alleviated. Since 2005, OTC drugs can be sold in specialized stores, which are not required to be pharmacies, and the price decision is up to the retailer. However, it is necessary that the person dispensing the OTC drugs in such a store be a pharmacist. Before 2005, only pharmacies were allowed to sell OTC drugs and prices were regulated by a maximum price limit set by the authorities. The aim of the regulation was to support competition within the market and decrease the price of OTC drugs19).
The year 2011 was marked by the signature of the Memorandum of Understanding21) between the European Union (EU) and Portugal. This move was preceded by the international financial crisis of 2008, which had considerable impact on the Portuguese economy. The memorandum stipulated that, in exchange for financial support from the EU to overcome the economic downturn, Portugal had to fulfil several conditions related to its budget22). The conditions spanned different sectors, including healthcare. Portugal promised to achieve healthcare savings by controlling costs within the health sector. The share of public spending on pharmaceuticals was aimed to be reduced to 1% in 2013. Regarding prices, the maximum price of the first generic entering the market was set to be at most 60% of the branded product with a similar active substance, and the reference-pricing system and the maximum profit margins were revised.
Price decisions on reimbursed prescription and nonprescription drugs continue to be strictly regulated, providing only limited space for price variations. Drugs are subject to the maximum approved retail price. In the first step, a maximum price is set for medicines at the production or import stage. This price is denoted PVA (derived from the term Preço de Venda ao Armazenista, standing for ex-factory price or approved wholesaler price) and represents the price for which the producer sells the medicine to the wholesaler or pharmacies. The Portuguese PVA cannot exceed the average PVA for three reference countries within the EU. The reference group changes every year. In the second step, maximum trading margins are applied. These consist of a fixed amount per dose and a percentage of the drug’s PVA. These margins were subject to changes over the years and allow limited space for price competition. Over the years, the allowed margins have tended to decrease. In general, the government implements legislation aimed at reducing public pharmaceutical expenditures19).
Table 1 provides an overview of the allowed margins before and after Decree No. 195-C/201523). Prior to this legislation, the wholesale margin was set at a maximum of 8% and the retail pharmacy margin was a maximum of 20%24, 25), motivating pharmacies to dispense the most expensive drugs26).
Tab. 1. Allowed maximum margins on reimbursed prescription and non-prescription drugs by regulation from 2011 and 2015 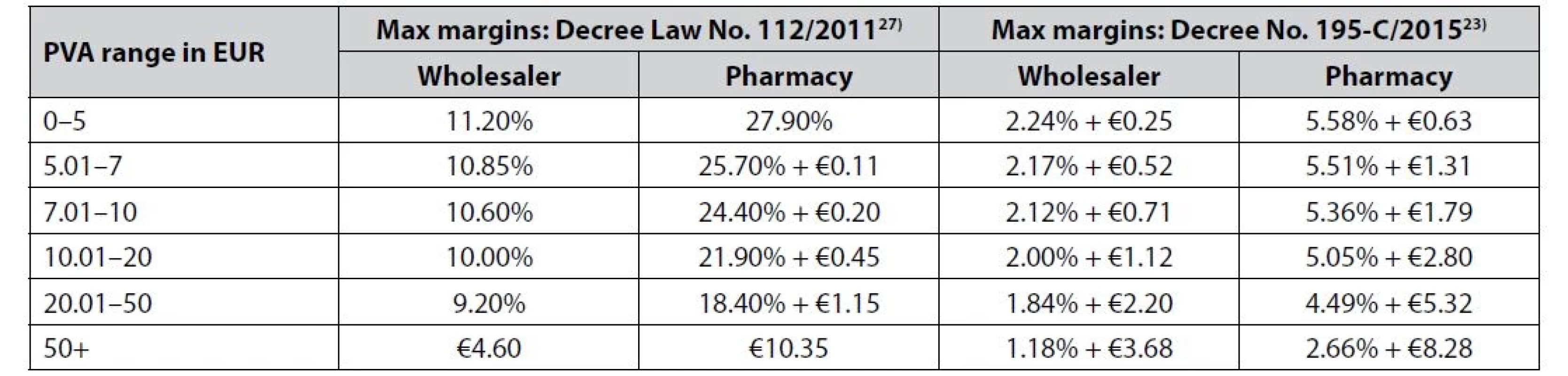
Note: Based on Decree Law No. 112/2011 and Decree No. 195-C/2015. All of these regulations led to a decrease in the average cost of a drug package, which fell from EUR 11.70 in 2010 to EUR 7.70 in 201628). It is worth mentioning that, regarding consumption, Donato et al.29) found that with the exemption of the financial crisis in 2009–2011 the consumption of pharmaceuticals was rising. This is of interest because the rise in consumption might offset the effect of lower prices on pharmacy profits. Moreover, there was a significant increase in the non-prescription medicine market, which increased in value from EUR 201.90 million in 2010 to more than EUR 290 million in 2016. Of these medicines, fewer than 20% were sold outside of pharmacies28). It is worth mentioning that in 2013 a new subcategory of medicine emerged on the Portuguese market. This is the category of medicinal products for human use and it is dispensed without a prescription exclusively by pharmacies30).
Gomes et al.31) assessed the competition on the retail pharmacy market in Portugal after the crisis in 2008 and the signature of the Memorandum of Understanding21) in reaction to it. After analysing the closures and openings of retail pharmacies within Portugal, the authors suggested that there is a dynamic and competitive market, the distribution of which seems to follow people’s needs, supporting access to medicine, and concluded that pharmacies were able to adapt to the regulation and are able to operate on the market.
Moura and Barros32) assessed the effect of OTC drug deregulation on product prices. Using the dataset of the prices for five common OTC drugs within Lisbon in 2006, 2010, and 2015 and implementing a differencein - differences strategy, the authors suggested that competitive pressure in the OTC market deeply affected prices. On average, supermarkets sold OTC drugs at prices 20% lower than those at pharmacies. Pharmacies in the vicinity of a supermarket reported decreases of 4–6% in the prices of OTC medicines. The authors also stipulated that the share of non-pharmacy OTC sellers in 2014 was 20%.
It is important to stress that there are other potential services that might lead to price competition and bring profit to pharmacies. In addition to the traditional sale of medicines, pharmacies also provide other services such as nutrition consultation33), influenza vaccination34, 35), and smoking cessation support36, 37). Martins and Quirós38) found that, when comparing market concentration indices, access to these special services tended to increase. Procompetitive measures led to greater supply of supportive services in competitive urban markets. These activities might provide additional space for price competition on the market. Gregório et al.39) estimated that for the dispensing of a medicine to be profitable for a pharmacy, the medicine has to be priced above EUR 18.30. This calculation was based on the 2014 remuneration system and the observed time costs for the pharmacies while dispensing. The average price for prescription medicine was EUR 25.97, but only 10% of drugs cost more than EUR 25 per package. On the other hand, the cost of consultations was estimated to be less than EUR 3, which in comparison to EUR 10.30 and EUR 20.60 at a general practitioner or emergency department, respectively, gives pharmacists a potential advantage in attaining the patients. The future importance of the services provided by pharmacies beyond medicine dispensing has also been emphasized by a recent paper by Gregório et al.40), who used scenario analysis to evaluate the future potential of retail pharmacies in Portugal. The authors expected a decrease in the demand for retail pharmacies, which would lead to their stronger orientation towards services. Nonetheless, services are likely to be a considerable source of profits for pharmacies.
Data and empirical framework
Data and descriptive evidence
All of the data used in this paper originate from the website of Statistics Portugal (https://www.ine.pt/) and are publicly available. We used data for all 308 Portuguese municipalities to study entry decisions and competition in the retail pharmacy market in Portugal. Municipalities stand for the second-level administrative subdivision of Portugal and can be seen as territorial units rather than cities per se. These can be further broken down to parishes, however our data are not precise enough to use parishes for analysis. We estimated an entry model for 2004, the last year before the deregulation took place, and 2020, as the most recently available data point. In total, there were 2,759 retail pharmacies in Portugal in 2004, and the number of pharmacies increased to 2,922 in 2020.2
Table 2 provides an overview of the observed market structures in 2004 and 2020. There are only a small number of markets (municipalities) with 0 pharmacies. In 2004, these were the municipalities of Corvo, Lajes das Flores, and Marvão. In 2020, Marvão had a retail pharmacy. Because of the small number of markets without pharmacies, the results for the entry threshold for the first pharmacy should be interpreted with caution.
Tab. 2. The amount and share of the markets with given No. of pharmacies in 2004 and 2020 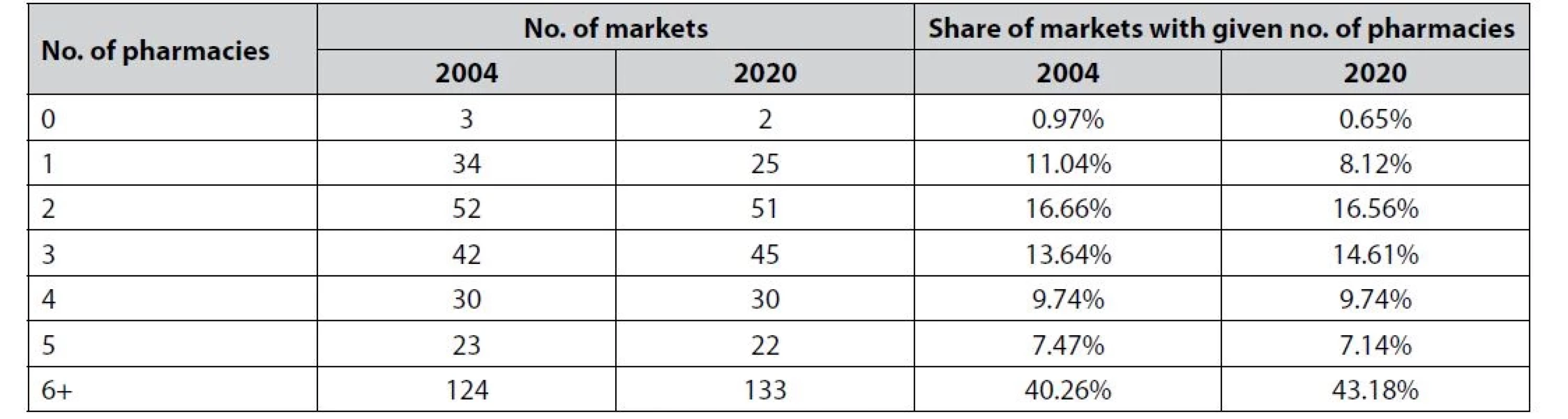
Note: Based on data from Statistics Portugal. In line with the previous application of entry models15, 16), the pharmacy data were merged with marketlevel information on the population and selected demographic and economic indicators. We controlled for age structure, density, and average wages. Table 3 reports the descriptive statistics. Between 2004 and 2020, the mean number of pharmacies per municipality increased from 9 to 9.50. However, this number is inflated by Lisbon, which has 256 pharmacies, and Porto, with 109. In reality, only 78 municipalities have over 9 pharmacies. When it comes to the population, we can see a high level of variance. Interestingly, the average population decreased by 500 during the period 2004–2020. Comparing 2004 to 2020, people earn on average EUR 265 more and the population is getting older. One indicator, suggesting a huge problem with ageing in Portugal, is the ageing index, which measures the number of elderly people per 100 young people. In 2004, the index was 106.58, while in 2020 it reached 165.1341).
Tab. 3. Descriptive statistics for 2004 and 2020, N = 308 (all municipalities) 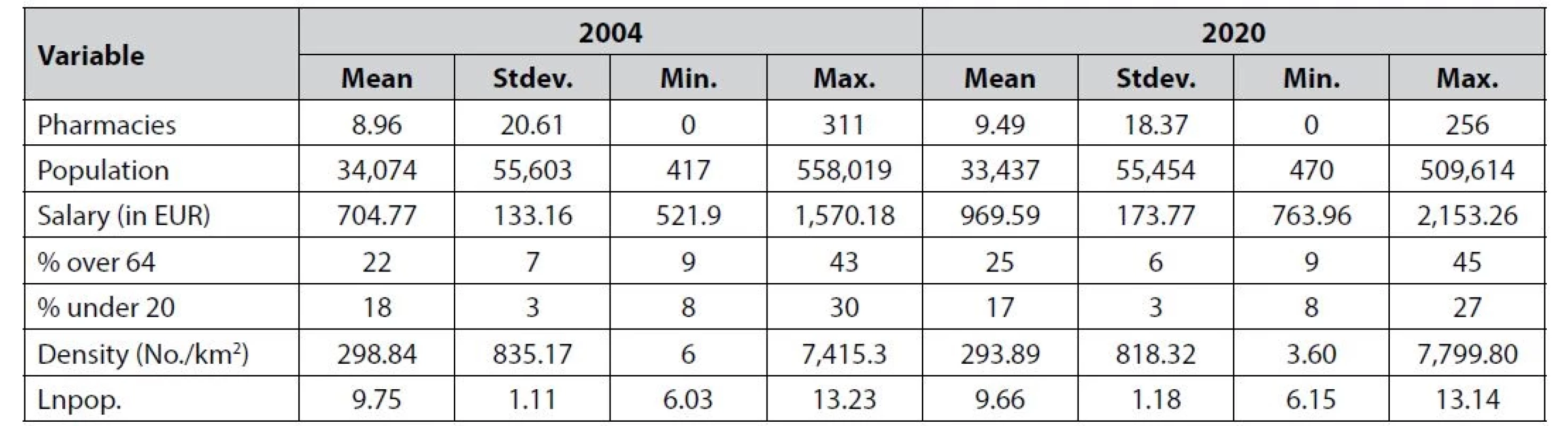
Note: Based on data from Statistics Portugal. Lnpop. represents the logarithm for the population headcount on the given market. Stdev. represents the standard deviation and min. and max. the minimum and maximum, respectively. Empirical framework
Entry models based on the original methodology of Bresnahan and Reiss11) were estimated to provide answers to the questions of competition and entry thresholds. Such models modified for use in the setup of retail pharmacies have been used by such studies as that by Lábaj et al.15).
We assume that the market has N competing pharmacies, reaching variable profit per-capita that depends on the number of firms in the market (ν(N)) and the size of the market (S). The fixed costs (f) are independent of the number of competing firms. Perfirm profit can then be derived as π(N) = ν(N)S – f. As we cannot observe variable income and fixed costs directly, we estimate the break-even points, given by the number of firms in a market of size S. The reasoning behind this approach is as follows. From observing a specific number of firms in a market of size S, we can infer that N incumbents break even, whereas the N+1th potential entrant does not.
Formally:
Rewriting and taking logs of [1] leads to the log-ratio of variable profits over fixed costs, which is characterized by a vector of observable market characteristics (X), firm fixed effects (θN), and an unobservable error term (ε):
Based on the entry conditions in Equation [1], the base model for the coefficient estimation can be specified as:
where θN and θN+1 represent the changes in the ratio of variable profits to fixed costs depending on the market structure, y* stands for the ratio of variable profit to fixed costs that is derived from the number of pharmacies y, and ε is the error term. β can be estimated from an ordered probit model where θN and θN+1 are the cut points measuring the change in the ratio of variable profits to fixed costs in log form.
Once θ is estimated, we proceed to derive the entry thresholds based on the following equation:
where Sj represents the minimum population necessary for entrant j to enter the market. θN stands for changes in the ratio of variable profits to fixed costs, and X is a vector on the market characteristics. These parameters allow us to draw conclusions on the market size (population) necessary for a pharmacy to break even on a market with respect to the number of competitors on that market.
Next, we calculate the entry threshold ratios (ETRN), which measure the fall in variable profits per customer between monopolies and competitive markets11). It is important to note that these ratios do not measure the level of competition, but rather the level of changes respective to the number of firms. The effect of each successive entrant is estimated by comparing the perfirm break-even population as:
We estimated and compared the entry thresholds and entry threshold ratios before and after liberalization. Comparing the two enabled us to see if competition got tenser with OTC drugs deregulated, and the results also provided insights into the relevance of the entry regulation set by the regulator in Portugal.
Changes in entry thresholds over time indicate the entry decisions of pharmacies based on market size (population). Lower thresholds show that pharmacies are more likely to enter markets with lower populations and are thus more likely to break even. The changes in competition in the market were observed by comparing entry threshold ratios over time. Higher entry thresholds ratios imply tougher competition as it is required to increase the population size more disproportionally for an additional pharmacy to enter the market. Regarding entry regulations, if we saw entry thresholds significantly lower than the set threshold of 3,500 clients per pharmacy, the regulation could be considered too restrictive as the market conditions would allow the pharmacy to break even in a smaller market, allowing customers to reap potential gains from price competition between retail pharmacies.
Results and discussion
Table A1 in the Appendix reports the estimates from the ordered probit model. The key parameters of interest are the population coefficients and cutpoint estimate, which are used to calculate the entry thresholds. Population and cut points are highly significant with a positive effect on the number of pharmacies in the market. Density and average salary in the municipality do not seem to play a significant role in determining the profitability of a retail pharmacy. It is not uncommon to find this result in similar empirical studies for other markets (see, for example, the discussion in Lábaj et al.15)). The share of the elderly population is positively correlated with the number of pharmacies, which is in line with intuition and the empirical literature.
Table 4 presents the entry thresholds, the break-even population for a new pharmacy entering a market in Portugal with a given number of competitors.
Tab. 4. Per-firm thresholds and entry threshold ratios for retail pharmacies 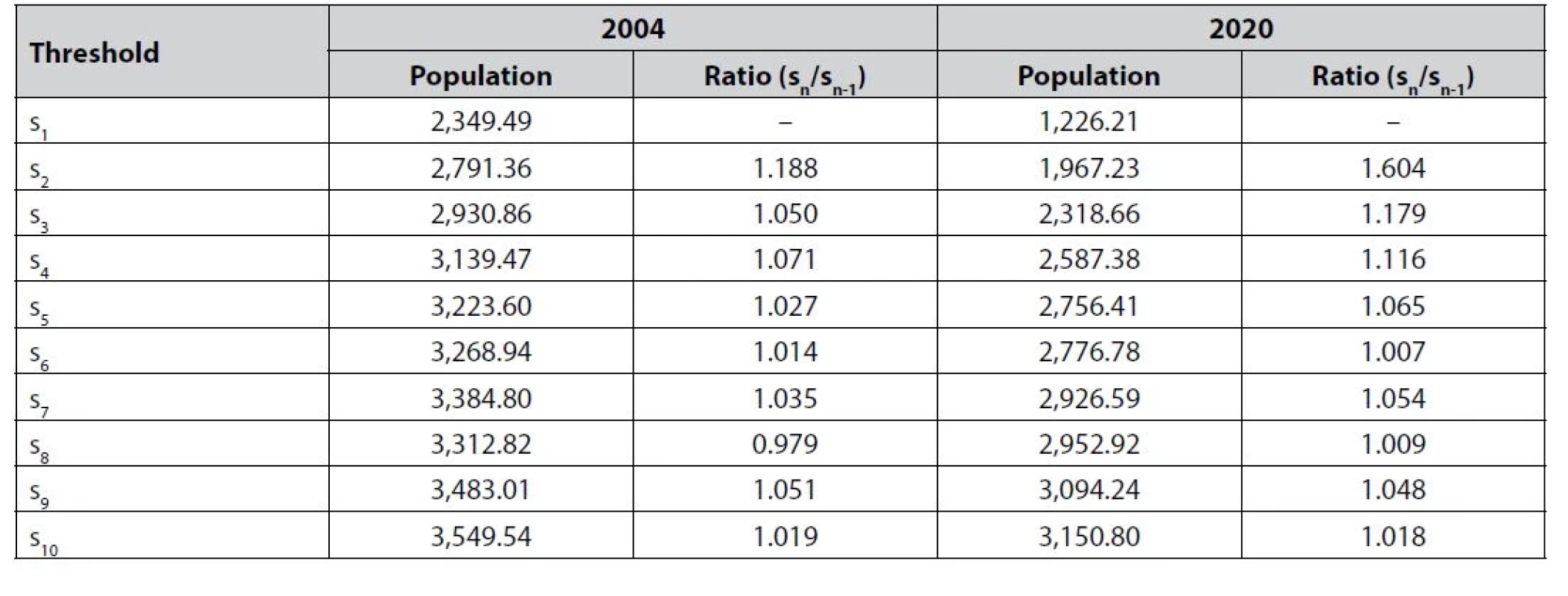
When compared over time, it can be seen that although the entry thresholds decreased between 2004 and 2020 considerably, deregulation brought competitive pressure on retail pharmacies as the threshold ratios increased. For five or more pharmacies in the market, entry thresholds are very close to one. This suggest that four or less pharmacies in the market led to competitive outcomes both in 2004 and 2020. Competitive effects increased significantly for the first four entrants.
In 2020, it was necessary to have at least 3,934 people in the market for two pharmacies to break even, representing 1,967 potential clients per pharmacy. Back in 2004, the necessary market size was 5,582, and thus 2,791 potential clients per pharmacy. We can observe a similar decrease in the entry thresholds over time for markets with other numbers of competitors. There are several driving forces behind this trend that we will discuss in more detail, but in general our findings indicate that the availability of retail pharmacies became better across markets over time.
There are a few possible explanations. Firstly, the market for medication has been expanding over the years. This is in line with the results of Donato et al.29), who documented a rise in the consumption of prescribed drugs over this period, and with the evidence of increased sales of OTC drugs published by Infarmed28). Secondly, as one owner can now own four pharmacies, it might be easier to enter new markets as the costs are shared between more units. Thirdly, as part of the legislation was aimed at price deregulation, allowing pharmacies to set their own profit margins on OTC drugs, the effect of margin setting might allow the pharmacies to overcome the effects of tenser competition.
We think that the last explanation is not likely as we see that even the thresholds for higher-order markets decreased, even though the ratios are very close to 1. If the decrease were a result of margins on OTC drugs, it would likely have disappeared for the bigger markets as competition would press the profit margins closer and closer to 0. In addition, Moura and Barros32) suggested that the prices of OTC drugs actually decreased after deregulation.
On the other hand, there is evidence suggesting that pharmacies might be able to reap more profits from a lower number of clients. Data show a long-term trend of increased per-capita expenditures on healthcare in Portugal. While in 2004 expenditures amounted to EUR 1,384.30, in 2020 they were EUR 1,989.1041). This is likely the result of the changed population structure leaning towards the elderly, who are less healthy and thus consume more medicine than the rest of the population. Donato et al.29) suggested that the consumption of prescription drugs is increasing permanently. This might offset the price decreases referred to by Infarmed28) and Teixeira et al.42). In addition, pharmacies within Portugal are allowed to provide health screening services and nutritional advisory, which may allow them to earn additional profits25).
Regarding the competition itself, once the thresholds ratios are examined, there is an obvious pattern of increased ratios in 2020 compared to 2004 that points to more intense competition among pharmacies. As price competition pushes prices down, we can see that while in 2004 it was necessary for the per-firm population to rise by only 5% for a third player to enter the market, in 2020 an increase of 18% was required. Similarly, for a fourth competitor to enter the per-firm population had to increase by 12% in 2020, instead of 7% in 2004. For further entrants, the ratios were never over 1.10, suggesting that the competition effects tend to fade away. This is sensible because prices and margins cannot be decreased under a certain vital level.
When compared to the entry restrictions set by the regulator at 3,500 clients per pharmacy, our results show that this level is overly restrictive as the population needed for pharmacies to break even is below it even for markets of 10 competitors. Relaxing entry restrictions or lowering the population requirements per pharmacy would allow patients to gain potential advantages from price competition between pharmacies and could make medicines more accessible, both spatially and financially. This is of increased interest because, as Padeiro20) suggested, there is existing pharmaceutical deprivation in certain areas, even in Lisbon.
Conclusion
This paper analysed the dynamics of competition in the Portuguese retail pharmacy market between 2004 and 2020. It was a period of important regulation changes. Price restrictions on OTC drugs were relaxed in 2004. The sale of OTC drugs by retailers other than pharmacies was allowed. In 2007, the ownership of pharmacies was liberalized. As the experiences of other countries with deregulation in the pharmacy market have differed, it is important to monitor and explore the entry decisions, competition, and availability of pharmaceutical services after such substantial regulatory changes. We applied well-established entry models in industrial organization to study entry behaviour and competition over this period.
Our results suggest three interesting points. First, the entry thresholds decreased during the period. Regardless of the number of pharmacies within a market, the entry threshold for a new player was lower in 2020 than it was in 2004. This comes as a surprise as the legislative changes in 2004 enabled other retailers to sell OTC drugs and thus increased competitive pressure within the industry. However, the other aspects of the market provide possible explanations. As the prices of non-OTC drugs are highly regulated and decreasing over recent years28), the decreased entry threshold can likely be attributed to the higher consumption of drugs per patient29), possibly arising from the increasing population age. Another contributing factor might be the broader scale of the services pharmacies currently provide, such as vaccinations and nutrition advisory, which generate additional profits and are not subject to price regulation.
Second, the threshold ratios increased during the period. While in 2004 a three-pharmacy market needed only 1.05 times more population per pharmacy than a two-pharmacy market did, in 2020 the ratio increased to almost 1.18. Similarly, the ratio between three - and four-pharmacy markets increased from 1.07 to 1.12. This is in line with expectations after deregulation, which was expected to lead to increased competition within a market, suggesting that a new entrant leads to a decreased profit margin set by pharmacy for its services. Thus, the retail pharmacy market, even though it is still regulated, show tendencies towards behaving as a competitive market, suggesting price competition between entrants.
Third, the estimated thresholds were well below the thresholds set by regulation. The requirement of 3,500 potential patients in the proximity of a new pharmacy might be too harsh, not considering the real aspects of the market. Even for a tenth pharmacy to enter, our model suggests that many fewer than 3,500 patients are needed to break even. It might be efficient for regulators to reconsider the legislative entry threshold as its current level might distort the competitive tendencies of the market and thus disadvantage patients.
Overall, our results suggest that there have been competitive tendencies within the retail pharmacy markets, which might not have been properly translated into the overly strict legislation. The lower entry thresholds, regardless of their main cause, point to the need to reconsider the current entry requirement, which limits competition within the market. However, liberalized entry with regulated prices can lead to an excessive entry of pharmacies and commercialization of the role of pharmacists (see for example Barbarisi et. al.8) and Grega et al.43)). Thus, any policy changes require to consider the effects on equity, efficiency, quality of services, and overall effects on social surplus.
Appendix
Tab A1. Parameter estimates from ordered probit model 
*** represents statistical significance at the level of α = 1%
** at α = 5%
* at α = 10%Conflict of interest: none.
Comment
1 This work has been funded by national funds through FCT – Fundação para a Ciência e a Tecnologia, I.P., Project UIDB/05037/2020, and project APVV 18-0425 #Pharmacies.
2 There are also approximately 200 mobile pharmaceutical spots in the market that are not included the empirical analysis. According to Statistics Portugal, such a medicine depot is defined as a unit targeted at dispensing medications to the general public, under the responsibility of a pharmacist, and depending on a pharmacy in its registration permit. Its establishment and operating conditions are special and duly regulated. As it depends on the pharmacy under which it is registered, we do not perceive it as an independent unit making its own decisions about market entry or exit.
Received November 21, 2022 / Accepted January 4, 2023
M. Bilka • doc. Ing. Martin Lábaj, PhD.
University of Economics in Bratislava
Faculty of Economics, Department of National Economy
Dolnozemská cesta 1, 852 35 Bratislava, Slovakia
e-mail: martin.labaj@euba.sk
A. P. Duarte
Univiversity of Coimbra, Center for Business and Economics Research
Faculty of Economics, Coimbra, Portugal
Zdroje
1. OECD. Focus on health spending. Technical report. OECD Health Statistics. 2015.
2. Berenbrok L. A., Gabriel N., Coley K. C. Evaluation of frequency of encounters with primary care physicians vs visits to community pharmacies among Medicare beneficiaries. JAMA Netw Open 2020; 3(7).
3. Schaumans C., Verboven F. Entry and regulation: Evidence from health care professions. Rand. J. Econ. 2008; 39(4), 949–972.
4. Brooks J. M., Doucette W. R., Wan S., Klepser D. G. Retail pharmacy market structure and performance. Inquiry 2008, 45(1), 75–88.
5. Chen J. The effects of competition on prescription payments in retail pharmacy markets. South. Econ. J. 2019; 85(3), 865–898.
6. Heinsohn J. G., Flessa S. Competition in the German pharmacy market: An empirical analysis. BMC Hlth Serv. Res. 2013; 13, 407.
7. Stargardt T., Schreyogg J., Busse R. Pricing behaviour of pharmacies after market deregulation for OTC drugs: The case of Germany. Health Policy 2007; 84(1), 30–38.
8. Barbarisi I., Bruno G., Diglio A., Elizalde J., Piccolo C. A spatial analysis to evaluate the impact of deregulation policies in the pharmacy sector: Evidence from the case of Navarre. Health Policy 2019; 123(11), 1108–1115.
9. Fernandez A. I., Lara R. P., Ugalde M. C., Sisodia G. S. Distinctive competencies and competency-based management in regulated sectors: a methodological proposal applied to the pharmaceutical retail sector in Spain. J. Retail. Consum. Serv. 2018; 42, 29–36.
10. Vogler S., Arts D., Sandberger K. Impact of pharmacy deregulation and regulation in European countries. Gesundheit Österreich. Vienna, 2012.
11. Bresnahan T. F., Reiss P. C. Entry and competition in concentrated markets. J. Polit. Econ. 1991; 99(5), 977 – 1009.
12. Nilsson I. Competition and market-entry into the Swedish pharmacy market: A study on municipality level between 2011–2016. Masters thesis, Umeå University, 2017.
13. Arentz O., Recker C., Vuong V. A., Wambach A. Entry in German pharmacy market. Otto-Wolff-Discussion Paper, 2016.
14. Aue R. Spatial effects of price regulations and competition. A dynamic approach to the German retail pharmacy market. University of Bonn and University of Mannheim, Germany, 2020.
15. Lábaj M., Silanič P., Weiss C., Yontcheva B. Market structure and competition in the healthcare industry: Results from a transition economy. Eur. J. Health Econ. 2018; 19(8), 1087–1110.
16. Lábaj M., Mandžák P. Entry and competition of healthcare providers in Slovakia: A spatial analysis. University of Economics in Bratislava, Department of Economic Policy Working Paper Series No. 23. 2020.
17. Mandžák P. Market structure, entry, and competition in transition economies. Dissertation, University of Economics in Bratislava, Faculty of National Economy, 2020.
18. Decree Law No. 307/2007, of 31 August. Legislação Farmacêutica Compilada. Ministry of Health, 2007.
19. De Almeida Simoes J., Figueiredo Augusto G., Fronteira I., Hernández-Quevedo C. Portugal: Health system review. Health syst. Transit. 2017; 19(2), 1–184.
20. Padeiro M. Geographical accessibility to community pharmacies by the elderly in metropolitan Lisbon. Res. Social Adm. Pharm. 2018; 14(7), 653–662.
21. European Commission. Portugal: Memorandum of understanding on specific economic policy conditionality, 2011.
22. Ferraz R., Duarte A. P. Economic growth and public indebtedness in the last four decades: Is Portugal different from the other PIIGS’ economies? Naše gospodarstvo/ Our Economy 2015; 61(6), 3–11.
23. Decree No. 195-C/2015 of 30 July 2015.
24. Barros P. P. Pharmaceutical market reforms in Portugal under the Memorandum of Understanding. Eurohealth 2012; 18(1), 33–36.
25. Ferreira B. R. The evolution of community pharmacy in Portugal: The case of Grupo Holon. MSc thesis, Católica - Lisbon School of Business and Economics, 2016.
26. Oliveira M. D., Pinto C. G. Health care reform in Portugal: An evaluation of the NHS experience. Health Econ. 2005; 14, 203–220.
27. Decree Law No. 112/2011, of 29 November. Legislação Farmacêutica Compilada. Ministry of Health, 2011.
28. Infarmed. The pharmaceutical industry in figures. Lisbon: Instituto Nacional da Farmácia e do Medicamento, 2016.
29. Donato A. A., Pita J. R., Batel-Marquez F. Development of medicines consumption in Portugal before and during the financial crisis. Eur. J. Public. Health 2021; 31(5), 974–979.
30. Martins A. P., Goncalves E., Marcelo A., Vilao S., Silva J. A. Pharmacy medicines not subject to medical prescription in Portugal: An underused access opportunity? Acta Med. Port. 2016; 29(9), 542–548.
31. Gomes M., Queirós S. I., Romano S., Mendes Z., Duarte P. Dynamic and regulated competition in the Portuguese pharmacy market. Rev. Port. Farmacoter. 2017; 9(4), 21–29.
32. Moura A., Barros P. P. Entry and price competition in the over‐the‐counter drug market after deregulation: Evidence from Portugal. Health Econ. 2020; 29(8), 865–877.
33. Borralho J., Gregório J. “Each one has their own role”: Exploratory study on consumers’ perceptions about nutritionists services provided in community pharmacies. Res. Social Adm. Pharm. 2021; 17(2), 475–479.
34. Kirkdale C. L., et al. Implementation of flu vaccination in community pharmacies: Understanding the barriers and enablers. Ann. Pharm. fr. 2017; 75(1), 9–16.
35. Rosado H., Bates I. An overview of current pharmacy impact on immunisation: A global report. International Pharmaceutical Federation, 2016.
36. Condinho M., Fernández-Llimos F., Figueiredo I. V., Sinogas C. Smoking cessation in a community pharmacy: Preliminary results of a pharmaceutical care programme. Vitae 2015; 22(1), 42–46.
37. Condinho M., Ramalhinho I., Sinogas C. Smoking cessation at the community pharmacy: Determinants of success from a real-life practice. Pharmacy 2021; 9(3), 143–153.
38. Martins L., Queirós S. Competition among pharmacies and the typology of services delivered: The Portuguese case. Health Policy 2015; 119(5), 640–647.
39. Gregório J., Russo G., Lapao L. V. Pharmaceutical services cost analysis using time-driven activity-based costing: A contribution to improve community pharmacies’ management. Res. Social Adm. Pharm. 2016; 12(3), 475–485.
40. Gregório J., Cavaco A., Lapao L. V. A scenario-planning approach to human resources for health: The case of community pharmacists in Portugal. Hum. Resour. Health 2014; 12, 58.
41. Pordata. Base de dados Portugal Contemporâneo, 2021.
42. Teixeira I., Mendes Z., Geurreiro J. P., Costa S. Troika in Portugal: Pharmaceutical sector from paper to reality. Value in Health 2013; 16(7), A456.
43. Grega D., Ambrus T., Matejovič A., Šutorová M., Kolář J. Analysis of the effectiveness of the pharmacy network. Farmacia 2021; 69(4).
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2023 Číslo 1- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Entry and competition of retail pharmacies: A case study of OTC drugs sales and ownership deregulation1
- Childhood obesity: causes, consequences, and prevention
- Contribution to the concept of polypharmacy II. Prescription and use of medicines
- New trends in advanced parkinson disease stage therapy
- Are some COVID-19 vaccines better than others regarding the short-term side effects?
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- New trends in advanced parkinson disease stage therapy
- Childhood obesity: causes, consequences, and prevention
- Contribution to the concept of polypharmacy II. Prescription and use of medicines
- Entry and competition of retail pharmacies: A case study of OTC drugs sales and ownership deregulation1
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání








