-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBiological role of copper as an essential trace element in the human organism
Biologická role mědi jako základního stopového prvku v lidském organismu
Tento článek popisuje přehledem fyziologických vlastností mědi (Cu) jako základního stopového prvku hrajícího důležitou roli v metabolismu člověka, a to především jako kofaktor mnoha metaloenzymů. Pro správnou funkci lidského těla je zásadní potřeba udržovat homeostázu Cu, protože při jejím narušení dochází k silným patologickým projevům. Příklady těžkých vrozených onemocnění jater, při kterých dochází k výraznému hromadění mědi v játrech, jsou Wilsonova choroba a idiopatická toxikóza. Naopak, vrozené onemocnění Menkesova choroba se projevuje závažným nedostatkem Cu v organismu. Ačkoliv je Cu nezbytná pro mnoho životních procesů, představuje také silnou zbraň používanou od starověku proti mnoha mikroorganismům. Nakonec jsou v příspěvku shrnuty teorie antimikrobiálního a antivirového působení Cu spolu s přehledem současných a možných budoucích využití v medicínských i nemedicínských oblastech lidského života.
Klíčová slova:
měď • metaloenzymy • toxicita mědi • nedostatek mědi • nemoci spojené s mědí • aplikace mědi
Authors: Miroslava Pavelková Jakub Vysloužil Kateřina Kubová David Vetchý
Published in the journal: Čes. slov. Farm., 2018; 67, 143-153
Category: Review article
Summary
This paper presents an overview of the physiological properties of copper (Cu), an essential trace element playing an important role in the human metabolism, primarily as a cofactor of many metalloenzymes. The maintenance of Cu homeostasis is required for proper functioning of the human body. However, when the disturbance of Cu homeostasis occurs, strong pathological manifestations may develop. Wilson’s disease and idiopathic toxicosis are examples of severe chronic liver diseases that are the results of genetic predisposition to the hepatic accumulation of copper. Conversely, congenital Menkes disease is manifested as serious Cu’s nutritional deficiency. Although Cu is necessary for many life processes, it is also a powerful weapon used since the ancient times against many microorganisms. Finally, the theories of Cu antimicrobial and antiviral mechanisms of action are summarized, including contemporary and potential future utilizations in medical and non-medical fields of human life.
Key words:
copper • metalloenzymes • copper toxicity • copper deficiency • copper-related diseases • copper applications
Introduction
Copper (Cu), a redox active metal, is an essential trace element in humans and animals and it is involved in a number of physiological and biochemical processes as a cofactor of numerous metalloenzymes1–3). These catalyze a large number of enzymatic processes – cellular respiration, biosynthesis of neurotransmitters and peptide hormones, protection against free radicals, cross-linking of elastin, collagen and keratin4). Cu is also essential for iron homeostasis and thus indirectly affects hematopoiesis and participates in blood coagulation and angiogenesis5–7).
In biological systems, Cu is predominantly present in the oxidised form of Cu2+ (cupric) ion. The ability of copper to accept and donate electrons reversibly plays the main role in the disposal of free radicals from the organism8).
The Cu’s body content is about 70–80 mg9); of which 10% is distributed in the plasma and blood elements and 90% in tissues. The Cu blood concentration is slightly different by gender; the male values range from 0.614 to 0.970 mg/L, in women from 0.694 to 1.030 mg/L. Cu concentration increases with age in healthy men, but not in women. In male smokers the blood Cu amount significantly decreases9, 10). The average intake of Cu by adults varies from 0.6 to 1.6 mg/d, a higher intake is needed during pregnancy and breastfeeding11).
Besides Cu indispensability for the basic life processes of most organisms, it is also important as a potent antimicrobial weapon against invading pathogens.
The finding that Cu is used by the innate immune system is a relatively recent discovery. The Cu appears to play a unique role in nutritional immunity by acting as a component of the antimicrobial arsenal produced by cells of the innate immune system12, 13). Although the exact mechanism of Cu antimicrobial effect is not fully understood, the benefit has been used throughout human civilization, from ancient Egyptian and Greek civilizations to the present time. Therefore, the Cu biocidal effect has become of scientific interest especially due to increasing antibiotic resistance against commonly used antibiotics14).
The Cu antibacterial effect (e.g. against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis, Bacillus subtillis), the antifungal effect (e.g. against Candida albicans, etc.)15, 16), antiviral (against bronchitis virus, polio virus, herpes simplex virus and HIV-117–20) and spermicidal activities21) have been demonstrated in many studies.
Despite the extreme sensitivity of microorganisms to Cu, humans exposure is considered safe, as it is demonstrated by the widespread use of long-term Cu intrauterine devices by women22–24).
This article discusses the overview of physiological effects of Cu, the mechanisms involved in maintaining the Cu homeostasis as well as the pathophysiological states associated with this trace element. Moreover, Cu antimicrobial and antiviral effects are described, including contemporary and potential future utilizations in the fight against serious pathogens.
The physiological importance of copper
Copper homeostasis
Cu homeostasis is a highly coordinated process on the intracellular and intercellular level involving the processes of intestinal absorption, distribution, efflux from cells and biliary excretion25).
The absorption of Cu in the body depends mainly on its chemical form; the highly soluble Cu compounds are readily absorbed. The factors inhibiting the absorption of Cu from the intestinal lumen and simultaneously reducing its general bioavailability tend either to reduce its intraluminal solubility or to provoke competitive interactions with the Cu transport through the mucosa26). Presence of many components can affect its absorption. Fibre, phytate27, 28), vitamin C29, 30), fructose31, 32) and other sugars decrease the bioavailability of Cu, but only at their highly elevated intake (fibre > 50 g/day; sugars > 35% of energy; ascorbic acid > 1500 mg/day). During normal diet, it is unlikely to affect the Cu utilization. Zinc and cadmium are the most powerful competitive inhibitors of Cu absorption33). Conversely, a high level of protein intake (above 100 g/day) enhances Cu bioavailability27).
The Cu absorption takes place in the small intestine either by diffusion or using transport proteins 1-DMT1 or 1-CTR1 (1-DMT1 is the divalent metal transporter, 1-CTR1 is the Cu2+ specific transporter). The DMT1 binds iron and other divalent metals, such as Cu, manganese, etc. The Cu affinity for DMT1 is relatively low. The transporter CTR1 plays the key role in Cu uptake4, 25).
In human cells, Cu is utilized in several cell compartments, and its intracellular distribution is regulated in response to metabolic demands and changes in cell environment34). The reduction Cu2+ to Cu+ ions is necessary for the transport across cell membranes35). The CTR1 is found in two cell locations: at the plasma membrane and in intracellular vesicles. The CTR1 distribution between these two compartments is cell-specific. A significant fraction of CTR1 in enterocytes is intracellular and is located in the vicinity of the apical membrane4, 36). The Cu transported across the apical membrane by CTR1 in intestinal enterocytes is shuttled to the ATP7A (Cu-transporting P-type ATPase) and pumped into the portal circulation for further utilization and to the liver, which is the major Cu storage organ37). In the liver, kidney, placenta, and mammary gland, the basolateral plasma membrane is the predominant CTR1 location28–39) and it transports Cu from circulation by, mostly, retrieving Cu from specific carriers40).
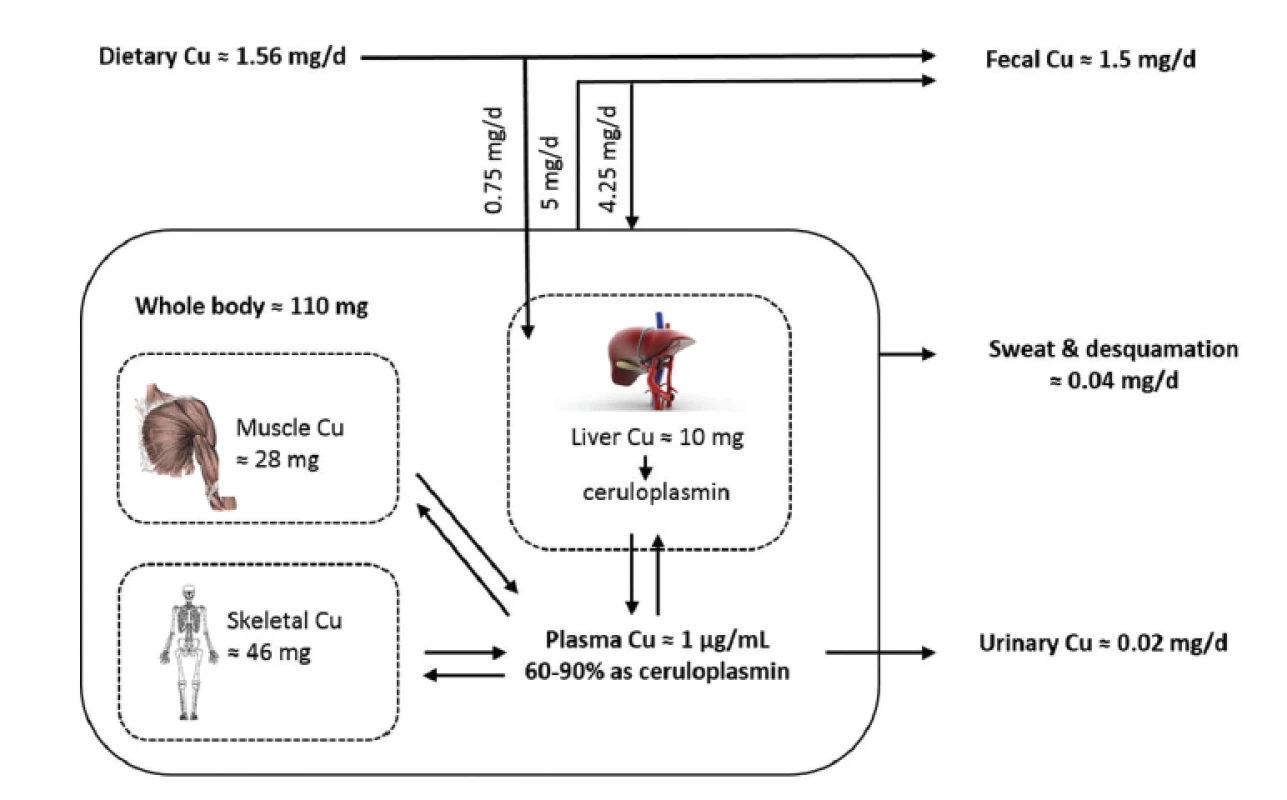
The ATP7A plays crucial role in moving Cu across many other polarized cell layers including the placenta and the blood-brain barrier to ensure adequate Cu concentration for the proper foetus development and especially of the brain. The ATP7A is also important for the delivery of Cu to nascent proteins in the Golgi apparatus. In mammals, ATP7A is expressed in many tissues except the liver. In the liver and several other tissues, ATP7B (homologous Cu-transporting ATPase) is important for loading Cu on the ceruloplasmin (ensuring peripheral Cu distribution). The ATP7B also mobilizes excess Cu into the bile to prevent tissue Cu overload. Cu excretion is almost exclusively by faeces, resulting from biliary excretion (70%); the rest is unabsorbed Cu and Cu from desquamation mucosal cells. The urine excretion is negligible25) (see Fig. 1).
The most important Cu-containing metalloenzymes necessary for physiological processes of humans include the following ones:
- Cu-Zn superoxide dismutase (Cu-Zn SOD) is the most abundant extracellular metalloenzyme, responsible for the removal of superoxide anions (highly toxic reactive oxygen species) and for its transformation to hydrogen peroxide (H2O2) for further disposal (by catalase and glutathione peroxidase). It is a ubiquitous and highly stable enzyme consisting of two subunits. Each subunit has a Cu and Zn site. Cu ions are bound on the four imidazole histidine residues; Zn stabilizes the protein structure. The brain, liver and renal cortex contain the high tissue amount of the Cu-Zn SOD4, 6).
- Lysyl oxidase is a key enzyme of the collagen and elastin synthesis and maturation. It catalyses the connective tissue formation by cross-linking between collagen fibres and elastin. Lysyl oxidase is a multimeric protein composed of 32 kDa subunits, requiring Cu for its activity11).
- Cytochrome-c oxidase sits within the inner mitochondrial membrane. It has four redox-active metal sites, two heme sites (hemes a and a3), and two Cu sites (CuA and CuB)11). The cytochrome-c oxidase is the terminal electron acceptor of the respiratory chain and reduces molecular oxygen (O2) to water. In addition, it pumps protons from the inside to the outside of the membrane, thereby a proton gradient across the membrane is formed42, 43).
- Dopamine β-hydroxylase is indispensable for the conversion of dopamine to norepinephrine in the adrenal ganglion and noradrenergic neurons. The presence of the Cu and ascorbic acid (as an oxygen transport coenzyme) is essential for its activity44).
- Tyrosinase is a transmembrane cuproenzyme, which catalyzes the oxidative conversion of tyrosine by many metabolic pathways to melanin pigment45).
- Ceruloplasmin (CP, α-2-globulin) as a single polypeptide chain (about 120 kDa with 12 kDa carbohydrate) is the main plasma Cu-transporting protein (carrying up to 90% of Cu), responsible for Cu delivery to cells and excretion of Cu from the body11). As CP is one of the positive protein reactants of the acute phase, the increased CP plasma concentration occurs during pathological conditions, such as inflammation, infection or injury46). However, it appears that the regulation of CP expression is controlled not only just by inflammatory cytokines, but also through the hypoxia-inducible factor, which is linked to iron metabolism47). Higher concentrations of CP are associated with the development of atherosclerosis4, 6). The other important roles of CP are the antioxidant, amine oxidase and ferroxidase activities (the oxidation of Fe2+ to Fe3+ before binding to transferrin)11).
Copper toxicity
Although Cu is an essential element in biology, it can be also a potentially dangerous and its dysregulation can lead to the development of many adverse health effects, including liver and kidney damage, anaemia, immunotoxicity and developmental toxicity48).
Fortunately, acute and chronic Cu toxicity occurs relatively rarely; as a result of an accident, occupational diseases, environmental pollution or congenital metabolic disorders of Cu49).
The most knowledge about the mechanisms of Cu-mediated biological damage comes from studies with DNA50, 51). Cu2+ ions bind specifically to DNA52), preferably to guanosine residues51). The products of these reactions include single and double DNA strand breaks as well as base modification, the main product being 8-oxo-2’-deoxyguanosine50, 51, 53, 54).
Moreover, Cu2+, as heavy metal ions, catalyse the generation of reactive oxygen species (ROS) and cause cellular damage51, 55) via depletion of enzyme activities through lipid peroxidation and reaction with nuclear proteins and DNA56). One of the most important metal-mediated mechanisms of the formation of free radical generation is by a Fenton-type reaction. Superoxide ion and hydrogen peroxide can interact with Cu, via the metal catalysed Haber–Weiss/Fenton reaction to form OH radicals57).
Cu+ + H2O2 → Cu2+ + OH– + ·OH Fenton reaction
Cu2+ + ·O2 → Cu+ + O2 Haber ─ Weiss
However, Cu-mediated DNA damage is only partially inhibited by free-radical scavengers51, 55); thus ROS formation is not the main way leading to biological damage.
Similar as Cu excess, severe deficiency of Cu also affects, directly or indirectly, the components of the oxidant defence system and as a result it increases ROS formation leading to oxidative damage of lipids, DNA, and proteins58).
Chronic exposure
Chronic Cu toxicity is primarily manifested in the liver (as liver cirrhosis) with episodes of haemolysis and with disorders of the immune system. The damages of the renal tubules, brain and other organs are the other accompanying symptoms and they may progress up to coma, liver necrosis, circulatory collapse and death.
The dialysis patients using devices with Cu tubing, workers using Cu pesticides and infants during long-term intravenous total parenteral nutrition49) are the risk groups for the development of chronic Cu toxicity. The patients with chronic liver disease can be also potentially susceptible to Cu25). Many of the chronic toxic effects of Cu are associated with oxidative damage of membranes of macromolecules59). Excessive Cu intake participates in the development of Cu toxicity also indirectly, through interaction with other nutrients (for example anaemia due to interference of Cu with iron transport)25).
Dermal exposure is not associated with systemic toxicity, but occasionally in susceptible individuals it may cause allergic reactions22).
Acute exposure
Acute intoxication can be caused by single or multiple ingestion of drinking water with an elevated Cu content (3–6 mg/L). The main acute symptoms are nausea, vomiting and gastric irritation. It is unclear whether these symptoms are a result of an acute Cu irritation or metal bitter taste of Cu. The progression of symptoms such as abdominal pain and headache, nausea, dizziness, vomiting, and diarrhoea, tachycardia, respiratory difficulties, haemolytic anaemia, haematuria, massive gastrointestinal bleeding, damage and failure of the kidney and liver was observed after an intentional or accidental ingestion of high concentrations of Cu salts (20–70 g)25, 49).
Copper deficiency
In humans, serious Cu deficiency is rare. However, unrecognized or marginal Cu deficiency (< 1 mg / day) may be widespread. The cause can be either inadequate dietary intake (primary Cu deficiency) or inadequate Cu absorption (secondary Cu deficiency). Also some heavy metals in foods can compete with Cu absorption and decrease its absorption2).
The group with potential risk of Cu deficiency includes the genetically susceptible population: newborns with low birth weight, infants fed cow’s milk, pregnant and lactating mothers, patients receiving total parenteral nutrition (when Cu intake is lower than 0.1 mg Cu/kg body weight/day), individuals with malabsorption syndrome, diabetics, alcoholics, people with eating disorders and also vegetarians25).
The symptoms of Cu deficiency (accompanied by a reduction of Cu concentration in serum and of CP level) are abnormalities of the bone marrow, neutropenia and mainly hypochromic microcytic anaemia, which is resistant to iron treatment25). The other symptoms include the growth disorders and also an increased incidence of infections due to the Cu necessity for the production of interleukin-2 by lymphocytes during infection60) and hypopigmentation of hair. Deficits of the nutrients during pregnancy can result in gross structural malformations in the conceptus, and persistent neurological and immunological abnormalities in the offspring61).
Interference of the oxidant defence system components and consequently increased ROS production and oxidative damage to lipid, DNA and proteins was demonstrated due to Cu deficiency in the animals and cell culture models25, 61).
A decrease of SOD activity and an increase of superoxide anions has been shown in Cu deficient rats62, 63). In addition, it is postulated that a decrease in cytochrome-c oxidase activity and oxidative inactivation of complex I (NADH: ubiquinone oxidoreductase) contribute to increased production of ROS in Cu deficient animals64). Increased lipid peroxidation has been observed in Cu deficient plasma, liver, heart, aorta, and erythrocytes65, 66).
Congenital disorders of copper metabolism
Menkes disease and Wilson’s disease are the most frequent diseases of Cu metabolism. Menkes disease is manifested as serious Cu´s nutritional deficiency, while Wilson’s disease as copper toxicosis25).
Menkes disease
Menkes disease is a rare disease linked to the X chromosome, caused by mutation of the gene encoding ATP7A, Menkes ATPase (Cu-transporting ATPase)2). ATP7A (protein in the trans-Golgi network) is the enzyme responsible for Cu incorporation into cuproenzymes (at a low Cu level), and for the efflux of Cu excess from the cells from the trans-Golgi network to the plasma membrane (at high Cu intracellular levels). Menkes disease mainly occurs in boys, and is often fatal in early childhood25). The typical symptoms are mental retardation, neurological abnormalities and degeneration of connective tissue (due to a lack of several Cu dependent enzymes needed for brain development)2).
A decreased activity of cytochrome-c oxidase leads to a serious neurological disorder too. Tyrosinase deficiency causes a lack of melanin, which is manifested by hypo-pigmented hair with a steel appearance, and brittle and frizzy hair (“curly hair syndrome”) due to the lack of an unidentified cuproenzyme required for cross-linking of keratin25).
The treatment is performed by intravenous Cu administration, but Cu cannot be transported to the brain. Nevertheless, early diagnosis and daily injection of Cu-histidine intraperitoneally and intrathecally into the central nervous system could prevent neurodegeneration and prolong life25).
Wilson’s disease
Wilson’s disease is a rare autosomal recessive disorder of Cu metabolism, caused by mutation of the gene encoding enzyme ATP7B – Wilson ATPase on a chromosome 132). ATP7B enzyme is mainly found in the liver, where it is primarily responsible for Cu delivery to cuproenzymes and for Cu biliary efflux.
Consequently, an accumulation of Cu in hepatocytes occurs, leading to cirrhosis2, 53) and defective incorporation of Cu into CP. The result is an increased plasma concentration of unbound Cu and subsequent Cu accumulation in the extrahepatic tissues, especially in the kidney, brain and cornea – as Kayser Fleischer-rings (diagnostic marker of Wilson’s disease)2, 25).
The neurological symptoms of the disease include the upper limbs tremor, slow movement and personality changes. Late complications are icterus and encephalopathy development, coagulation disorders, occasionally associated with intravascular coagulation and renal insufficiency9, 25).
In an early diagnosis, Wilson’s disease can be treated by many different ways (using chelating agents, low income Cu diet and high intake of zinc supplements)59). The antidote of choice is zinc (in form of ZnSO4 or (CH3COO)2Zn); it partially blocks Cu absorption by induction of metallothionein, which binds Cu in the mucosal cells until they are not desquamated and eliminated25). Moreover, zinc is fully effective, non-toxic, body’s own substance. On the other hand, zinc exhibits a too slow effect especially in the acute phase of Cu toxicity with neurological symptoms. Therefore, chelating agents are used, including D-penicillamine, trientine (risk of toxicity) and tetrathiomolybdate. These create complexes with dietary protein and Cu, and make Cu to be non-toxic. Subsequently, patients are treated by maintenance zinc therapy67). Concomitant dietary restrictions removing foods with high content of copper (for example chocolate, oysters and fungi) are required. With early diagnosis and treatment, these people can live a normal life25).
Idiopathic copper toxicosis
This rare disorder is a hereditary genetic disease of Cu metabolism, called also non-Indian childhood cirrhosis. It is characterized by abnormally high Cu levels in the liver, normal or elevated plasma concentrations of copper and CP, clinical onset of cirrhosis by 2 years of age, and death within 5 years.
Early initiated D-penicillamine treatment prevents fatal consequences and often renewed liver histology49).
Copper and neurodegenerative diseases
The disturbance of Cu homeostasis leading to oxidative stress and formation of free radicals contributes to the development of Alzheimer’s disease and Creutzfeldt-Jakob disease2). In Alzheimer’s disease, we often encounter an increased Cu concentration in the cerebrospinal fluid, the Cu concentration in the plasma is either normal or elevated59). Alzheimer’s disease aetiology is associated with the accumulation of β-amyloid protein. Under normal conditions, its precursor binds Cu (in reduced state) and facilitates its transport across the entire length of the neuron (from the cell body to the surface of the axon and to the plasma membrane of the dendrites). In Alzheimer’s disease, the function of β-amyloid protein precursor is impaired, leading to Cu oxidation in the presence of H2O2 and to production of free oxygen radicals. At the same time, β-amyloid protein precursor fragmentation occurs, these aggregate and damage the neurons by free oxygen radicals2). In addition, Cu extracellularly accumulates in amyloid plaques, which leads59) to reduction of the cytochrome-c oxidase and superoxide dismutase activity. The consequences of these events are reduction of the key metabolic and defence mechanisms and damages to the neurons2).
In the case of Creutzfeldt-Jakob disease, another neuronal membrane protein (prion), allowing Cu entry by endocytosis, is damaged, the same as in the scrapie in humans and bovine spongiform encephalopathy (mad cow disease) in bovine animals too. As in the previous case, a protein conformational change alters its function. The structural change involves the transition of a natural α-helical prion into a β-leaf conformation that confers the pathogenic potential of the protein and simultaneously aggregates it. Moreover, the mutant prion possesses a markedly reduced ability for Cu transport, making neuronal cells susceptible to oxidative stress2).
The relationship between Cu and the development of Parkinson’s disease is also described. Cu is associated with accelerated aggregation of α-synuclein protein in the formation of Lewy bodies3, 68). Similarly, Cu has been shown to promote aggregation of mutant Huntington disease polyglutamine repeat proteins. If Cu dysregulation is a cause or a consequence of these neurodegenerative diseases is under considerable investigation3).
Copper and cancer
In cancer, Cu (and simultaneously CP) concentrations are almost always significantly increased (2–3 times), while concentrations of zinc, iron and selenium are significantly decreased69).
Serum Cu concentrations correlate with tumour development, its size, occurrence, progression and recurrence and it can be said that the malignant tumours have often higher Cu concentrations2, 70). ROS production and oxidative stress are probably the major mechanisms involved in the development of cancer59). Moreover, Cu induces proliferation and migration of endothelial cells by activating various angiogenic factors (e.g., endothelial growth factor, basic fibroblast growth factor, tumour necrosis factor, and interleukin 1). As a result, new blood vessels are formed, because tumours require a rich supply of oxygen and nutrients. The formation of new bloodstream can be reversed by chelating agents2), such as D-penicillamine, tetrathiomolybdate, clioquinol and trientine, which are antiangiogenic agents still in clinical trials69).
In cancer, the level of plasma CP positively correlates with the stage of disease, and it can be said that malignant tumours have often higher Cu concentrations70).
Antimicrobial and antiviral activity of copper
Although Cu is necessary for many life processes, it is also a powerful antimicrobial weapon against many microorganisms. For over a hundred years it is used as a bactericidal and fungicidal agent. Cu antimicrobial activity is also utilized by the immune cells of eukaryotes. Many studies have shown that activated macrophages accumulate Cu within the phagosome that captures and prohibits microbial attack. In addition, levels of ATP7A protein are increased and Cu is transferred from the secretory space into the phagosome, resulting in a parallel increase of the levels of phagosomal Cu concentration.
These observations suggest responsibility of ATP7A for the increase in phagosomal Cu concentrations during infection12, 13, 71).
Several reports demonstrate that bacterial cells upregulate the expression of Cu ATPases during infection in dependency on metalloregulatory transcription factors of Cu, resulting in their ability to export Cu. Observing that bacterial pathogens actively exclude intracellular accumulation of Cu or alter the distribution of Cu in mammalian hosts suggests that the Cu status is a key battleground during infections12).
Biocidal mechanism of copper
The exact mechanisms of the biocidal effect of Cu are a source of ongoing investigation. It is assumed that the cause of cell death is multifactorial rather than the result of a single universal mechanism72). A key property of Cu which significantly contributes to its toxic effect is its ability to accept and donate single electrons as it changes the oxidation state between Cu+ and Cu2+. This allows Cu to act as a catalyst for the generation of ROS, such as hydroxyl radicals and superoxide anions. These ROS have a potential to cause oxidative damage to proteins, nucleic acids and lipids (including those in the cell membrane). The formation of ROS is the main mechanism of the Cu antibacterial effect73).
Free Cu ions may compete with zinc or other metal ions for important binding sites on proteins, leading to conformational change and the loss of protein function74, 75).
In addition, Cu ions can lead to the depletion of the sulfhydryl groups, for example in cysteines or glutathione in cytoplasmic enzymes needed to make branched-chain amino acids76).
Bacteria have developed a number of protection mechanisms against the toxic effects of Cu ions: extracellular Cu ion sequestration, relative impermeability of the outer and inner bacterial membrane to Cu ions, Cu absorbing metallothionein-like proteins in the cytoplasm and periplasm, and active cell mediated secretion75).
However, when they exceed a certain level of Cu and an exposure time (they are different between organisms), they cannot cope with Cu overload and can die. Due to the multifactorial death mechanism of Cu and especially its non-specific mechanism of damage their tolerance to Cu is relatively low. The bacterial Cu resistance systems do not usually provide protection but only prolong their survival75).
Cu possesses a potent antiviral (virucidal) activity. The inactivation of the enveloped or non-enveloped, single - or double-stranded DNA or RNA viruses by Cu and Cu compounds has been reported in many studies18, 20, 77–79).
Cu2+ ions may inactivate viruses in a number of ways by binding electron donor groups on proteins or nucleic acids80). The results are a spiral structure breakdown of nucleic acids and their denaturation. In the single chain DNA, the Cu binding site occurs on average in every third nucleotide. Moreover, formation of ROS can affect the peptide backbone of the capsid proteins of the virions81).
The viruses generally have no copper tolerance74). Moreover, researchers Sagripanti et al., demonstrated, specifically in the Herpes simplex virus, the Cu enhanced antiviral effect with the following reducing agents in the order: ascorbic acid ˃˃ hydrogen peroxide > cysteine19).
Health related copper applications
Copper and copper-based compounds, due to their potent biocidal properties, are currently used in several medical and in many nonmedical areas, which affect human health (see Table 1, 2).
Tab. 1. Current and future potential applications of copper and copper compounds in the medical area 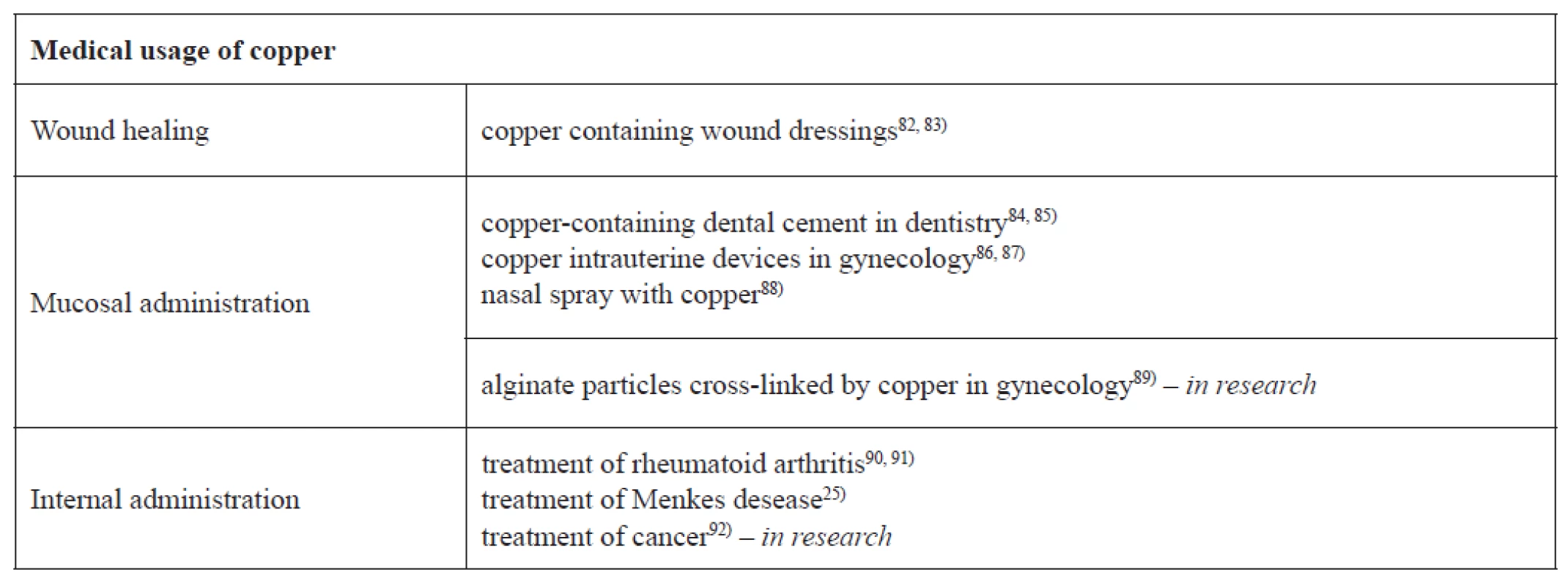
Tab. 2. Current and future potential applications of copper and copper compounds in with health related areas 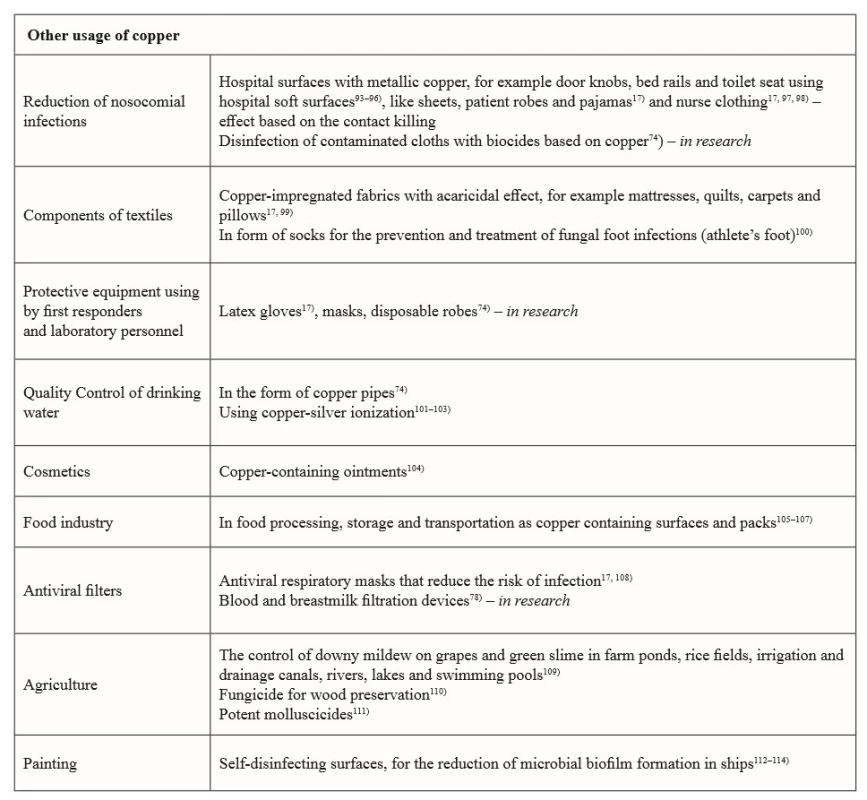
Although copper is widely used in many areas of human life, the main disadvantages of copper medical uses are: inapplicability in systemic infections, mainly due to its potential toxicity after orally administration and its price74).
Conclusion
In conclusion, copper is an essential mineral for human life and its homeostasis is strictly maintained by many physiological processes. Although the Cu safety is often discussed, potent biocidal properties against microorganisms destine its widespread use in many areas related to human health. Many of these utilizations (for example intrauterine devices, dental cement) are already being commonly used. Therefore, it is expected that other novel approaches using Cu antimicrobial properties could bring new positive effects on health and life of humans. These new technologies, using for example non-soluble copper compounds into polymeric yarns, from which many textile and other consumer products for protection health workers and patients are produced, are in the research phase. Also, use of copper surfaces leading to the contact killing of pathogenic microorganisms in hospitals, followed by reduction of nosocomial infections occurrence, offers a great potential to the future. Reduced antibiotic consumption is ultimately a huge benefit. Nevertheless, in spite of the fact that Cu is a very attractive active material used for maintaining and even improving human health, its use should be very carefully considered, especially with regard to its possible toxic effects at the cellular and tissue level.
Conflicts of interest: none.
Received August 6, 2018 / Accepted August 21, 2018
M. Pavelková • J. Vysloužil • doc. PharmDr. Kateřina Kubová, Ph.D. (✉) • D. Vetchý
University of Veterinary and Pharmaceutical Sciences Brno
Department of Pharmaceutics
Palackého 1, 602 00 Brno, Czech Republic
e-mail: kubovak@vfu.cz
Zdroje
1. Olivares M., Uauy R. Copper as an essential nutrient. Am. J. Clin. Nutr. 1996; 63, 791S–796S.
2. Krupanidhi S., Sreekumar A., Sanjeevi C. B. Copper & biological health. Indian J. Med. Res. 2008; 128, 448–461.
3. Gaetke L. M., Chow-Johnson H. S., Chow Ch. K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014; 88, 1929–1938.
4. Sharp P. A. Ctr1 and its role in body copper homeostasis. Int. J. Biochem. Cell B. 2003; 35, 288–291.
5. Chillappagari S., Seubert A., Trip H., Kuipers O. P., Marahiel M. A., Miethke M. Copper Stress Affects Iron Homeostasis by Destabilizing Iron-Sulfur Cluster Formation in Bacillus subtilis. J. Bacteriol. 2010; 192, 2512–2524.
6. Ferns G. A. A., Lamb D. J., Taylor A. The possible role of copper ions in atherogenesis: the Blue Janus. Atherosclerosis 1997; 133, 139–152.
7. Arredondo M., Núñez M. T. Iron and copper metabolism. Mol. Aspects Med. 2005; 26, 313–327.
8. Uauy, R., Olivares M., Gonzalez M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998; 67, 952S–959S.
9. Angelova M., Asenova S., Nedkova V., Koleva-Kolarova R. Copper in the human organism. Trakia J. Sci. 2011; 9, 88–98.
10. Wang K., Chen X., Cui Y., Gao X. The reference interval of zinc, copper, selenium and zinc/copper ratio of healthy adult in Licang. Trace Elem. Electroly. 2011; 28, 1–10.
11. Tapiero H., Townsend D. M., Tew K. D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003; 57, 386–398.
12. Festa R. A., Thiele D. J. Copper: an Essential Metal in Biology. Curr. Biol. 2011; 21, R877–R883.
13. Ladomersky E., Petris M. J. Copper tolerance and virulence in bacteria Metallomics. 2015; 7, 957–964.
14. O’Gorman J., Humphreys H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012; 81, 217–223.
15. Cai X., Zhang B., Liang Y., Zhang J., Yan Y., Chen X., Wu Z., Liu H., Wen S., Tan S., Wu T. Study on the antibacterial mechanism of copper ion - and neodymium ion-modified α-zirconium phosphate with better antibacterial activity and lower cytotoxicity. Colloid. Surface B. 2015; 132, 281–289.
16. Ren G., Hu D., Cheng E. W., Vargas-Reus M. A., Reip P., Allaker R. P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Ag. 2009; 33, 587–590.
17. Borkow G., Gabbay J. Putting copper into action: copper impregnated products with potent biocidal activities. FASEB J. 2004; 18, 1728–1730.
18. Sagripanti J.-L., Routson L. B., Lytle C. D. Virus Inactivation by Copper or Iron Ions Alone and in the Presence of Peroxide. Appl. Environ. Microbiol. 1993; 59, 4374–4376.
19. Sagripanti J. L., Routson L. B., Bonifacino A. C., Lytle C. D. Mechanism of Copper-Mediated Inactivation of Herpes Simplex Virus. Antimicrob. Agents Ch. 1997; 41, 812–817.
20. Sagripanti J. L., Lightfoote M. M. Cupric and Ferric Ions Inactivate HIV. AIDS Res. Hum. Retrov. 1996; 12, 333–337.
21. Roblero L., Guadarrama A., Lopez T., Zegers-Hochschild F. Effect of copper ion on the motility, viability, acrosome reaction and fertilizing capacity of human spermatozoa in vitro. Reprod. Fertil. Dev. 1996; 8, 871–874.
22. Hostynek J. J., Maibach H. I. Copper hypersensitivity: dermatologic aspect – an overview. Rev. Environ. Health 2003; 18, 153–183.
23. Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception 2007; 75, S70–S75.
24. Hubacher D., Lara-Ricalde R., Douglas M. D., Taylor J., Guerra-Infante F., Guzmán-Rodríguez R. Use of Copper Intrauterine Devices and the Risk of Tubal Infertility among Nulligravid Women. N. Engl. J. Med. 2001; 345, 561–567.
25. Stern B. R., Solioz M., Krewski D., Aggett P., Aw T.-Ch., Baker S., Crump K., Dourson M., Haber L., Hertzberg R., Keen C., Meek B., Rudenko L., Schoeny R., Slob W., Star T. Copper and Human Health: Biochemistry, Genetics, and Strategies for Modeling Dose-response Relationships. J. Toxicol. Environ. Health B Crit. Rev. 2007; 10, 157–222.
26. Mills C. F. Dietary interactions involving the trace elements. Annu. Rev. Nutr. 1985; 5, 173–193.
27. Sandstead H. H. Copper bioavailability and requirements. Am. J. Clin. Nutr. 1982; 35, 809–814.
28. Turnlund J. R., King J. C., Gong B., Keyes W. R., Michel M. C. A stable isotope study of copper absorption in young men: effect of phytate and α-cellulose. Am. J. Clin. Nutr. 1985; 42, 18–23.
29. Milne D. B., Klevay L. M., Hunt J. R. Effects of ascorbic acid supplements and a diet marginal in copper on indices of copper nutriture in women. Nutr. Res. 1988; 8, 865–873.
30. Jacob R. A., Skala J. H., Omaye S. T., Turnlund J. R. Effect of Varying Ascorbic Acid Intakes on Copper Absorption and Ceruloplasmin Levels in Young Men. J. Nutr. 1987; 117, 2109–2115.
31. Redman R. S., Fields M., Reiser S., Smith J. C. Jr. Dietary fructose exacerbates the cardiac abnormalities of copper deficiency in rats. Atherosclerosis 1988; 74, 203–214.
32. Holbrook J. T., Smith J. C. Jr., Reiser S. Dietary fructose or starch: effects on copper, zinc, iron, manganese, calcium and magnesium balances in humans. Am. J. Clin. Nutr. 1989; 49, 1290–1294.
33. World Health Organization Geneva. Trace elements in human nutrition and health. WHO Library Cataloguing in Publication Data 1996.
34. Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217.
35. Collins J. F., Prohaska J. R., Knutson M. D. Metabolic crossroads of iron and copper. Nutr. Rev. 2010; 68, 133–147.
36. Nose Y., Wood L. K., Kim B.-U., Prohaska J. R., Fry R. S., Spears J. W., Thiele D. J. Ctr1 Is an Apical Copper Transporter in Mammalian Intestinal Epithelial Cells in Vivo That Is Controlled at the Level of Protein Stability. J. Biol. Chem. 2010; 285, 32385–32392.
37. Nose Y., Kim B.-E., Thiele D. J. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006; 4, 235–244.
38. Kim H., Son H. Y., Bailey S. M., Lee J. Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am. J. Physiol.-Gastr. L. 2009; 296, G356–G364.
39. Zimnicka A. M., Maryon E. B., Kaplan J. H. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J. Biol. Chem. 2007; 282, 26471–26480.
40. Moriya M., Ho Y.H., Grana A., Nguyen L., Alvarez A., Jamil R., Ackland M.L., Michalczyk A., Hamer P, Ramos D., Kim S., Mercer J. F., Linder M. C. Copper is taken up efficiently from albumin and alpha2-macroglobulin by cultured human cells by more than one mechanism. Am. J. Physiol.-Cell Ph. 2008; 295, C708–C721.
41. Bost M., Houdart S., Oberli M., Kalonji E., Huneau J.-F., Margaritis I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Bio. 2016; 35, 107–115.
42. Ferguson-Miller S., Babcock G. T. Heme/Copper Terminal Oxidases. Chem. Rev. 1996; 96, 2889–2908.
43. Wikstrom M. K. F. Proton pump coupled to cytochrome-c oxidase in mitochondria. Nature 1977; 266, 271–273.
44. Jepma M., Deinum J., Asplund Ch. L., Rombouts S. A., Tamsma J. T., Tjeerdema N., Spapé M. M., Garland E. M., Robertson D., Lenders J. W. M., Nieuwenhuis S. Neurocognitive Function in Dopamine-β-Hydroxylase Deficiency. Neuropsychopharmacol. 2011; 36, 1608–1619.
45. Biesemeier A., Kreppel F., Kochanek S., Schraermeyer U. The classical pathway of melanogenesis is not essential for melanin synthesis in the adult retinal pigment epithelium. Cell Tissue Res. 2010; 339, 551–560.
46. Saenko E. L., Yaropolov A. I., Harris E. D. Biological functions of ceruloplasmin expressed through copper-binding sites and a cellular receptor. J. Trace Elem. Exp. Med. 1994; 7, 69–88.
47. Mukhopadhyay Ch. K., Mazumder B., Fox P. L. Role of Hypoxia inducible Factor-1 in Transcription Activation of Ceruloplasmin by Iron Deficiency. J. Biol. Chem. 2000; 275, 21048–21054.
48. Bonham M., O’Connor J. M., Hannigan B. M., Strain J. J. The immune system as a physiological indicator of marginal copper status? Brit. J. Nutr. 2002; 87, 393–403.
49. Gaetke L. M., Chow Ch. K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163.
50. Dizdaroglu M., Rao G., Halliwel B., Gajewski E. Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions. Arch. Biochem. Biophys. 1991; 285, 317–324.
51. Sagripanti J.-L., Kraemer K. H. Site-specific Oxidative DNA Damage at Polyguanosine Produced by Copper Plus Hydrogen Peroxide. J. Biol. Chem. 1989; 264, 1729–1734.
52. Sagripanti J.-L., Goering P. L., Lamanna A. Interaction of copper with DNA and antagonism by other metals. Toxicol. Appl. Pharm. 1991; 110, 477–485.
53. Li Y., Trush M. A. DNA damage resulting from the oxidation of hydroquinone by copper: role for a Cu(II)Cu(I) redox cycle and reactive oxygen generation. Carcinogenesis. 1993; 14, 1303–1311.
54. Toyokosni S., Sagripanti J.-L. Increased 8-hydroxyguanosine in kidney and liver of rats continuously exposed to copper. Toxicol. Appl. Pharm. 1994; 126, 91–97.
55. Samuni A., Aronovitch J., Godinger D., Chevion M., Czapski G. On the cytotoxicity of vitamin C and metal ions. A site-specific Fenton mechanism. Eur. J. Biochem. 1993; 137, 119–124.
56. Stohs S. J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Bio. Med. 1995; 18, 321–336.
57. Birben E., Sahiner U. M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012; 5, 9–19.
58. Wang X., Moulla D., Wright J.A., Brown D. R. Copper binding regulates intracellular alpha-synuclein localisation, aggregation and toxicity. J. Neurochem. 2010; 113, 704–714.
59. Iakovidis I., Delimaris I., Piperakis S. M. Copper and Its Complexes in Medicine: A Biochemical Approach. SAGE-Hindawi Access to Research. Mol. Biol. Int. 2011; Article ID 594529, 13p.
60. Muñoz C., López M., Olivares M., Pizarro F., Arredondo M., Araya M. Differential response of interleukin-2 production to chronic copper supplementation in healthy humans. Eur. Cytokine Netw. 2005; 16, 261–265.
61. Uriu-Adams J. Y., Keen C. L. Copper, oxidative stress, and human health. Mol. Aspects Med. 2005; 26, 268–298.
62. Hawk S. N., Lanoue L., Keen C. L., Kwik-Uribe C. L., Rucker R. B., Uriu-Adams J. Y. Copper-deficient rat embryos are characterized by low superoxide dismutase activity and elevated superoxide anions. Biol. Reprod. 2003; 68, 896–903.
63. Lynch S. M., Frei B., Morrow J. D., Roberts L. J., Xu A., Jackson T., Reyna R., Klevay L. M., Vita J. A., Keaney J. F. Jr. Vascular superoxide dismutase deficiency impairs endothelial vasodilator function through direct inactivation of nitric oxide and increased lipid peroxidation. Arterioscl. Throm. Vas. 1997; 17, 2975–2981.
64. Johnson W. T., Thomas A. C. Copper deprivation potentiates oxidative stress in HL-60 cell mitochondria. Proc. Soc. Exp. Biol. Med. 1999; 221, 147–152.
65. Chen Y., Saari J. T., Kang Y. J. Weak antioxidant defenses make the heart a target for damage in copper-deficient rats. Free Radical Bio. Med. 1994; 17, 529–536.
66. Nelson S. K., Huang C. J., Mathias M. M., Allen K. G. Copper-marginal and copper-deficient diets decrease aortic prostacyclin production and copper-dependent superoxide dismutase activity, and increase aortic lipid peroxidation in rats. J. Nutr. 1992; 122, 2101–2108.
67. Brewer G. J. Copper Control as an Antiangiogenic Anticancer Therapy: Lessons from Treating Wilson’s Disease. Exp. Biol. M. 2001; 226, 665–673.
68. Xu L., Pu J. Alpha-Synuclein in Parkinson’s Disease: From Pathogenetic Dysfunction to Potential Clinical Application. Hindawi Publishing Corporation Parkinson’s Disease. 2016; Article ID 1720621, 10p.
69. Gupte A., Mumper R. J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009; 35, 32–46.
70. Zowczak M., Iskra M., Paszkowski J., Mańcyak M., Torliński L., Wysocka E. Oxidase activity of ceruloplasmin and concentrations of copper and zinc in serum of cancer patients. J. Trace Elem. Med. Bio. 2001; 15, 193–196.
71. Samanovic M. I., Ding C., Thiele D. J., Darwin K. H. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe 2012; 11, 106–115.
72. Warnes S. L., Caves V., Keevil C. W. Mechanism of copper surface toxicity in Escherichia coli 0157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ. Microbiol. 2012; 14, 1730–1743.
73. Santo C. E., Lam E. W., Elowsky C. G., Quaranta D., Domaille D. W., Chang C. J., Grass G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microb. 2011; 77, 794–802.
74. Borkow G., Gabbay J. Copper, An Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Bio. 2009; 3, 272–278.
75. Grass G., Rensing C., Solioz M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microb. 2011; 77, 1541–1547.
76. Macomber L., Imlay J. A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. P. Natl. Acad. Sci. USA. 2009; 106, 8344–8349.
77. Sagripanti J.-L. Metal-based formulations with high microbicidal activity. Appl. Environ. Microb. 1992; 58, 3157–3162.
78. Borkow G., Sidwell R. W., Smee D. F., Barnard D. L., Morrey J. D., Lara-Villegas H. H. , Shemer-Avni Y., Gabbay J. Neutralizing Viruses in Suspensions by Copper Oxide-Based Filters. Antimicrob. Agents Ch. 2007; 51 : 2605–2607.
79. Borkow G., Lara H. H., Covington C. Y., Nyamathi A., Gabbay J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob. Agents Ch. 2008; 52, 518–525.
80. Abad F. X., Pinto R. M., Diez J. M., Bosch A. Disinfection of human enteric viruses in water by copper and silver in combination with low levels of chlorine. Appl. Environ. Microb. 1994, 60, 2377–2383.
81. Samuni A., Chevion M., Czapski G. Roles of copper and superoxide anion radicals in the radiation-induced inactivation of T7 bacteriophage. Radiat. Res. 1984; 99, 562–572.
82. Borkow G., Okon-Levy N., Gabbay J. Copper oxide impregnated wound dressings: biocidal and safety studies. Wounds 2010; 22, 301–310.
83. Sen C. K., Khanna S., Venojarvi M., Trikha P., Ellison E. C., Hunt T. K., Roy S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol.-Heart C. 2002; 282, H1821–H1827.
84. Thneibat A., Fontana M, Cochran M. A., Gonzalez-Cabezas C., Moore B. K., Matis B. A., Lund M. R. Anticariogenic and antibacterial properties of a copper varnish using an in vitro microbial caries model. Oper. Dent. 2008; 33, 142–148.
85. Foley J., Blackwell A. In vivo cariostatic effect of black copper cement on carious dentine. Caries Res. 2003; 37, 254–260.
86. Wu J. P., Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception 2014; 89, 495–503.
87. Bilian X. Intrauterine devices. Best Pract. Res. Cl. Ob. 2002; 16, 155–168.
88. Stérimar. https://sterimar.com/en/our-products/nez-sujet-aux-infections/ (26. 4. 2018)
89. Pavelková M., Kubová K., Vysloužil J., Kejdušová M., Vetchý D., Celer V., Molinková D., Lobová D., Pechová A., Vysloužil J., Kulich P. Biological effects of drug-free alginate beads cross-linked by copper ions prepared using external ionotropic gelation. AAPS Pharmscitech. 2017; 18, 1343–1354.
90. Jackson G. E., May P. M., Williams D. R. Metal-ligand complexes involved in rheumatoid arthritis-I: justifications for copper administration. J. Inorg. Nucl. Chem. 1978; 40, 1189–1194.
91. El-Gammal O. A., Elmorsy E. A., Sherif Y. E. Evaluation of the anti-inflammatory and analgesic effects of Cu(II) and Zn(II) complexes derived from 2-(naphthalen-1-yloxy)-N‘-(1-(pyridin-2-1)ethylidene)acetohydrazide. Spectrochim. Acta A. 2014; 120, 332–339.
92. Tisato F., Marzano C., Porchia M., Pellei M., Santini C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010; 30, 708–749.
93. Weaver L., Michels H. T., Keevil C. W. Survival of Clostridium difficile on copper and steel: futuristic options for hospital hygiene. J. Hosp. Infect. 2008; 68, 145–151.
94. Mehtar S., Wiid I., Todorov S. D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: an in vitro study. J. Hosp. Infect. 2008; 68, 45–51.
95. Noyce J. O., Michels H., Keevil C. W. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006; 63, 289–297.
96. Noyce J. O., Michels H., Keevil C. W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007; 73, 2748–2750.
97. Borkow G., Gabbay J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008; 70, 990–994.
98. Gabbay J., Borkow G., Mishal J., Magen E., Zatcoff R., Shemer-Avni Y. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Text. 2006; 35, 323–335.
99. Mumcuoglu K. Y., Gabbay J., Borkow G. Copper oxide-impregnated fabrics for the control of house dust mites. Int. J. Pest Manage. 2008; 54, 235–240.
100. Zatcoff R. C., Smith M. S., Borkow G. Treatment of tinea pedis with socks containing copper-oxide impregnated fibers. Foot. 2008; 18, 136–141.
101. Lin Y. E., Vidic R. D., Stout J. E., Yu V. L. Legionella in water distribution systems. J. AWWA. 1998; 90, 112–121.
102. Rohr U., Weber S., Selenka F., Wilhelm M. Impact of silver and copper on the survival of amoebae and ciliated protozoa in vitro. Int. J. Hyg. Envir. Heal. 2000; 203, 87–89.
103. Cachafeiro S. P., Naveira I. M., García I. G. Is copper-silver ionisation safe and effective in controlling legionella? J. Hosp. Infect. 2007; 67, 209–216.
104. Gorter R. W., Butorac M., Cobian E. P. Examination of the cutaneous absorption of copper after the use of copper-containing ointments. Am. J. Ther. 2004; 11, 453–458.
105. Mulligan A. M., Wilson M., Knowles J. C. The effect of increasing copper content in phosphate-based glasses on biofilms of Streptococcus sanguis. Biomaterials 2003; 24, 1797–1807.
106. Faúndez G., Troncoso M., Navarrete P., Figueroa G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 2004; 4, 7p.
107. Hassan A. A., Shoukary N. M., Ismail N. M. Efficacy of temperature, and two commonly used molluscicides and fertilizers on Fasciola gigantica eggs. J. Egypt. Soc. Parasitol. 2008; 38, 621–634.
108. Borkow G., Zhou S. S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE. www.plosone.org. 2010; 5, e11295.
109. La Torre A., Talocci S., Spera G., Valori R. Control of downy mildew on grapes in organic viticulture. Commun. Agric. Appl. Biol. Sci. 2008; 73, 169–178.
110. Schultz T. P., Nicholas D. D., Preston A. F. A brief review of the past, present and the future of wood preservation. Pest. Manag. Sci. 2007; 63, 784–788.
111. Ragab F., Shoukry N.M. Influence of certain fertilizers on the activity of some molluscicides against Biomphalaria alexandrina and Lymnaea natalensis snails. J. Egypt. Soc. Parasitol. 2006; 36, 959–977.
112. UK Marine Special Areas of Conservation. Copper-based antifouling paints. www.ukmarinesac.org.uk/activities/ports/ph4_3_1.htm (27. . 018).
113. Cooney T. E. Bactericidal activity of copper and noncopper paints. Infect. Cont. Hosp. Ep. 1995; 16, 444–450.
114. Cooney J. J., Tang R. J. Quantifying effects of antifouling paints on microbial biofilm formation. Method. Enzymol. 1999; 310, 637–644.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2018 Číslo 4- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Biologická role mědi jako základního stopového prvku v lidském organismu
- Studie vlivu derivátu 1,3-oxazol-4-yl-fosfonové kyseliny na systémové indikátory aktivity oxidu dusnatého u potkanů s arteriální hypertenzí
- Studium obsahových látek kořenu a nati Smallanthus sonchifolius pomocí plynové chromatografie – hmotnostní spektrometrie
- PRACOVNÍ DEN SEKCE TECHNOLOGIE LÉKŮ S NÁZVEM „Pokroky ve farmaceutické technologii“
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Biologická role mědi jako základního stopového prvku v lidském organismu
- PRACOVNÍ DEN SEKCE TECHNOLOGIE LÉKŮ S NÁZVEM „Pokroky ve farmaceutické technologii“
- Studium obsahových látek kořenu a nati Smallanthus sonchifolius pomocí plynové chromatografie – hmotnostní spektrometrie
- Studie vlivu derivátu 1,3-oxazol-4-yl-fosfonové kyseliny na systémové indikátory aktivity oxidu dusnatého u potkanů s arteriální hypertenzí
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání